Abstract
The osteophyte associated with osteoarthritis (OA) is a bony outgrowth formed at the margins of the affected joint through endochondral ossification-like processes. However, the mechanism of osteophyte formation and its pathogenesis are unclear. Perlecan (Hspg2), a heparan sulfate proteoglycan, is expressed in many extracellular tissues and plays critical roles in skeletal development and diseases. The aim of the present study is to identify the role of synovial perlecan in osteophyte formation using perinatal lethality rescued perlecan-knockout mice (Hspg2−/−-Tg) wherein perlecan expression is lacking in the synovial and other tissues, except for cartilage. We analyzed the development of osteophytes in joints of Hspg2−/−-Tg mice in two different animal models: the surgical OA model, in which the medial collateral ligament was transected and the medial meniscus was resected, and the TGF-β-induced osteophyte formation model. In the surgical OA model, the osteophyte size and maturation were significantly reduced in the OA joints of Hspg2−/−-Tg mice compared with control mice, while OA developed on the medial side of the knee joints with no differences in the cartilage degradation score or synovitis score between control and Hspg2−/−-Tgmice. The reduced osteophyte formation in Hspg2−/−-Tg mice was associated with reduced cell proliferation and chondrogenesis. In the TGF-β model, the osteophyte size and maturation were also significantly reduced in Hspg2−/−-Tg mice compared with control mice. Our findings suggest that synovial perlecan plays an important role in osteophyte development in OA, and they provide insights that may facilitate the development of OA therapy.
Keywords: Synovial perlecan, Osteophyte, Osteoarthritis, TGF-β Synovium
1. Introduction
Osteoarthritis (OA) is a disease characterized primarily by cartilage degradation (Wieland et al., 2005). Synovitis and osteophyte formation were considered secondary phenomena in OA, as degenerated articular cartilage affects subchondral bone and synovial membrane. In synovitis, the synovial membrane produces proteases and cytokines, which enhance cartilage degradation. In addition, while synovitis occurs throughout the affected joint in rheumatic arthritis, synovitis occurs locally and more mildly in OA (Ayral et al., 2005; Goldring and Goldring, 2007; Liu et al., 2010; Ning et al., 2011).
The osteophyte is a bony outgrowth that forms at the margins of the affected joint. Osteophytes that develop from the periosteum and synovium are located on the outside of the cortical bone in the joint. Mesenchymal stem cells (MSCs) in the periosteum and synovium are stimulated to proliferate by various triggers, such as mechanical factors and cytokines (van der Kraan and van den Berg, 2007). Cells inside the developing osteophyte undergo chondrogenesis and differentiate to mature hypertrophic chondrocytes where vascular invasion occurs. Chondrocytes are subsequently replaced with osteoblasts to form bone with marrow cavities. These developmental processes in the osteophyte are similar to those seen during endochondral ossification in the growth plate (van der Kraan and van den Berg, 2007).
Growth factors are expressed in osteophytes of humans and animals (van der Kraan and van den Berg, 2007). Factors such as transforming growth factor beta (TGF-β) (Uchino et al., 2000; Blaney Davidson et al., 2006; van der Kraan and van den Berg, 2007), bone morphogenetic proteins (BMPs) (Blaney Davidson et al., 2006), fibroblast growth factors (FGFs) (Jingushi et al., 2006), and insulin-like growth factor 1 (IGF-1) (Okazaki et al., 1999) have all been implicated in osteophyte developmental processes. After repeated injections into normal mouse joints, BMP-2 and TGF-β1 have been shown to induce chondrogenesis at specific sites (van Beuningen et al., 1994, 1998). Osteophytes induced by BMP-2 are found predominantly in the region where the growth plates meet the joint space, while those triggered by TGF-β1 are located on the outside of the cortical bone in the margins of the joint where osteophytes were usually found in OA (van Beuningen et al., 1994, 1998). These reports suggest that TGF-β plays a particularly important role in osteophyte formation. However, the molecular mechanisms that regulate osteophyte formation are still largely unknown.
Perlecan (Hspg2) is a heparan sulfate proteoglycan found in all basement membranes and in cartilage (Noonan et al., 1991; Iozzo, 1994). Perlecan interacts with extracellular matrix molecules, growth factors, and receptors and is implicated in cell growth, differentiation, and signaling (Whitelock et al., 2008). Perlecan knockout (Hspg2−/−) mice develop dwarfism with short limbs and die at birth (Arikawa-Hirasawa et al., 1999; Costell et al., 1999; Ishijima et al., 2012). Perlecan-deficiency in humans also causes lethal chondrodysplasia, dyssegmental dysplasia, and Silverman–Handmaker type dyssegmental dysplasia (Arikawa-Hirasawa et al., 2001). Thus, perlecan is essential for normal cartilage development in humans and mice.
In the synovial joint, perlecan is expressed in not only cartilage but also synovium (Dodge et al., 1995). The synovium from patients with OA expresses perlecan, and primary synovial cells upregulate the expression of perlecan in response to TGF-β (Dodge et al., 1995). However, the role of synovial perlecan, either in normal synovial functions or in pathological conditions like osteophyte formation in OA, has not been established.
In the present study, we examined the role of synovially-expressed perlecan in knee OA, using perinatal lethality rescued perlecan gene knockout mice (Hspg2−/−-Tg), in which perlecan expression is lacking in the synovium and other tissues except for cartilage (Xu et al., 2010; Ishijima et al., 2012).
2. Results
2.1. Perlecan is expressed in cartilage but not in the synovium of Hspg2−/−-Tg mice
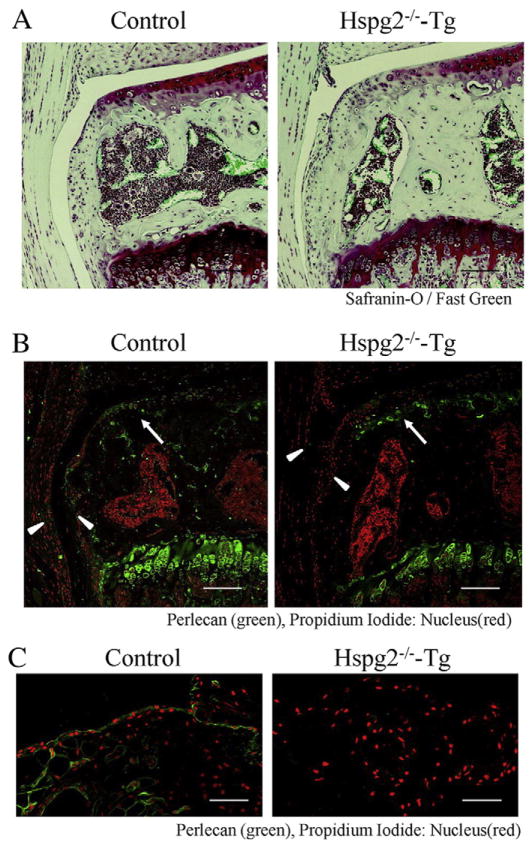
We first examined perlecan expression in knee joints in Hspg2−/−-Tg mice. In these mice, the perinatal lethality of perlecan knockout (Hspg2−/−) mice (Arikawa-Hirasawa et al., 1999) was rescued to allow survival by expressing recombinant perlecan specifically in cartilage under the control of the Col2a1 collagen promoter and enhancer (Hspg2−/−; Col2a1-Hspg2Tg/−) (Xu et al., 2010; Ishijima et al., 2012). Safranin-O and fast green staining of the knee joint showed no significant histological abnormalities in Hspg2−/−-Tg mice when compared to control heterozygous Hspg2+/−-Tgmice (Fig. 1A). Immunostaining revealed that perlecan was expressed in the articular cartilage of the joints of both mouse types (green, arrows in Fig. 1B), as expected. RT-PCR analysis also showed that there was no significant difference between the levels of perlecan mRNA in articular cartilage of control and Hspg2−/−-Tg mice (Supplemental Fig. 1). Perlecan was expressed in the synovium of control mice, which covers the whole joint internally along with the fibrous capsule (Cooper, 1964) (arrowhead, Fig. 1B). In contrast, perlecan was not expressed in the synovium of Hspg2−/−-Tgmice (arrowhead, Fig. 1B). The absence of perlecan was also demonstrated by immunostaining of the synovium, which was isolated from Hspg2−/−-Tg mice (Fig. 1C). Similarly, synovial cells were prepared from the synovium of Hspg2−/−-Tg mice, using the method we recently reported (Futami et al., 2012), and this showed no perlecan expression in culture. Synovial cells from control mice, however, expressed perlecan (data not shown).
Fig. 1.
Perlecan is expressed in cartilage but not in the synovium of Hspg2−/−-Tg mice. Safranin-O staining with fast green counterstaining (A) and perlecan immunostaining (B) in the knee joint (sham side of the OA surgical model in the medial tibial plateau) of control and Hspg2−/−-Tg mice. Perlecan immunostaining in the synovium (C) isolated from control and Hspg2−/−-Tgmice. Perlecan (green)was expressed in articular cartilage (arrow) and synovium(arrowhead) in control mice. However, perlecan was not expressed in synovium (arrowhead) in Hspg2−/−-Tg mice, although it was expressed in articular cartilage of Hspg2−/−-Tg mice. Scale bars=100 μm.
2.2. Inhibition of osteophyte formation of Hspg2−/−-Tg mice in the surgical OA model
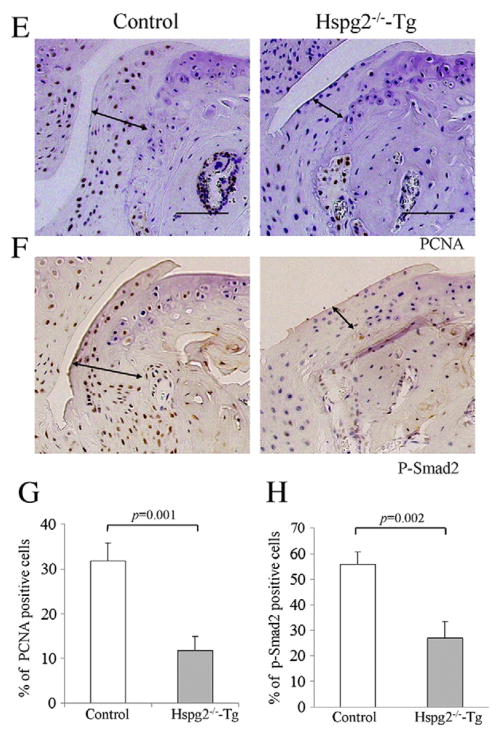
We performed surgery to induce OA. In this surgical OA model (Kamekura et al., 2005), the medial collateral ligament was transected, and the medial meniscus was resected on the left knee of Hspg2−/−-Tg (n=10) and control mice (n=9). All mice developed OA in the knee joints by 4 weeks after surgery (OA in Fig. 2A). The OA operation side for control and Hspg2−/−-Tg mice showed increased areas of fibrillation or partial cartilage loss, and lacked Safranin-O staining and synovitis (arrows in Fig. 2Ba,b) when compared to the sham-operation side, confirming OA induction. In contrast, osteophyte formation, which is also one of the characteristics of OA, was substantially inhibited in Hspg2−/−-Tg mice compared to control mice (arrow lines, Fig. 2Bc).
Fig. 2.
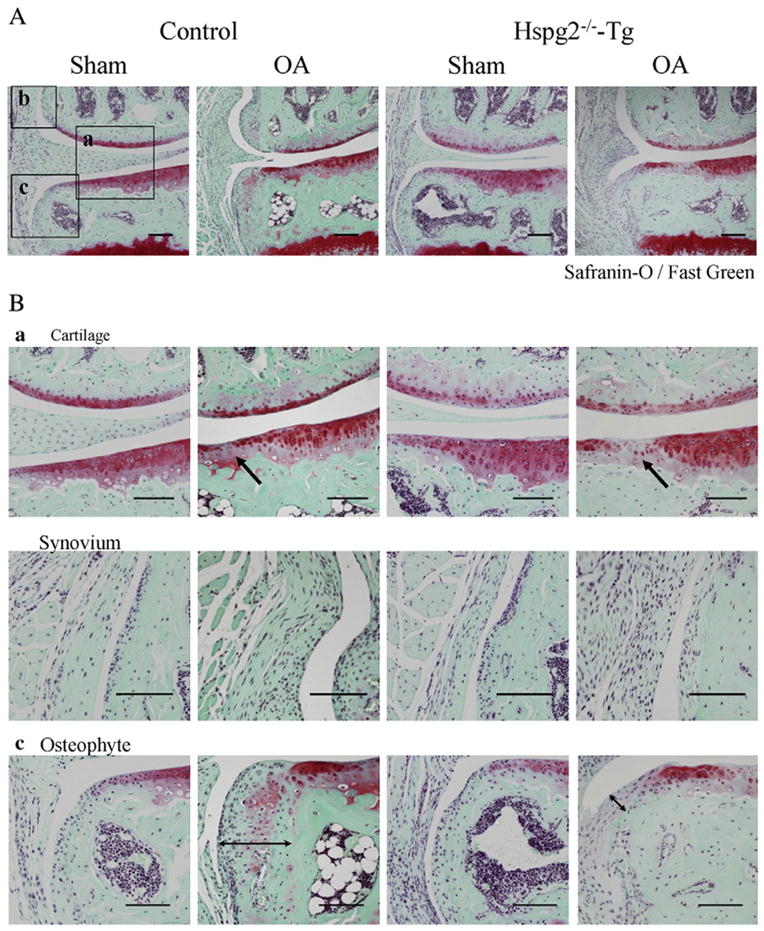
Inhibition of osteophyte formation in Hspg2−/−-Tg mice in the surgical OA model. Histological sections of knee joints 4 weeks after either OA operation (OA) or sham operation (Sham) in control mice and Hspg2−/−-Tg mice (A). High magnifications of cartilage, synovium, and osteophyte areas are shown in (B) a, b, and c, respectively. Boxed areas a, b, and c in (A) indicate representatives of these tissues in each sample. In OA cartilage, the Safranin-O staining regions were reduced in control mice and in Hspg2−/−-Tg mice (arrows in Ba). No histological differences were observed between the synovium in the sham and OA groups of and Hspg2−/−-Tg mice (Bb). Osteophyte formation was inhibited in Hspg2−/−-Tg mice (Bc). Quantitative scores of cartilage degradation (Ca), synovitis (Cb), and osteophyte size and maturity (Cc) were measured according to previous reports (Kamekura et al., 2005; Mapp et al., 2008; Little et al., 2009; Glasson et al., 2010). Cartilage degradation (structural change, Ca, left panel) and proteoglycan staining (Ca, right panel) were significantly increased in control and Hspg2−/−-Tg mice on the OA operation sides (OA) compared with the sham operation sides (Sham). No significant differences were found in the scores between control and Hspg2−/−-Tg mice. However, the osteophyte size and maturity in the OA operation sides (OA) were significantly reduced in Hspg2−/−-Tg mice compared with control mice. The osteophytes are shown with black lines (Bc). Scale bars, 100 μm. All data represent means and SEM.
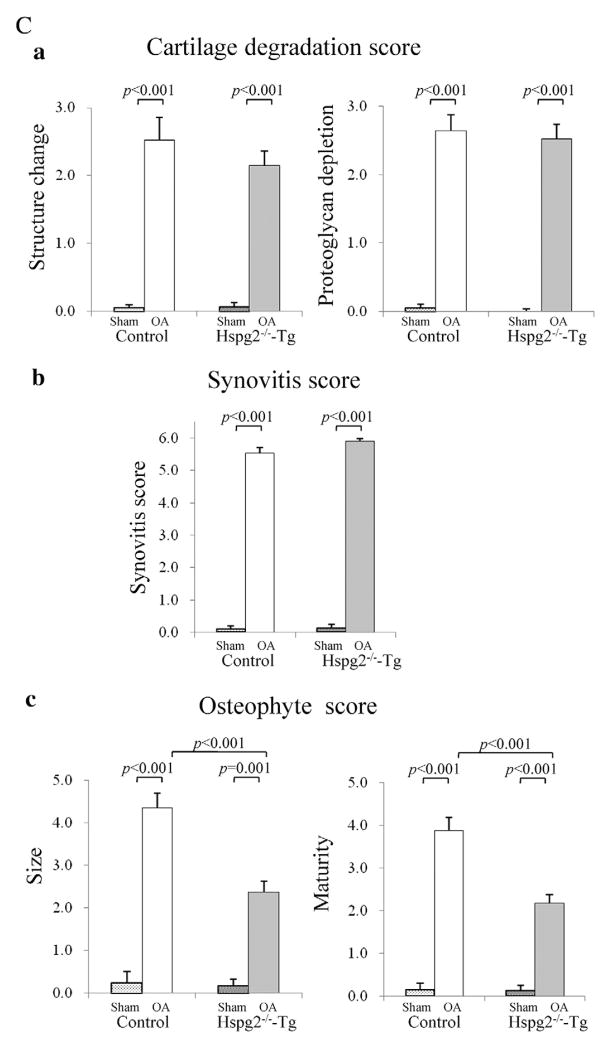
Statistical analysis for OA severity showed that the structure score (Fig. 2Ca), proteoglycan depletion score (Fig. 2Ca), and synovitis score (Fig. 2Cb) were significantly increased in control and Hspg2−/−-Tg mice in the OA operation side compared with the sham operation side (Fig. 2Ca,b). No significant differences were observed in these scores between control and Hspg2−/−-Tg mice (Fig. 2Ca,b). Osteophyte size and maturity were also increased in the OA operation side compared to the sham operation side in control and Hspg2−/−-Tg mice (Fig. 2Cc). However, the osteophyte size and maturity levels in the OA operation sides were significantly reduced in Hspg2−/−-Tg mice compared to control mice (Fig. 2Cc).
2.3. Comparison of osteophyte development between 4 and 8 weeks after OA surgery
Next, we compared osteophyte formation at 4 and 8 weeks after the surgery. In control mice, osteophytes developed in 4 weeks (arrow lines in Fig. 3A, left panel) as shown in Fig. 2. At 8 weeks after surgery, the osteophyte maturity and size were increased compared to that at 4 weeks after surgery in control mice (Fig. 3A, right panel, and Fig. 3C). In contrast, in Hspg2−/−-Tg mice, the osteophyte maturity and size did not significantly change during 4 to 8 weeks after surgery (Fig. 4B and C). These results indicate that osteophyte size in control mice increased in the first 4 weeks and continued to mature during 8 weeks after surgery. In contrast, the osteophyte growth in Hspg2−/−-Tg mice was significantly inhibited.
Fig. 3.
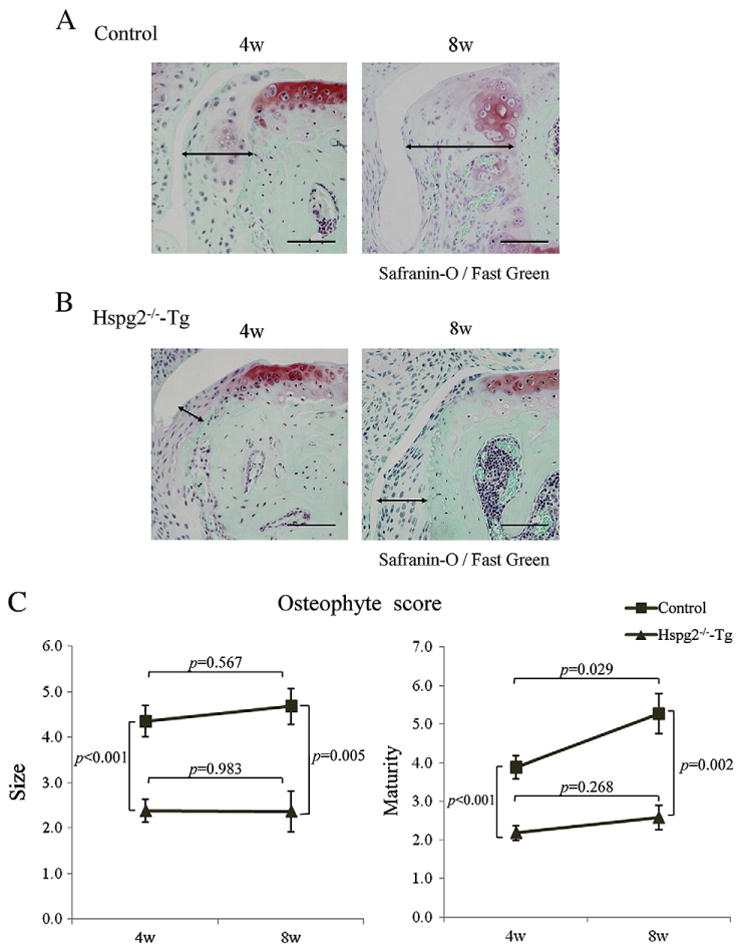
Perlecan deficiency inhibits diachronic osteophyte development in the surgical OA model. Histological sections of OA-induced knee joints 4 and 8 weeks after surgery in control mice (A) and Hspg2−/−-Tg mice (B). The size of osteophytes in Hspg2−/−-Tg knee joints (B) did not change between 4 weeks and 8 weeks after surgery while in control mice the size of osteophytes increased at 8 weeks compared with 4 weeks after surgery (A). In control mice, osteophyte maturity at 8 weeks after surgery was significantly increased compared to that at 4 weeks after surgery (C). At 4 or 8 weeks after surgery, the osteophyte size and maturity levels were significantly reduced in Hspg2−/−-Tg mice (triangle line) compared with the control mice (square line) (C). The osteophytes are shown with black lines (A, B). Scale bar, 100 μm. All data represent means and SEM.
Fig. 4.
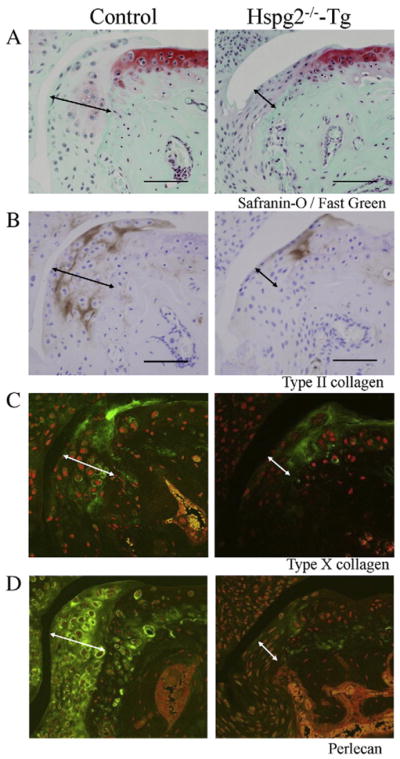
Perlecan deficiency reduces chondrocyte differentiation, cell proliferation, and Smad2 phosphorylation during osteophyte development. Histological sections of the knee joints of control and Hspg2−/−-Tg mice 4 weeks after surgery (OA). Safranin-O staining (red) with fast green (green) counter staining (A), immunohistochemistry for type II collagen (COLII) (B), type X collagen (COLX) (C), perlecan (D), PCNA (E), and phosphorylated Smad2 (p-Smad2) (F). COLII (brown, B) and COLX (green, C) expression in osteophytes was reduced in Hspg2−/−-Tg mice compared with control mice. Perlecan expression (green, D) was detected in synovial cells and chondrocytes of osteophytes with higher expression levels in hypertrophic chondrocytes of control mice. In Hspg2−/−-Tg mice, perlecan expression was absent in osteophytes but was detected in COLII-expressing chondrocytes (B and D) because these cells express the Hspg2-transgene driven by the Col2a1 promoter/enhancer. Fewer PCNA-positive cells (brown) were observed in osteophytes of Hspg2−/−-Tg mice compared with control mice (E). Quantitative analysis showed that the number of PCNA-positive cells in osteophytes was significantly reduced in Hspg2−/−-Tg mice compared with control mice (G). P-Smad2 staining (brown) was reduced in Hspg2−/−-Tg mice compared with control mice (F). Quantitative analysis confirmed the significant reduction of p-Smad2 in osteophytes of Hspg2−/−-Tg mice (H). The osteophytes are shown with black lines (A, B, E and F) and white lines (C and D). Scale bars, 100 μm. All data represent means and SEM.
2.4. Perlecan deficiency reduced chondrocyte differentiation, cell proliferation, and Smad2 phosphorylation in osteophyte developmental processes
Next, we analyzed chondrogenic processes by immunostaining type II collagen (COLII), a marker of proliferative chondrocytes, and type X collagen (COLX), a marker of hypertrophic chondrocytes in surgically induced knee OA (Fig. 4B, C). COLII (brown) and COLX (green) expression were reduced in osteophytes of Hspg2−/−-Tg mice compared to control mice (Fig. 4A, B, C). Perlecan expression (green) was detected in synovial cells and chondrocytes of osteophytes with higher expression levels in hypertrophic chondrocytes of control mice (Fig. 4D, left panel). In Hspg2−/−-Tg mice, perlecan expression was absent in osteophytes but was detected in COLII-expressing chondrocytes (Fig. 4D, right panel), because these cells expressed the Hspg2-transgene driven by the Col2a1 promoter/enhancer (Xu et al., 2010; Ishijima et al., 2012). We examined the effects of perlecan deficiency on synovial cell proliferation by staining tissue sections of the joints for proliferating cell nuclear antigen (PCNA). Fewer PCNA-positive cells were detected in osteophytes in Hspg2−/−-Tg mice than in control mice (Fig. 4E). Quantitative analysis showed that cell proliferation in osteophytes was significantly reduced in Hspg2−/−-Tg mice compared with control mice (Fig. 4G). To further elucidate the molecular mechanisms, Smad2 phosphorylation in osteophytes was examined because TGF-β/Smad signaling is upregulated during osteophyte development (Blaney Davidson et al., 2006). Phosphorylated Smad2 (p-Smad2) staining was reduced in Hspg2−/−-Tg mice compared with control mice (Fig. 4F). Quantitative analysis confirmed the significant reduction of p-Smad2 in the osteophytes of Hspg2−/−-Tg mice (Fig. 4H).
2.5. Requirement for synovial perlecan in TGF-β-induced osteophyte formation
We further examined the role of synovial perlecan in TGF-β-induced osteophyte development in knee joints using a TGF-β injection model (van Beuningen et al., 1998; van Lent et al., 2004). TGF-β induced osteophyte formation in control mice, but the deficiency in synovial perlecan expression reduced osteophyte formation in the Hspg2−/−-Tg mice (arrow lines, Fig. 5A). Quantitative analysis revealed that the osteophyte size and maturity were reduced in the joints of Hspg2−/−-Tg mice compared with control mice (Fig. 5B). These results indicate that perlecan expressed in the synovium is required for TGF-β-induced osteophyte development.
Fig. 5.
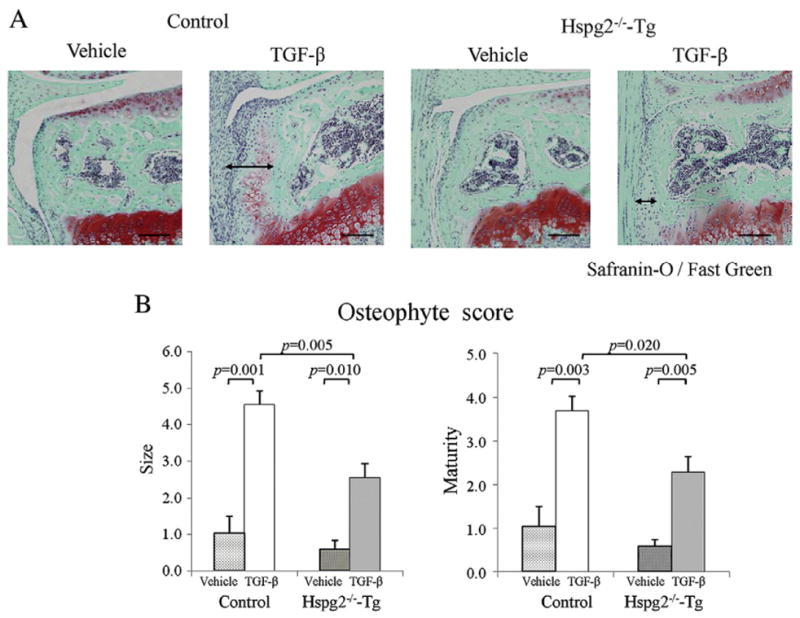
Reduction in TGF-β-induced osteophyte formation in Hspg2−/−-Tg mice. Histological sections of the knee joint 1 week after injection with either TGF-β or vehicle in control and Hspg2−/−-Tg mice (A). Safranin-O staining and osteophyte size and maturity were reduced in the joints of Hspg2−/−-Tg mice compared to control mice (A). Quantification showed that osteophyte size and maturity on the TGF-β injection side (TGF-β) of Hspg2−/−-Tg mice were significantly reduced (B). The osteophytes are shown with black lines (A). Scale bar, 100 μm. All data represent means and SEM.
3. Discussion
Osteophyte formation in OA occurs through a series of highly coordinated biological processes that include MSC proliferation and differentiation, deposition and remodeling of the cartilage matrix, vascular invasion, and bone marrow formation. In this study, we examined the role of synovial perlecan in osteophyte formation using synovial perlecan-deficient mice. We demonstrate that perlecan expression in the synovium is required for osteophyte formation in a surgical OA model and a TGF-β-induced model.
The osteophyte is developed at the cartilage–synovial junction in the synovial joint, where the synovium covers the periosteum (Cooper, 1964; van der Kraan and van den Berg, 2007). MSCs prepared from the synovium and the periosteum are able to differentiate to chondrocytes in vitro (De Bari et al., 2001, 2004; Sakaguchi et al., 2005; van der Kraan and van den Berg, 2007). However, it is still unclear whether periosteum, synovium, or both are involved in osteophyte formation (van der Kraan and van den Berg, 2007; Kurth et al., 2011). When macrophages expressed in synovium are selectively inhibited, osteophyte formation is inhibited (Bakker et al., 2001; Blom et al., 2004; van Lent et al., 2004). These results suggest that the synovium plays a role in osteophyte formation.
It remains unclear whether synovitis is involved in osteophyte formation, although less osteophyte formation is observed in patients with rheumatoid arthritis, wherein synovial inflammation plays a crucial role (Cabral et al., 1989). However, our results, which show that histological synovitis in the OA model of Hspg2−/−-Tg mice are similar in comparison with control mice (Fig. 2Cb), suggest that synovial perlecan may not affect osteophyte formation via inflammation, although further study is required.
Perlecan plays a role in neural cell proliferation in the niche in the central nervous system through modulating signal transduction of growth factors such as FGFs (Park et al., 2003; Kerever et al., 2007; Soulez et al., 2010). We found that cell proliferation was reduced in osteophytes of Hspg2−/−-Tg mice (Fig. 4E, G), suggesting that synovial perlecan also modulates cell proliferation. Perlecan deficiency reduced chondrocyte proliferation (Arikawa-Hirasawa et al., 1999), disorganized cartilage matrix deposition (Kvist et al., 2006), and reduced vascular invasion during endochondral ossification (Ishijima et al., 2012) in growth plates. We showed that synovial perlecan is required for differentiation of the osteophyte, as type II and X collagen expression in the osteophyte was reduced by the absence of synovial perlecan expression (Fig. 4B, C, D). These responses are similar to perlecan-deficient responses seen during early stage of growth plate development (Arikawa-Hirasawa et al., 1999). It is known that Col2a1 promoter/enhancer-driven transgene expression gradually decreases with age. Although synovial perlecan primarily contributes to osteophyte formation in OA joints, it is possible that the reduced perlecan transgene expression in cartilage may secondarily affect reduced osteophyte formation in Hspg2−/−-Tg mice with age.
TGF-β plays an essential role in initiating chondrogenesis, and BMPs affect the terminal differentiation of chondrocytes and endochondral ossification during the osteophyte developmental process (Uchino et al., 2000; Blaney Davidson et al., 2006; van der Kraan and van den Berg, 2007). FGF2 and IGF-1 are also implicated in osteophyte formation (Iwasaki et al., 1995; Okazaki et al., 1999; Mierisch et al., 2002; Fukumoto et al., 2003). Perlecan binds to growth factors and/or their receptors such as FGFs and VEGF and modulates growth factor activities (Aviezer et al., 1994; Brown et al., 1997; Whitelock et al., 2008; Ishijima et al., 2012). It is also suggested to interact with TGF-β and its receptors and modulators (Sengle et al., 2010), but whether perlecan modulates TGF-β signaling remains to be established. We found that synovial perlecan deficiency reduced the activity of Smad-2, a TGF-β downstream molecule (Fig. 4F, H) and inhibited osteophyte formation induced by the intra-articular injection of TGF-β in the osteophyte induction model (Fig. 5). These data suggest that synovial perlecan may modulate TGF-β signaling pathways that are necessary for osteophyte formation.
The function of the osteophyte and its clinical relevance in OA remain unclear. It has been speculated that the osteophyte develops as a secondary effect of cartilage destruction in OA (van der Kraan and van den Berg, 2007). A large-scale epidemiological study using a population-based cohort shows that the osteophyte formation volume is significantly associated with the physical functioning of patients with knee OA, while the medial joint space width reflected by the remaining articular cartilage volume is associated with pain (Muraki et al., 2011). We showed that synovial perlecan deficiency inhibited osteophyte formation, while no significant differences in articular cartilage degradation were noted in control and Hspg2−/−-Tg mice (Fig. 2). These data, combined with those from previous studies (Blaney Davidson et al., 2007; van der Kraan and van den Berg, 2007), indicated that osteophyte formation and articular cartilage destruction are independent phenomena in OA. While osteophytes reduced varus–valgus instability in knee OA, removal of osteophytes significantly increased joint motion (Pottenger et al., 1990). In addition to the reduction in knee pain, maintaining and improving physical function are also important for the better management of OA (Ishijima et al., 2011). Reducing osteophyte formation by regulating synovial perlecan expression may facilitate better management of OA by maintaining the physical function of patients with knee OA.
4. Materials and methods
4.1. Mice
We used a mouse (Hspg2−/−-Tg) model, in which the perinatal lethality of perlecan knockout (Hspg2−/−) is rescued by expression of the perlecan (Hspg2) transgene (Hspg2Tg/−), specific to cartilage and under the control of the chondrocyte-specific Col2a1 collagen chain promoter and enhancer (Arikawa-Hirasawa et al., 1999; Tsumaki et al., 1999; Xu et al., 2010; Ishijima et al., 2012). In Hspg2−/−-Tg (Hspg2−/−; Col2a1-Hspg2Tg/−) mice, perlecan is expressed in the cartilage but not in the synovium. We used the littermate heterozygous (Hspg2+/−-Tg, Hspg2+/−; Col2a1-Hspg2Tg/−) (Xu et al., 2010; Ishijima et al., 2012) as controls. Eleven- to twelve-week-old female adult Hspg2−/−-Tg mice and control mice (18–24 g in body weight) were used. All experimental procedures were performed following the guidelines for the care and use of animals and received ethical approval from the animal ethics committee at our university.
4.2. Procedures of osteoarthritis models
We performed two procedures to induce OA—the surgical OA model (Kamekura et al., 2005) and the TGF-β injection model (van Beuningen et al., 1998). In the surgical OA model, the medial collateral ligament was transected, and the medial meniscus was resected on the left knee of Hspg2−/−-Tg (n=10) and control mice (n=9). The contralateral right knee joint was subjected to a sham operation by the same approach, but without ligament transection and meniscectomy. Four weeks and 8 weeks after surgery, the mice were sacrificed. In the TGF-β injection model (van Beuningen et al., 1998; van Lent et al., 2004), a TGF-β1 solution (200 ng in 6 μl; R&D Systems, Minneapolis, MN, USA) was administered intra-articularly for a total of three times every other day at the left knee joint (n=5 in each group). The vehicle [saline with 1% bovine serum albumin (BSA)] was administered at the right knee joint. One week after the final injection, the mice were sacrificed.
4.3. Tissue preparation and histological grading score
The entire knee joints were dissected and fixed in 4% paraformaldehyde buffered with phosphate-buffered saline for 48 h at 4 °C. The specimens were decalcified for 10 days with 10% EDTA (pH 7.4) at room temperature. The specimens were dehydrated with an increasing concentration of ethanol and embedded in paraffin. The samples were cut into 3.5-μm coronal sections and stained with safranin-O and fast green. Development of OA in the medial femoral condyle (MFC) and the medial tibial plateau (MTP) was quantified with histological grading scores, according to the OARSI histopathology initiative (Kamekura et al., 2005; Mapp et al., 2008; Little et al., 2009; Glasson et al., 2010). The cartilage degradation score consisted of two domains: structure, with 8° (0, 0.5, 1–6) (Glasson et al., 2010), and proteoglycan depletion by using safranin-O and fast green section, with 6° (0–5) (Glasson et al., 2010). The synovial status was semi-quantified by the number of synovial lining layers, for 4° (0–3) (Mapp et al., 2008; Glasson et al., 2010). The osteophyte was evaluated semiquantitatively using osteophyte formation scores consisting of two domains, size and maturity (Little et al., 2009; Glasson et al., 2010). In osteophyte size, the thickness of the osteophyte was compared with that of the adjacent articular cartilage and classified for 4° (0; none, 1; small, the same thickness as the adjacent articular cartilage, 2; medium, among one time to three times the thickness of the adjacent articular cartilage, 3; large, more than three times the thickness of the adjacent articular cartilage) (Little et al., 2009; Glasson et al., 2010). Osteophyte maturity was scored with 5° (0; none, 1; precartilagious lesion; 2; predominantly cartilage, 3; mixed cartilage and bone, 4; predominantly bone) (Kamekura et al., 2005; Little et al., 2009; Glasson et al., 2010). The average histological grading scores in MFC and MTP were evaluated among 5 sections at intervals of 70 μm in each mouse knee, and the average scores were summed (Little et al., 2009).
4.4. Synovial tissue preparation
Synovial tissues from knee joints of 11-week-old mice were extracted according to the method we recently reported (Futami et al., 2012). One part of the synovium was immediately frozen in isopentane cooled with liquid nitrogen. Briefly, frozen sections were cut in a cryostat on microscope slides. The sections were fixed in acetone for 10 min at 4 °C before immunohistochemistry.
4.5. Immunohistochemistry
Immunohistochemistry for type II collagen (COLII), type X collagen (COLX), perlecan, PCNA, and phosphorylated Smad2 (p-Smad2) were performed in deparaffinized knee sections and synovial frozen sections. For epitope retrieval for COLII, COLX, and perlecan, the knee sections were treated with 2.5% hyaluronidase (Sigma, St. Louis, MO, USA) for 1 h at 37 °C, and for PCNA and p-Smad2 immunostaining, the sections were boiled with 0.1 M citrate buffer for 15 min. For the localization of COLII (1:300; the University of Iowa, Iowa City, IA, USA), we used a mouse on a mouse kit (Invitrogen Corporation, Camarillo, CA, USA), according to the manufacturer’s recommendations. For the localization of PCNA, we used a PCNA staining kit (Invitrogen Corporation, Camarillo, CA, USA), according to the manufacturer’s recommendations. For other proteins, after blocking with 5% goat serum and 2% BSA in PBS for 30 min at room temperature, the sections were incubated with antibodies to perlecan domain V (1:500; from Dr. Sasaki), COLX (1:500; from Dr. Lunstrum), and p-Smad2 (1:100; Millipore, Billerica, MA, USA) for 18 h at 4 °C. Visualization of immunoreactivity for perlecan and COLX was performed using Alexa Fluor 488 fluorescence (1:300; Molecular Probes, Carlsbad, CA, USA). For p-Smad2, biotin-labeled goat antibodies against rabbit IgG (1:300; Dako, Carpinteria, CA, USA) and biotin–streptavidin horseradish peroxidase (1:300; Dako, Carpinteria, CA, USA) were used and visualized using diaminobenzidine staining with hematoxylin counterstaining. Sections that showed positive staining for PCNA and p-Smad2 were scored by a blinded observer in a modified previous report (Blaney Davidson et al., 2006). The percentage of cells staining positive for PCNA and p-Smad2 on the osteophyte developed on the margin of the medial tibia were calculated. These cells were counted in at least three sections per knee (n=3 in each group).
4.6. Statistical analysis
Group means were compared with analysis of variance, and the significance of differences was determined by using an unpaired t-test. P values less than 0.05 were considered significant.
Method used for Supplemental Fig. 1: Total RNA was isolated from articular cartilage of knee joints from 10 to 15-week-old control and Hspg2−/−-Tg mice using TRIzol reagent (Invitrogen), according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized using cDNA Synthesis Kit (TOYOBO, Osaka, Japan). RT-PCR analyses were performed using Ex Taq Polymerase (Takara, Tokyo, Japan) with thermal cycler (Applied Biosystems, Foster City, CA, USA) under the following condition: initial denaturation for 3 min at 94 °C followed by 30 cycles consisting of 30 s at 94 °C, 1 min at 60 °C and 30 s 72 °C. RT-PCR products were electrophoresed on a 2% agarose gel and stained with ethidium bromide. Levels of expression were quantified densitometrically using the public domain Java image processing program ImageJ. The amounts of mRNA were shown as relative quantities in comparison to those of GAPDH mRNA. The PCR primers included perlecan (forward, 5′-TGCTTGCCA CAGCTATAATGAGTGTGTGG-3′; reverse, 5′-CACAGCGCCACAACTTGAGA GCACAG-3′) and GAPDH (forward, 5′-TGTGTCCGTCGTGGATCTGA-3′; reverse, 5′-TTGCTGTTGAAGTCGCAGGAG-3′).
Supplementary Material
Acknowledgments
We would like to thank Dr. T. Sasaki and Dr. G. Lunstrum for providing the antibodies, Dr. Z. Xu, Dr. S. de Vega and Dr. R. Nonaka for mouse management and technical assistance, and T. Sueyoshi, Y. Kojima, J. Furuhata, Y. Toui, K. Miyahara, and H. Sakai for technical assistance. This study was funded in part by a High Technology Research Center Grant from the Ministry of Education, Culture, Sport, Science and Technology, Japan (19791047 and 21791418 for M.I. and 22300223 for E.A.-E.) and the Intramural Program of National Institute of Dental and Craniofacial Research, the National Institutes of Health (to Y.Y.).
Abbreviations
- BMP
bone morphogenetic protein
- BSA
bovine serum albumin
- COLII
type II collagen
- COLX
type X collagen
- FGF
fibroblast growth factor
- IGF-1
insulin-like growth factor 1
- MSCs
mesenchymal stem cells
- OA
osteoarthritis
- PCNA
proliferating cell nuclear antigen
- p-Smad2
phosphorylated Smad2
- SEM
standard error of mean
- TGF-β
transforming growth factor beta
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.matbio.2013.01.004.
Contributor Information
Haruka Kaneko, Email: harukago@juntendo.ac.jp.
Muneaki Ishijima, Email: ishijima@juntendo.ac.jp.
Ippei Futami, Email: ippei@juntendo.ac.jp.
Naoki Tomikawa-Ichikawa, Email: naoichi@fmu.ac.jp.
Keisuke Kosaki, Email: kkosaki@t3.rim.or.jp.
Ryo Sadatsuki, Email: rsadatsu@juntendo.ac.jp.
Yoshihiko Yamada, Email: yyamada@dir.nidcr.nih.gov.
Hisashi Kurosawa, Email: kuro@juntendo.ac.jp.
Kazuo Kaneko, Email: k-kaneko@juntendo.ac.jp.
Eri Arikawa-Hirasawa, Email: ehirasaw@juntendo.ac.jp.
References
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Wilcox WR, Le AH, Silverman N, Govindraj P, Hassell JR, Yamada Y. Dyssegmental dysplasia, Silverman–Handmaker type, is caused by functional null mutations of the perlecan gene. Nat Genet. 2001;27:431–434. doi: 10.1038/86941. [DOI] [PubMed] [Google Scholar]
- Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79:1005–1013. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis — results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr Cartil. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Bakker AC, van de Loo FA, van Beuningen HM, Sime P, van Lent PL, van der Kraan PM, Richards CD, van den Berg WB. Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthr Cartil. 2001;9:128–136. doi: 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- Blaney Davidson EN, Vitters EL, van der Kraan PM, van den Berg WB. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis. 2006;65:1414–1421. doi: 10.1136/ard.2005.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Vitters EL, van Beuningen HM, van de Loo FA, van den Berg WB, van der Kraan PM. Resemblance of osteophytes in experimental osteoarthritis to transforming growth factor beta-induced osteophytes: limited role of bone morphogenetic protein in early osteoarthritic osteophyte formation. Arthritis Rheum. 2007;56:4065–4073. doi: 10.1002/art.23034. [DOI] [PubMed] [Google Scholar]
- Blom AB, van Lent PL, Holthuysen AE, van der Kraan PM, Roth J, van Rooijen N, van den Berg WB. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr Cartil. 2004;12:627–635. doi: 10.1016/j.joca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Brown JC, Sasaki T, Gohring W, Yamada Y, Timpl R. The C-terminal domain V of perlecan promotes beta1 integrin-mediated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans. Eur J Biochem. 1997;250:39–46. doi: 10.1111/j.1432-1033.1997.t01-1-00039.x. [DOI] [PubMed] [Google Scholar]
- Cabral AR, Loya BL, Alarcon-Segovia D. Bone remodeling and osteophyte formation after remission of rheumatoid arthritis. J Rheumatol. 1989;16:1421–1427. [PubMed] [Google Scholar]
- Cooper NS. Arthritis and the rheumatic diseases. Pathologic considerations. Phys Ther. 1964;44:574–583. doi: 10.1093/ptj/44.7.574. [DOI] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fassler R. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bari C, Dell’Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- De Bari C, Dell’Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50:142–150. doi: 10.1002/art.11450. [DOI] [PubMed] [Google Scholar]
- Dodge GR, Boesler EW, Jimenez SA. Expression of the basement membrane heparan sulfate proteoglycan (perlecan) in human synovium and in cultured human synovial cells. Lab Invest. 1995;73:649–657. [PubMed] [Google Scholar]
- Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, O’Driscoll SW. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthr Cartil. 2003;11:55–64. doi: 10.1053/joca.2002.0869. [DOI] [PubMed] [Google Scholar]
- Futami I, Ishijima M, Kaneko H, Tsuji K, Ichikawa-Tomikawa N, Sadatsuki R, Muneta T, Arikawa-Hirasawa E, Sekiya I, Kaneko K. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS One. 2012;7:e45517. doi: 10.1371/journal.pone.0045517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI Histopathology Initiative — Recommendations for Histological Assessments of Osteoarthritis in the Mouse, Osteoarthritis Cartilage. Osteoarthritis Cartilage; England: 2010. pp. S17–23. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Perlecan: a gem of a proteoglycan. Matrix Biol. 1994;14:203–208. doi: 10.1016/0945-053x(94)90183-x. [DOI] [PubMed] [Google Scholar]
- Ishijima M, Watari T, Naito K, Kaneko H, Futami I, Yoshimura-Ishida K, Tomonaga A, Yamaguchi H, Yamamoto T, Nagaoka I, Kurosawa H, Poole RA, Kaneko K. Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res Ther. 2011;13:R22. doi: 10.1186/ar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishijima M, Suzuki N, Hozumi K, Matsunobu T, Kosaki K, Kaneko H, Hassell JR, Arikawa-Hirasawa E, Yamada Y. Perlecan modulates VEGF signaling and is essential for vascularization in endochondral bone formation. Matrix Biol. 2012;31:234–245. doi: 10.1016/j.matbio.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M, Nakahara H, Nakata K, Nakase T, Kimura T, Ono K. Regulation of proliferation and osteochondrogenic differentiation of periosteum-derived cells by transforming growth factor-beta and basic fibroblast growth factor. J Bone Joint Surg Am. 1995;77:543–554. doi: 10.2106/00004623-199504000-00007. [DOI] [PubMed] [Google Scholar]
- Jingushi S, Shida J, Iwamoto Y, Kinoshita T, Hiyama Y, Tamura M, Izumi T. Transient exposure of fibroblast growth factor-2 induced proliferative but not destructive changes in mouse knee joints. Connect Tissue Res. 2006;47:242–248. doi: 10.1080/03008200600883146. [DOI] [PubMed] [Google Scholar]
- Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr Cartil. 2005;13:632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- Kurth TB, Dell’accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63:1289–1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- Kvist AJ, Johnson AE, Morgelin M, Gustafsson E, Bengtsson E, Lindblom K, Aszodi A, Fassler R, Sasaki T, Timpl R, Aspberg A. Chondroitin sulfate perlecan enhances collagen fibril formation. Implications for perlecan chondrodysplasias. J Biol Chem. 2006;281:33127–33139. doi: 10.1074/jbc.M607892200. [DOI] [PubMed] [Google Scholar]
- Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Ishijima M, Futami I, Kaneko H, Kubota M, Kawasaki T, Matsumoto T, Kurihara H, Ning L, Xu Z, Ikeda H, Takazawa Y, Saita Y, Kimura Y, Xu S, Kaneko K, Kurosawa H. Correlation between synovitis detected on enhanced-magnetic resonance imaging and a histological analysis with a patient-oriented outcome measure for Japanese patients with end-stage knee osteoarthritis receiving joint replacement surgery. Clin Rheumatol. 2010;29:1185–1190. doi: 10.1007/s10067-010-1522-3. [DOI] [PubMed] [Google Scholar]
- Mapp PI, Avery PS, McWilliams DF, Bowyer J, Day C, Moores S, Webster R, Walsh DA. Angiogenesis in two animal models of osteoarthritis. Osteoarthr Cartil. 2008;16:61–69. doi: 10.1016/j.joca.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Mierisch CM, Anderson PC, Balian G, Diduch DR. Treatment with insulin-like growth factor-1 increases chondrogenesis by periosteum in vitro. Connect Tissue Res. 2002;43:559–568. [PubMed] [Google Scholar]
- Muraki S, Oka H, Akune T, En-yo Y, Yoshida M, Suzuki T, Yoshida H, Ishibashi H, Tokimura F, Yamamoto S, Nakamura K, Kawaguchi H, Yoshimura N. Independent association of joint space narrowing and osteophyte formation at the knee with health-related quality of life in Japan: a cross-sectional study. Arthritis Rheum. 2011;63:3859–3864. doi: 10.1002/art.30641. [DOI] [PubMed] [Google Scholar]
- Ning L, Ishijima M, Kaneko H, Kurihara H, Arikawa-Hirasawa E, Kubota M, Liu L, Xu Z, Futami I, Yusup A, Miyahara K, Xu S, Kaneko K, Kurosawa H. Correlations between both the expression levels of inflammatory mediators and growth factor in medial perimeniscal synovial tissue and the severity of medial knee osteoarthritis. Int Orthop. 2011;35:831–838. doi: 10.1007/s00264-010-1045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan DM, Fulle A, Valente P, Cai S, Horigan E, Sasaki M, Yamada Y, Hassell JR. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem. 1991;266:22939–22947. [PubMed] [Google Scholar]
- Okazaki K, Jingushi S, Ikenoue T, Urabe K, Sakai H, Ohtsuru A, Akino K, Yamashita S, Nomura S, Iwamoto Y. Expression of insulin-like growth factor I messenger ribonucleic acid in developing osteophytes in murine experimental osteoarthritis and in rats inoculated with growth hormone-secreting tumor. Endocrinology. 1999;140:4821–4830. doi: 10.1210/endo.140.10.7053. [DOI] [PubMed] [Google Scholar]
- Park Y, Rangel C, Reynolds MM, Caldwell MC, Johns M, Nayak M, Welsh CJ, McDermott S, Datta S. Drosophila perlecan modulates FGF and hedgehog signals to activate neural stem cell division. Dev Biol. 2003;253:247–257. doi: 10.1016/s0012-1606(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Pottenger LA, Phillips FM, Draganich LF. The effect of marginal osteophytes on reduction of varus–valgus instability in osteoarthritic knees. Arthritis Rheum. 1990;33:853–858. doi: 10.1002/art.1780330612. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability. J Biol Chem. 2010;286:5087–5099. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulez M, Sirois I, Brassard N, Raymond MA, Nicodeme F, Noiseux N, Durocher Y, Pshezhetsky AV, Hebert MJ. Epidermal growth factor and perlecan fragments produced by apoptotic endothelial cells co-ordinately activate ERK1/2-dependent antiapoptotic pathways in mesenchymal stem cells. Stem Cells. 2010;28:810–820. doi: 10.1002/stem.403. [DOI] [PubMed] [Google Scholar]
- Tsumaki N, Tanaka K, Arikawa-Hirasawa E, Nakase T, Kimura T, Thomas JT, Ochi T, Luyten FP, Yamada Y. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol. 1999;144:161–173. doi: 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino M, Izumi T, Tominaga T, Wakita R, Minehara H, Sekiguchi M, Itoman M. Growth factor expression in the osteophytes of the human femoral head in osteoarthritis. Clin Orthop Relat Res. 2000;377:119–125. doi: 10.1097/00003086-200008000-00017. [DOI] [PubMed] [Google Scholar]
- van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994;71:279–290. [PubMed] [Google Scholar]
- van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB. Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthr Cartil. 1998;6:306–317. doi: 10.1053/joca.1998.0129. [DOI] [PubMed] [Google Scholar]
- van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthr Cartil. 2007;15:237–244. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- van Lent PL, Blom AB, van der Kraan P, Holthuysen AE, Vitters E, van Rooijen N, Smeets RL, Nabbe KC, van den Berg WB. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum. 2004;50:103–111. doi: 10.1002/art.11422. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis — an untreatable disease? Nat Rev Drug Discov. 2005;4:331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- Xu Z, Ichikawa N, Kosaki K, Yamada Y, Sasaki T, Sakai LY, Kurosawa H, Hattori N, Arikawa-Hirasawa E. Perlecan deficiency causes muscle hypertrophy, a decrease in myostatin expression, and changes in muscle fiber composition. Matrix Biol. 2010;29:461–470. doi: 10.1016/j.matbio.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.