Abstract
Purpose
The titanium mesh cage (TMC) is a typical metal cage device which has been widely used in cervical reconstruction for decades. Nano-hydroxyapatite/polyamide-66 (n-HA/PA66) cage is a novel biomimetic non-metal cage device growing in popularity in many medical centres in recent years. There has been no comparison of the efficacy between these two anterior reconstructing cages. The purpose of this study was to compare the radiographic and clinical outcomes of these two different devices.
Methods
Sixty-seven eligible patients with single-level ACCF using TMC or n-HA/PA66 cage for cervical degenerative diseases, with four-year minimum follow-up, were included in this prospective non-randomised comparative study. Their radiographic (cage subsidence, fusion status, segmental sagittal alignment [SSA]) and clinical (VAS and JOA scales) data before surgery and at each follow-up was recorded completely.
Results
The fusion rate of the n-HA/PA66 group was higher than TMC at one year after surgery (94 % vs. 84 %) though their finial fusion rates were similar (97 % vs. 94 %). Finial n-HA/PA66 cage subsidence was 1.5 mm with 6 % of severe subsidence over three millimetres, which was significantly lower than the respective 2.9 mm and 22 % of TMC (P < 0.0001). Lastly, SSA, VAS and JOA in TMC group were worse than in the n-HA/PA66 group (P = 0.235, 0.034 and 0.007, respectively).
Conclusions
The n-HA/PA66 cage is associated with earlier radiographic fusion, less subsidence and better clinical results than TMC within four years after one-level ACCF. With the added benefit of radiolucency, the n-HA/PA66 cage may be superior to TMC in anterior cervical construction.
Keywords: Anterior cervical corpectomy, Fusion, Nano-hydroxyapatite/polyamide 66 cage, Titanium mesh cage
Introduction
Anterior cervical corpectomy and fusion (ACCF) is proposed as a surgical alternative technique to anterior cervical discectomy and fusion (ACDF) when the anterior compression is not limited to the disc level. Anterior corpectomy can provide direct decompression of the fractured vertebral body, proliferating osteophyte or ossification of the posterior longitudinal ligament. However, the stability of the anterior vertebral column is destroyed following resection, and it subsequently makes reconstruction another critical procedure in these operations. Use of autogenous bone grafts from the iliac crest for cervical reconstruction has been considered as the gold standard for decades [1]. The tricortical iliac crest graft can achieve satisfactory osseous fusion but it brings around 25 % of donor-site morbidities, including haematoma, persistent donor-site pain and infection [2, 3]. To prevent these complications, structural allograft is used as an alternative transplant in anterior fusion. However, lower fusion rates, higher rates of breakage and the risk of disease transmission have greatly limited the use of this graft [4, 5].
Recently, the application of cages has become a popular method of anterior reconstruction. On the one hand, the material strength and toughness of cages can provide sufficient immediate stability for a reconstructed segment, ensuring later bone graft fusion. On the other hand, the cages can be filled by crumbled autograft, allograft, artificial bone graft or even hybrid graft as required. It provides more choices for anterior reconstruction to ensure satisfactory osseous fusion and reduce the complications. Currently available cage devices can be divided into metallic and non-metallic cages. The titanium mesh cage (TMC) is a typical metallic cage and has been widely used in spinal reconstruction since it was first introduced in 1986 [6]. The TMC has adequately demonstrated the superiorities of cage devices to structural autograft or allograft with respect to their good mechanical behaviour and satisfactory clinical outcomes [7, 8]. However, the stress shielding, postoperative radiographic interference, and high rate of subsidence of TMCs are still of great concern which hinders this cage becoming an ideal reconstructing device [6, 9]. To prevent these material defects, recent studies have tended to focus on the non-metallic cage as an alternative device to traditional metallic cages. More recently, PMMA (polymethyl methacrylate), stackable PEEK (polyetheretherketone) and stackable CFRP (carbon fibre reinforced polymer) cages have been developed for reconstruction following anterior cervical corpectomy [10, 11].
The hollow cylindrical n-HA/PA66 cage (nano-hydroxyapatite/polyamide-66) is a bionic non-metallic cage made by covalent miscibility of the nano-hydroxyapatite and polyamide-66 which simulates the constituent form of natural bone (Fig. 1). The n-HA/PA66 cage for cervical reconstruction is eight to 14 mm in outer diameter and three to eight millimetres in inner diameter while its length is adjusted as clinically required. Each cage has wide rims (nearly three millimetres) with several shallow recesses designed to prevent subsidence and migration by decreasing the cutting action and increasing the friction between the cage edges with vertebral endplates. This cage has been approved for clinical use by the State Drug and Food Administration of China since 2005. In recent years, the use of this cage for anterior cervical fusion has become more and more popular in many medical centres, and the short-term clinical effects are satisfactory [12, 13]. However, detailed comparative assessments of the n-HA/PA66 cage with other cage devices have not been available. In this study, we observed the results of patients who had undergone single-level anterior cervical corpectomy and fusion with n-HA/PA66 cage or TMC to compare the differences in radiographic and clinical outcomes between these two cage devices.
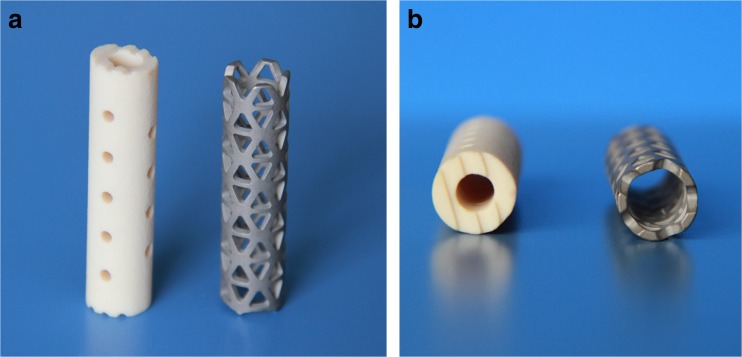
Fig. 1.
Frontal (a) and transverse (b) view of cervical nano-hydroxyapatite/polyamide 66 cage (n-HA/PA66) (left side) and titanium mesh cage (TMC) (right side). There are several shallow recesses designed on the rims of the n-HA/PA66 cage to prevent cage translocation (a). The rims of the n-HA/PA66 cage are wider than TMC which reduce cutting of the cage into the endplates and preventing subsidence (b)
Patients and Methods
This was a prospective, non-randomised, comparative study of patients with cervical myelopathy or radiculopathy who underwent single-level anterior cervical corpectomy and fusion (ACCF) with one cage and anterior plate. The study was both approved by the Institutional Review Board and the Ethics Committee of West China Hospital of Sichuan University and was performed at our department from January 2008 to January 2009.
During this period, 80 consecutive patients were eligible for inclusion, however only 67 patients (84 %) with complete data sets (preoperative and more than four years follow-up data) were included in the final analysis. The inclusion criteria were: (1) myelopathy or radiculopathy with failure of conservative treatment (of at least three months), (2) the radiographs documented cervical degenerative diseases with compression of the cervical spinal cord or nerve roots, or whichever best explains the clinical symptoms and signs, and (3) the spinal cord or nerve roots could be adequately decompressed by single-level ACCF surgery. The exclusion criteria were: (1) cervical deformities or neoplasia, (2) severe osteoporosis, and (3) patients with psychiatric disorders. Each patient gave a signed informed consent for entry into this study. The level of corpectomy was determined mainly on the clinical presentation and radiological findings. The anterior fusion was performed using either TMC (Medtronic Sofamor Danek Inc. Memphis, TN) or n-HA/PA66 cage (Sichuan National Nano Technology Co., Ltd. Chengdu, SC), and the cage was chosen at the discretion of the surgeon.
Surgical Technique and Cage Preparation
All the operations were performed by one of four senior spine surgeons. The patients were placed in the supine position with neck extension. Standard right-side anterior cervical approach, cervical corpectomy and decompression were performed. A high-speed burr and curette were used to prepare the adjacent endplates. Then a TMC or n-HA/PA66 cage was cut to the proper length and contour according to the angle of proximal and distal endplates. The cage was filled with morsellised autologous bone from local resected vertebral body before being inserted into the intervertebral space (Fig. 2). The Atlantis anterior cervical plate system (Medtronic Sofamor Danek Inc. Memphis, TN) was used in each patient for further stabilisation. After surgery, all patients were requested to wear a soft cervical collar for approximately four weeks.
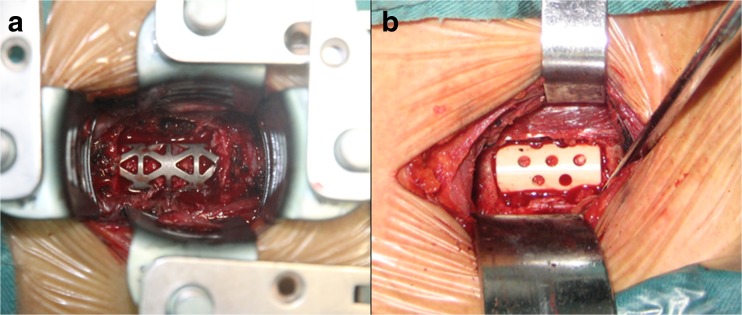
Fig. 2.
Implanting of the cages after cervical decompression. a Titanium mesh cage. b Nano-hydroxyapatite/polyamide 66 cage
Outcomes assessment
The patients had radiological follow-up immediately, at one, three, and six months and then annually after surgery. The cervical plain radiographs were taken at each follow-up examination. The following parameters were evaluated on lateral plain radiographs: cage subsidence and preoperative and postoperative segmental sagittal alignment (SSA). The height of the fusion segment was measured as the distance between the midpoint of the superior endplate of the upper vertebra and the midpoint of the inferior endplate of the lower vertebra. Subsidence was defined as the difference of height of the fusion segment between immediately after surgery and the later follow-up. Once cage subsidence exceeded three millimetres it would be considered as severe subsidence [10, 14]. The SSA was defined as the Cobb angle between the superior endplate of the upper vertebra and the inferior endplate of the lower vertebra. We defined the lordosis angle as positive and the kyphosis angle as negative. The changes in sagittal alignment before surgery and at postoperative follow-ups were determined. All these radiographic parameters were measured by three independent senior spine surgeons who were not involved in the primary surgery and were unaware of the clinical results of the patients, and their average value was adopted for final analysis.
Three-dimensional CT scans were taken at six months and annually after surgery to evaluate the fusion status. Fusion was deemed to have occurred if (1) bridging bony trabeculae appeared across the interfaces between the endplate and the bone graft, (2) the bone in the fusion area was radiographically denser and more mature than originally achieved in surgery, and (3) absence of pseudoarthrosis, which was determined by the lucent line at the interfaces. The fusion status was also evaluated by the three senior spinal surgeons.
In addition, the clinical outcome of each patient was assessed by comparing the scores on a 10-point visual analogue scale (VAS) and the Japanese Orthopaedic Association (JOA) scale before surgery and at six months and annual postoperative follow-ups.
SPSS 17.0 statistic software (SPSS, Inc, Chicago, Illinois, USA) was used for all statistical analyses in this study. Quantitative data including age, subsidence, SSA, JOA scores and VAS points are presented as the mean ± standard deviation. The mean comparison at different time points in the same group was conducted with one-way ANOVA, and the pairwise comparison was performed with the LSD method. Mean comparison in two groups was analysed by the Mann–Whitney test. Categorical data such as sex, smoking, diagnosis, pathological level and fusion status in two groups were analysed by chi-square test. All P values were two-sided, and levels of significance reaching >95 % were accepted.
Results
Of the 80 patients eligible for inclusion in this study, we obtained complete data sets (preoperative-, immediately post-, one-year post-, and four-year post-operative follow-up data) for 67 patients (84 %). It contained 42 men and 25 women with a mean age of 47.2 years (range 32–62 years). Thirty-two patients underwent the single-level ACCF with a TMC (TMC group) (Fig. 3) and the other 35 patients with n-HA/PA66 cages (n-HA/PA66 group) (Fig. 4). The demographics of both groups of patients are shown in Table 1. There were no statistically significant differences in sex, age, smoking, diagnosis or pathogenic level between these two groups (all P > 0.05).
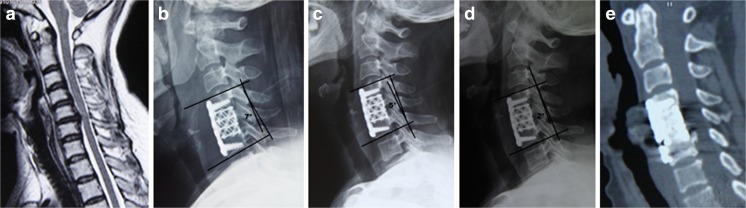
Fig. 3.
A 46-year-old woman underwent cervical corpectomy and fusion with a titanium mesh cage for OPLL. The compression arising in the C5 level was found on the preoperative cervical MRI (a), lateral plane radiograph immediately after surgery showed the Cobb angle of segmental sagittal alignment (SSA) was corrected to 7° after surgery (b). Gradually, the SSA reduced to 5° and 2° at one- (c) and four-years (d) after surgery, respectively. Finally, a solid fusion associated with marked subsidence (in the posterior lower footprint) was found on the four-year postoperative sagittal CT (e)
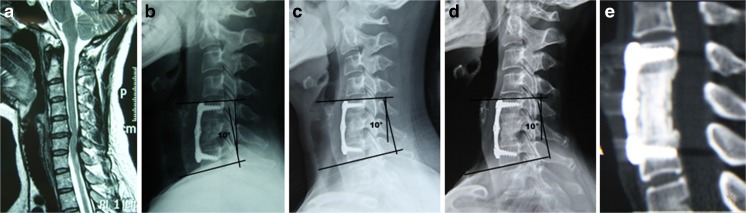
Fig. 4.
A 45-year-old woman underwent cervical corpectomy and fusion with a n-HA/PA66 cage for intervertebral disc herniation. The protruded C5-6 disc was shown on cervical MRI before surgery (a). The sagittal alignment of the fusion segment was corrected to 10° after surgery (b), and satisfactorily maintained at 10° at both the one- (c) and four-year (d) follow-ups. Finally, a satisfactory osseous fusion without severe subsidence was clearly shown on the four-year postoperative CT scan (e)
Table 1.
Patient demographic data
| Characteristic | TMC group (32 patients) | n-HA/PA66 group (35 patients) | P |
|---|---|---|---|
| Men/women | 20/12 | 22/13 | 0.976 |
| Mean age (y) | 46.8 ± 7.2 | 47.6 ± 7.1 | 0.633 |
| Smoking (n) | 10 | 14 | 0.456 |
| Diagnosis (n) | 0.818 | ||
| OPLL | 6 | 8 | |
| CS | 18 | 17 | |
| IVDH | 8 | 10 | |
| Pathological level (n) | 0.857 | ||
| C3 | 3 | 3 | |
| C4 | 8 | 11 | |
| C5 | 15 | 13 | |
| C6 | 6 | 8 | |
TMC titanium mesh cage, n-HA/PA66 nano-hydroxyapatite/polyamide-66, OPLL ossification of posterior longitudinal ligament, CS cervical spondylosis, IVDH intervertebral disc herniation
There was no case of cage migration or breakage in either group at the last follow-up. At one year after surgery, 27 patients’ cages were fused with a fusion rate of 84 % in the TMC group that, although insignificant, were markedly lower than the fusion rate of 94 % (33 of 35) in the n-HA/PA66 group (P = 0.185). At the four-year follow-up, the final fusion rate was 94 % (30 of 32) in the TMC group and similar to the fusion rate of 97 % (34 of 35) of the n-HA/PA66 group (P = 0.502). The titanium cage subsidence had a mean of 2.5 mm with a rate of severe subsidence of 19 % (six of 32) at one-year follow-up and a mean of 2.9 mm with a rate of 22 % (seven of 32) respectively at four-year follow-up. However, n-HA/PA66 cage subsidence had a mean of 1.2 mm with a rate of severe subsidence of 3 % (one of 35) at one-year follow-up and 1.5 mm with a rate of 6 % (two of 35) at four-year follow-up. The n-HA/pa66 cage subsidence at one- or four-year follow-up was significantly lower than TMC subsidence, respectively (Table 2).
Table 2.
Radiographic and clinical outcomes
| Variable measure | TMC group (32 patients) | n-HA/PA66 group (35 patients) | P |
|---|---|---|---|
| Subsidence (mm) | |||
| One-year post-op | 2.5 ± 1.0 | 1.2 ± 0.8a | 0.0001 |
| Four-year post-op | 2.9 ± 1.1 | 1.5 ± 0.9a | 0.0001 |
| SSA (°) | |||
| Pre-op | 5.4 ± 5.2 | 5.5 ± 4.5 | 0.955 |
| Immediately post-op | 8.7 ± 3.8 | 8.8 ± 3.6 | 0.786 |
| One-year post-op | 7.1 ± 3.6 | 7.8 ± 3.4 | 0.567 |
| Four-year post-op | 6.5 ± 3.3 | 7.6 ± 3.0 | 0.235 |
| VAS (point) | |||
| Pre-op | 7.1 ± 1.4 | 7.1 ± 1.7 | 0.747 |
| One-year post-op | 3.1 ± 1.4 | 2.3 ± 1.5a | 0.026 |
| Four-year post-op | 2.2 ± 1.3 | 1.3 ± 1.0a | 0.034 |
| JOA (point) | |||
| Pre-op | 8.4 ± 2.4 | 8.5 ± 2.8 | 0.684 |
| One-year post-op | 15.0 ± 1.8 | 15.9 ± 1.3 | 0.034 |
| Four-year post-op | 14 ± 1.9 | 15.3 ± 1.8a | 0.007 |
TMC titanium mesh cage, n-HA/PA66 nano-hydroxyapatite/polyamide-66, op operation, SSA segmental sagittal alignment, VAS visual analogue scale, JOA Japanese Orthopedic Association scale
a TMC group vs. n-HA/PA66 group, p value less than 0.05
In the TMC group, the mean SSA before surgery was 5.4°, and significantly improved to 8.7° immediately after surgery (P = 0.001) while in the n-HA/PA66 group, the mean preoperative SSA was 5.5° and significantly improved to 8.8° immediately after surgery (P < 0.001). The change of SSA around surgery in the TMC group and n-HA/PA66 group was similar. However, we found in later follow-ups the mean SSA of the TMC group was lower than the n-HA/PA66 group. The same phenomenon was also observed in the VAS and JOA scores, whereby the preoperative VAS and JOA scores of the TMC group were similar to the n-HA/PA66 group, respectively; however, the patients fused with n-HA/PA66 cages had significantly greater improvement of their VAS and JOA scores than patients with TMC over the follow-up period (Table 2).
Discussion
The currently available devices applied in clinical anterior reconstruction are varied, including structural autografts, allografts and cages. However in anterior cervical corpectomy and fusion (ACCF), there is abundant morsellised bone from the corpectomy to fill the cage device which makes the cage the best choice for reconstruction. The cage filled with local bone can effectively avoid the donor site morbidity from iliac autograft harvesting and the risk of disease transmission corrected with allograft [2–5], while at the same time ensuring a high rate of osseous fusion [7, 8].
The titanium mesh cage (TMC) is a typical metal cage device which has been widely used for decades. The previous results of using TMC and anterior plating after cervical corpectomy were favourable [7, 15–18]. The initial report of this technique by Majd et al. showed a 97 % solid fusion rate and an 80 % excellent or good clinical outcome for the patients [7]. Dorai et al. reported a fusion rate of 97 % in their 45 patients who underwent ACCF with TMC [15]. Subsequently, Nakase et al. reported a fusion rate of 100 % in 22 patients with the same surgical technique over a mean 54.3 months follow-up [16]. Castellvi et al. found a solid bony fusion in 97.5 % of patients at six months and 100 % at one year after cervical corpectomy and reconstruction with a titanium cage and anterior plate [17]. Although the high rate of TMC fusion was encouraging, the same high rate of cage subsidence could not be ignored. In a large sample study on ACCF with titanium cages by Chen et al., they found TMC subsidence (more than one millimetre) occurred in 79.7 % of patients and severe subsidence (more than three millimetres) in 19 % of cases. Meanwhile they found severe TMC subsidence was correlated with bad neurological outcomes and subsidence-related complications which noticeably diminished the benifits of surgery [9]. To find the more ideal cage device and better clinical results, non-metallic cages have recently become extremely popular with spine surgeons.
The hollow cylindrical nano-hydroxyapatite/polyamide-66 cage (n-HA/PA66) is a novel biomimetic non-metallic cage device. The design concept of this material is very good, combining the strength properties of hydroxyapatite (HA) with the elasticity properties of polyamide-66 (PA66), which is very close to the inherent attributes of human bone [18, 19]. The n-HA/PA66 cage has been designed and used since 2005, and the clinical results of this cage have been gradually reported in recent years. In our early study, we found a fusion rate of 90.2 % in 51 patients who underwent lumbar or thoracic corpectomy with an n-HA/PA66 cage fusion [12]. Zhao et al. showed a 94.3 % bony fusion rate and a 2.9 % cage subsidence rate in their 35 patients who had an n-HA/PA66 cage fusion following cervical corpectomy [13]. However, until now no comparative study has been reported between the n-HA/PA66 cage and the TMC. In this study, we have presented our results using these two different types of cages used for reconstruction following single-level cervical corpectomy.
In our series, solid bony fusion was found in 84 % of patients (27/32) in the TMC group at one year and in 94 % (30/32) four years after surgery. Under the same conditions, solid fusion was found in 94 % of patients (33/35) in the n-HA/PA66 group at one year and in 97 % (34/35) four years after the operations. Though the final fusion rates of the two groups were similar, it was not difficult to discover that bony fusion occurred sooner in patients who underwent anterior cervical corpectomy with the n-HA/PA66 cage than with the TMC. Immediate stability from the anterior plate and intervertebral support device are a prerequisite for later osseous fusion. The excellent mechanical strength of TMC is well known and documented in a series of studies. The strength of bending, tensing and compressing of n-HA/PA66 was 95, 79 and 117 MPa, respectively, matching well with cortical bone [18, 19]. When implanting into intervertebral space, both cages provide sufficient support for the anterior cervical column. It is worth mentioning that Young’s modulus of the TMC was 110 GPa, significantly higher than the approximate 12 GPa of bone graft inside the cage. However, Young’s modulus of the n-HA/PA66 cage was 5.6 GPa, much lower than bone graft. As described in Wolff’s law, bone grows in response to applied stress and is reabsorbed if a mechanical stimulus is lacking. The superior elasticity of the n-HA/PA66 cage to the TMC helps to prevent the stress shielding and promotes earlier bony fusion. Moreover, the implanted n-HA/PA66 cage in theory can provide a micro-ion-exchange of Ca2+ and PO43- between the cage and surrounding tissue and then form a crystal layer on the cage surface [13, 20]. This layer then becomes an optimal trestle bridge assisting in the creeping growth of bone graft.
The most common complication of TMC is subsidence. Severe cage subsidence will lead to the loss in height of the fusion segment, correlating with kyphotic deformity, instrument failure or postoperative neurological deterioration, which becomes a potential problem of postoperative patients [10, 13]. In our patients with TMC fusion, the rate of severe cage subsidence (more than three millimetres) was 22 % at the last follow-up. In the n-HA/PA66 group, the final rate of severe cage subsidence was 6 %, which was significantly lower than the TMC group. The risk factors related to the development of subsidence of the cages are varied, including not only intraoperative end-plate preparation and osteoporosis but also cage material and cage shape [14, 21, 22]. The titanium mesh cage has sharp footprints designed to anchor the cage into the adjacent endplates and avoid a translation; however, the sharp footprints lead to a smaller cage–endplate interface contact area, increasing the cutting and penetration from the cage into the vertebra. The shape converted from TMC to n-HA/PA66 cage with much larger footprints (approximate three millimetres) is beneficial for distributing the loads on the endplates and decreasing the incidence and amount of cage subsidence. Meanwhile, the modulus of elasticity of the TMC is much higher than bone graft inside the cage as stated above and leads to a concentration of the compressive stress on the cage–endplate interface rather than bone graft–endplate interface which on the other hand facilitates cage subsidence. As opposed to this, the lower elasticity modulus of the n-HA/PA66 cage distributes the stress on the implant–endplate interface area, thereby reducing the subsidence. In addition, cage subsidence usually occurrs before bony fusion is achieved [9]. Therefore, the faster bony fusion time of the n-HA/PA66 cage compared to the TMC also results in less subsidence.
In this study, the VAS and JOA scales were used to assess the clinical outcomes after ACCF. We found both the postoperative VAS and JOA points in patients with TMC fusion were poorer than the ones with n-HA/PA66 fusion even though both the initial surgical correction and finial fusion status of these two groups were almost equivalent. It indicated that, compared to the n-HA/PA66 group, the higher rate of cage subsidence associated with the greater loss of surgical correction in the TMC group may greatly diminish the acquired advantages from initial decompressive surgery.
Moreover, fusion assessment in the TMC is much more difficult, because the titanium mesh is not radiolucent and prevents the direct observation of bony bridging inside cage on plain radiographs [23, 24]. The radiographic penetrability of the n-HA/PA66 cage is slightly lower than cortical bone. On plain radiographs, the evaluation of the fusion status inside the n-HA/PA66 cage is much easier and more reliable than the TMC; however, evaluation of the profile and integrity of the n-HA/PA66 cage is limited, due to its radiolucent nature and lack of display markers [12]. Therefore, three-dimensional CT was used in all patients in this study to attempt to assess the graft fusion and cage integrity accurately. CT scans have been known as the surest direct radiographic examination for fusion assessment [8, 24]. In fact, even if we used CT scans, the imaging interference from titanium mesh often influenced the observation of bony bridging at specific positions and affected the judgment of fusion. But this has not appeared in the fusion evaluation in the n-HA/PA66 cage group. It showed that the n-HA/PA66 cage, as a non-metallic device, has more advantages than the TMC or other metallic cages in terms of the postoperative radiographic assessment of the fusion.
This was a prospective study of two different types of cages: the TMC and the n-HA/PA66 cages. From our experience, both these cages provide satisfactory solid anterior column reconstruction with high finial fusion rates following single-level anterior cervical corpectomy. However, n-HA/PA66 cages have superior mechanical properties and shape design compared to TMC cages, with the added advantages of facilitating earlier fusion and causing less cage subsidence. We now prefer this novel cage for ACCF surgery because of its better finial clinical results.
However, several limitations still exist in this prospective study. We have included a total of 67 patients for evaluation. The number of patients in each group was quite small. And the mean follow-up time was relatively short (only four years). Furthermore, the choice of the two different cages was not randomised and the final results are influenced by physician factors to a certain degree. In future, a prospective, randomised, multicentre study with larger samples and longer follow-ups is required to compare the long-term effects of these two cages.
Acknowledgments
Conflicts of interest
The authors declare that they have no conflicts of interest concerning this article.
Device status/drug statement
The device(s)/drug(s) contained in this work is/are FDA approved or approved by corresponding national agency for this indication.
Funding sources/benefits received
No funds were received in support of this work. No benefits in any form have been or will be received from any commercial party related directly or indirectly to the subject of this article.
Contributor Information
Xi Yang, Email: formosa88@163.com.
Limin Liu, Phone: +86-28-85422430, Email: llm_cd@126.com.
References
- 1.Malloy KM, Hilibrand AS. Autograft versus allograft in degenerative cervical disease. Clin Orthop Relat Res. 2002;394:27–38. doi: 10.1097/00003086-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqui AA, Jackowski A. Cage versus tricortical graft for cervical interbody fusion. A prospective randomised study. J Bone Joint Surg Br. 2003;85(7):1019–1025. doi: 10.1302/0301-620X.85B7.13398. [DOI] [PubMed] [Google Scholar]
- 3.Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2003;28(2):134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Tomford WW. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am. 1995;77(11):1742–1754. doi: 10.2106/00004623-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Zdeblick TA, Ducker TB. The use of freeze-dried allograft bone for anterior cervical fusions. Spine. 1991;(Phila Pa 1976) 16:726–729. doi: 10.1097/00007632-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Riew KD, Rhee JM. The use of titanium mesh cages in the cervical spine. Clin Orthop Relat Res. 2002;394:47–54. doi: 10.1097/00003086-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Majd ME, Vadhva M, Holt RT. Anterior cervical reconstruction using titanium cage with anterior plating. Spine (Phila Pa 1976) 1999;24(15):1604–1610. doi: 10.1097/00007632-199908010-00016. [DOI] [PubMed] [Google Scholar]
- 8.Eck KR, Bridwell KH, Ungacta FF, et al. Analysis of titanium mesh cages in adults with minimum two-year follow-up. Spine (Phila Pa 1976) 2000;25(18):2407–2415. doi: 10.1097/00007632-200009150-00023. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Chen D, Guo Y, et al. Subsidence of titanium mesh cage: a study based on 300 cases. J Spinal Disord Tech. 2008;21(7):489–492. doi: 10.1097/BSD.0b013e318158de22. [DOI] [PubMed] [Google Scholar]
- 10.Chen JF, Lee ST, Wu CT. A hollow cylindrical PMMA strut for cervical spine reconstruction after cervical multilevel corpectomy. J Spinal Disord Tech. 2010;23(5):321–327. doi: 10.1097/BSD.0b013e3181b15bc8. [DOI] [PubMed] [Google Scholar]
- 11.Kabir SM, Alabi J, Rezajooi K, et al. Anterior cervical corpectomy: review and comparison of results using titanium mesh cages and carbon fibre reinforced polymer cages. Br J Neurosurg. 2010;24(5):542–546. doi: 10.3109/02688697.2010.503819. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Song Y, Liu L, et al. Anterior reconstruction with nano-hydroxyapatite/polyamide-66 cage after thoracic and lumbar corpectomy. Orthopedics. 2012;35(1):e66–e73. doi: 10.3928/01477447-20111122-10. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Z, Jiang D, Ou Y, et al. A hollow cylindrical nano-hydroxyapatite/polyamide composite strut for cervical reconstruction after cervical corpectomy. J Clin Neurosci. 2012;19(4):536–540. doi: 10.1016/j.jocn.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 14.Barsa P, Suchomel P. Factors affecting sagittal malalignment due to cage subsidence in standalone cage assisted anterior cervical fusion. Eur Spine J. 2007;16:1395–1400. doi: 10.1007/s00586-006-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorai Z, Morgan H, Coimbra C. Titanium cage reconstruction after cervical corpectomy. J Neurosurg. 2003;99(1 suppl):3–7. doi: 10.3171/spi.2003.99.1.0003. [DOI] [PubMed] [Google Scholar]
- 16.Nakase H, Park YS, Kimura H, et al. Complications and long-term follow-up results in titanium mesh cage reconstruction after cervical corpectomy. J Spinal Disord Tech. 2006;19:353–357. doi: 10.1097/01.bsd.0000210113.09521.aa. [DOI] [PubMed] [Google Scholar]
- 17.Castellvi AE, Castellvi A, Clabeaux DH. Corpectomy with titanium cage reconstruction in the cervical spine. J Clin Neurosci. 2012;19(4):517–521. doi: 10.1016/j.jocn.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Li Y, Wei J, et al. Development of biomimetic nano- hydroxyapatite/poly(hexamethyleneadipamide) composites. Biomaterials. 2002;23(24):4787–4791. doi: 10.1016/S0142-9612(02)00229-6. [DOI] [PubMed] [Google Scholar]
- 19.Huang M, Feng J, Wang J, et al. Synthesis and characterization of nano-HA/PA66 composites. J Mater Sci Mater Med. 2003;14(7):655–660. doi: 10.1023/A:1024087410890. [DOI] [PubMed] [Google Scholar]
- 20.Xu Q, Lu H, Zhang J, et al. Tissue engineering scaffold material of porous nanohydroxyapatite/polyamide 66. Int J Nanomedicine. 2010;5:331–335. doi: 10.2147/ijn.s9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu CC, Liao JC, Chen WJ, Chen LH. Outcomes of interbody fusion cages used in 1 and 2-levels anterior cervical discectomy and fusion: titanium cages versus polyetheretherketone (PEEK) cages. J Spinal Disord Tech. 2010;23(5):310–316. doi: 10.1097/BSD.0b013e3181af3a84. [DOI] [PubMed] [Google Scholar]
- 22.Ordway NR, Rim BC, Tan R, et al. Anterior cervical interbody constructs: effect of a repetitive compressive force on the endplate. J Orthop Res. 2010;30(4):587–592. doi: 10.1002/jor.21566. [DOI] [PubMed] [Google Scholar]
- 23.Narotam PK, Pauley SM, McGinn GJ. Titanium mesh cages for cervical spine stabilization after corpectomy: a clinical and radiological study. J Neurosurg. 2003;99(2 Suppl):172–180. doi: 10.3171/spi.2003.99.2.0172. [DOI] [PubMed] [Google Scholar]
- 24.Chuang HC, Cho DY, Chang CS, et al. Efficacy and safety of the use of titanium mesh cages and anterior cervical plates for interbody fusion after anterior cervical corpectomy. Surg Neurol. 2006;65(5):464–471. doi: 10.1016/j.surneu.2005.12.021. [DOI] [PubMed] [Google Scholar]