Abstract
In this issue of Blood, Hobbs et al use a JAK2 V617F knock-in mouse model to interrogate the impact of JAK2 V617F on thrombosis and demonstrate altered function of megakaryocytes and platelets in the context of JAK2 V617F expression.1
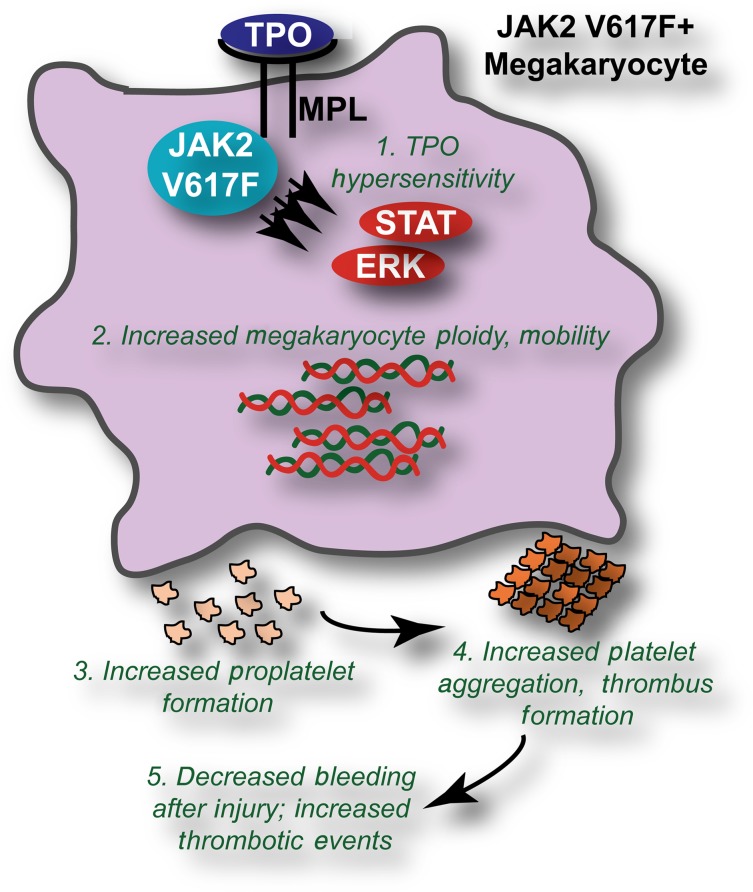
JAK2 V617F induces biological changes to megakaryocytes and platelets leading to increased thrombotic events. The presence of JAK2 V617F leads to (1) hypersensitive signaling through the thrombopoietin (TPO)/MPL pathway in megakaryocytes, leading to increased activation of downstream molecules such as signal transducer and activator of transcription 3 and extracellular signal-regulated kinase. Phenotypically, this manifests in (2) increased ploidy and mobility of JAK2 V617F megakaryocytes; (3) increased formation of proplatelets; and (4) increased aggregation, spreading, and thrombus formation of platelets. The ultimate consequence of these biological changes is (5) decreased bleeding volumes in response to injury and increased thrombotic events.
Thrombosis is a major cause of disease-related morbidity and mortality in myeloproliferative neoplasm (MPN) (reviewed in Barbui et al2). Accordingly, reduction of thrombotic risk is a central therapeutic goal for this disease. The increased risk of thrombosis observed in MPN patients is a consequence of not only quantitative but also qualitative cellular changes. JAK2 V617F–positive MPN patients have been shown to exhibit biological differences in platelet cell function, such as increased P-selectin expression on platelets.3 As such, it has been long been suspected that JAK2 V617F has direct effects on platelet function, but the exact nature of these effects has remained elusive until now. The use of a mouse knock-in model in which all hematopoietic cells are heterozygous for human JAK2 V617F and express physiological levels of JAK2 V617F provides a rare opportunity to systematically investigate the effect of JAK2 V617F on platelet function that is not feasible using primary cells from MPN patients because of the genetic diversity and nonuniform treatment regimens of the patient population.
Employment of this JAK2 V617F knock-in mouse model led to a number of important observations regarding the impact of JAK2 V617F on megakaryocyte and platelet biology and, thus, on the qualitative impacts of JAK2 V617F on thrombosis (summarized in figure). First, it was confirmed that expression of endogenous levels of JAK2 V617F in megakaryocytes led to hypersensitive signaling through the TPO receptor, myeloproliferative leukemia virus oncogene (MPL), after stimulation with low concentrations of TPO. Phenotypically, these JAK2 V617F–positive megakaryocytes exhibited increased ploidy and were more mobile as compared with megakaryocytes expressing wild-type Janus kinase 2 (JAK2). In addition, JAK2 V617F–expressing megakaryocytes exhibited increased proplatelet formation when incubated on a fibrinogen surface concomitant with increased activation of the spleen tyrosine kinase and phospholipase C γ-2 signaling pathways downstream of the fibrinogen receptor, integrin αIIbβ3. The altered biology observed in the context of JAK2 V617F was not limited to megakaryocytes but also extended to platelets, which exhibited increased thrombus formation, increased reactivity in response to collagen-related peptide and thrombin, increased aggregation, and increased spreading to the extent that spontaneous full lamellipodia spreading could be observed in JAK2 V617F–derived platelets. To exclude the possibility that the altered biological activity of JAK2 V617F platelets was simply attributable to a higher concentration of platelets, Hobbs and coauthors carefully repeated key experiments with blood that was diluted with plasma to equilibrate platelet concentrations between JAK2 V617F and wild type. Even with equivalent platelet numbers, the increased biological activity of JAK2 V617F platelets was still evident.
Cumulatively, the aberrant activity of megakaryocytes and platelets in the setting of JAK2 V617F led to decreased bleeding volumes after tail injury of JAK2 V617F knock-in mice compared with JAK2 wild type, a good approximation of increased thrombosis. Interestingly, a prior report of mouse modeling of JAK2 V617F, using an ectopically expressing retroviral bone marrow transplant model, found increased bleeding time in the setting of JAK2 V617F,4 although it is difficult to directly compare these model systems because of differences in the expression level of JAK2 V617F as well as measurement of bleeding volume in this study vs bleeding time in the prior report. Nonetheless, the decreased bleeding volume observed with the JAK2 V617F knock-in mouse model reported here is consistent with the increased thrombotic episodes experienced by patients with JAK2 V617F–positive disease. Finally, Hobbs and colleagues used gene expression profiling to identify genes that were significantly up- or downregulated in the JAK2 V617F setting compared with JAK2 wild type. These gene expression profiles confirmed certain pathways known to be important in thrombosis (such as Pecam15) and also revealed a number of new candidate genes that could be implicated in this process through follow-up analyses.
The impact of JAK2 V617F on thrombotic risk likely extends beyond its effect on platelets. The presence of JAK2 V617F mutant liver endothelial cells but not hematopoietic cells in patients with Budd-Chiari syndrome6 suggests that expression of JAK2 V617F also directly affects nonhematopoietic cells to augment thrombotic risk. The manner by which JAK2 V617F promotes thrombosis is no doubt complex and multilayered with much left to uncover. Thanks to Hobbs et al, we are now beginning to unravel this mystery.
These findings could lead to important clinical applications. For example, laboratory tests could be developed to detect the previously described JAK2 V617F–induced platelet abnormalities. These tests could serve as a tool to risk stratify MPN patients for thrombotic risk and to monitor the impact of therapy. The human JAK2 V617F knock-in mouse model could be exploited as a system to test the ability of novel MPN drugs to reduce thrombotic risk. Collectively, this report by Hobbs and colleagues has shed light on an important and long-unanswered question in MPN/JAK2 V617F clinical biology. Future work can be expected to build on these findings to further investigate the mechanisms underlying these phenomena and to identify new clinical modalities that could be employed to the benefit of patients with MPN.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.Hobbs CM, Manning H, Bennett C, et al. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock-in mouse model of essential thrombocythemia. Blood. 2013;122(23):3787–3797. doi: 10.1182/blood-2013-06-501452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122(13):2176–2184. doi: 10.1182/blood-2013-03-460154. [DOI] [PubMed] [Google Scholar]
- 3.Arellano-Rodrigo E, Alvarez-Larrán A, Reverter JC, Villamor N, Colomer D, Cervantes F. Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica. 2006;91(2):169–175. [PubMed] [Google Scholar]
- 4.Zaleskas VM, Krause DS, Lazarides K, et al. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS ONE. 2006;1(1):e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99(1):3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sozer S, Fiel MI, Schiano T, Xu M, Mascarenhas J, Hoffman R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood. 2009;113(21):5246–5249. doi: 10.1182/blood-2008-11-191544. [DOI] [PMC free article] [PubMed] [Google Scholar]