Abstract
The regulatory T (Treg) cells play an important role in the maintenance of homeostasis and the prevention of autoimmune diseases. Although most studies are focusing on the role of Treg cells in T cells and T cells-mediated diseases, these cells also directly affect B cells and other non-T cells. This manuscript updates the role of Treg cells on the B cells and B cell-mediated diseases. In addition, the mechanisms whereby Treg cells suppress B cell responses have been discussed.
Keywords: Treg, Foxp3, B cells, antibodies, autoimmune diseases
Introduction
Accumulated evidence has indicated that regulatory T (Treg) cells play an essential role in the maintenance of homeostasis and prevention of autoimmune responses [1-5]. Most of these cells are CD4+ cells that express CD25, an IL-2 receptor alpha chain and other Treg cells related molecular markers [6-8]. Foxp3, a transcript factor, has been recognized to be a key factor for Treg cell development and function. Foxp3 has also been considered as a specific marker to define and identify Treg cells from other T cell subpopulations although this has been challenged on its specificity in human Treg cells [9-12]. In addition to CD4+ Treg cells, CD8+ Treg cells represent another cell population and Foxp3 may not be so crucial for their development and function when compared to CD4+ Treg cells [13-15].
Treg cells originate from thymus, being called natural Treg cells. These cells move to periphery to exert their roles. In the periphery, these cells can be emigrated from thymus or differentiated in the local places. Thus, Treg cells can be classified into natural and induced Treg cells. It is unclear what percentages of Treg cells are natural or induced Treg cells in the periphery since both Treg populations share similar phenotypes and functional characteristics, and no specific molecular marker can distinguish natural Treg from induced Treg cells so far. Although recent efforts have demonstrated that neuropilin-1 and helios could be valuable to distinguish two populations [16-18], however, other studies have also demonstrated that their specificity may not be what we previously expected since helios is also highly expressed on Th2 and T follicular helper cells and is associated with the differentiation of these cells [4,19].
Induced Treg cells can be induced ex vivo from non-Treg cells [20-23]. During this induction, TGF-β and TGF-β receptor signal pathway is essential for the development and differentiation of induced Treg cells [24-26]. Other factors, like IL-2 and all-trans retinoic acid (atRA), can promote Treg cell development and function [27-32]. These iTreg cells not only mostly share the phenotypic and functional characteristics with nTreg cells, but also display some advantageous features. For example, these cells are more stable in the inflammatory conditions than natural Treg cells, demonstrating their superior capacity on treating inflammatory and autoimmune diseases [33-37].
B cells and immune responses
Both B- and T-lymphocytes consist of important players in this adaptive immune response. B-cells exert their effect through the production of antibodies, antigen-presenting ability and cytokines production.
B cells usually need the help provided by T cells to get activated upon encountering antigens to differentiate into effector plasma cells. Plasma cells produce or secrete antibodies that subsequently circulate in the blood, lymph, and tissues where they can target specific antigens or pathogens and promote their elimination [38]. B cells can also be activated independent upon T cells, as B cells express Toll-like receptors (TLRs), primarily TLR4 and TLR9, which recognize additional signals in the form of microbial viral components, to affect like innate immune cells [39].
Like dendritic cells (DCs), B cells have also antigen-presenting cell ability. B cell receptor expressed on B cell surface can bind specific antigen containing major histocompatibility complex (MHC). When MHC is presented to T cell surface, B cells have elicited T cell immune-mediated response. Unlike DCs, B cells present low loses of antigens whereas DCs present high levels of antigens that both may have a concordant role in presenting antigens to T cells [38,40].
Additionally, B cells also produce cytokines, for example, activated B cells can produce IL-4, IL-6, IL-10, IL-21, IL-23, TNF-α, and lymphotoxin. These cytokines further regulates innate and adaptive immune responses [38,40].
B cells and autoimmune diseases
Aside from their role in immune defense, the dysfunction of B cells also contributes three classes of B-cells diseases: congenital immunodeficiencies, autoimmunity, and leukemia and lymphoma [41,42].
B lymphocytes have been classically recognized to contribute to the pathogenesis of autoimmune diseases through autoantibody production [40]. Self-reactive B cells are responsible for the autoantibody production and autoimmunity. Self-reactive B cells are mostly eliminated in the bone marrow through a process termed negative selection. Nonetheless, some of self-reactive B cells escape negative selection in the bone marrow and migrate to periphery. These self-reactive B cells are kept under check by other mechanisms including deletion, anergy and immune modulation in the periphery [38,40]. Genetic defects may promote a loss of B cell tolerance [43]. Dysregulated apoptotic genes increase B-cell lifespans and thereby promote survival of self-reactive B-cell clones, leading to autoantibodies and multiple autoimmune syndromes [44]. Treg cells play an important role in controlling the immune responses of these self-B and self-T cells and then prevention of autoimmune diseases. Dysfunction of Treg cells contributes autoimmune responses.
Although B cells are generally considered to be crucial for the pathogenesis of autoimmune diseases, it actually has an extent difference of role in the pathogenesis of various autoimmune diseases. In general, systemic lupus erythematosus (SLE) appears to be highly dependent upon B cells for their development. Using MRL/lpr animal model of SLE, B cells are crucial for the lupus pathogenesis since B-cell-deficient MRL/lpr mice have no pathology at an age whereas B-cell-intact MRL/lpr mice have an evident disease [45]. Conversely, in other autoimmune diseases, such as rheumatoid arthritis (RA), systemic sclerosis (SSc), multiple sclerosis (MS), and type 1 diabetes (T1D), B cells may plan an adjuvant role in their development. Additionally, B cells play an important role in the early stage of diseases during the initiation of T-cell activation and the generation of the autoreactive long-lived plasma cells, thus using therapy on B-cell depletion should be considered on initial phase of diseases but not late stage of diseases.
A major role B cell played is the production of autoantibodies. When the disease is initiated, the self-reactive B cells recognize self-tissues and produce autoantibodies. For example, the levels of anti-dsDNA, anti-ANA, anti-SM, anti-phospholipid, anti-cardioplin, anti-Ro antibodies are elevated and corrected the clinical features of disease activity in SLE [46]. These autoantibodies are also indicatives of diagnosis and progress in SLE. Autoantibodies will bind to self-antigens to form an immune complex in tissues that locally activate the complement cascade and induce type III immune complex reactions with inflammation [40].
B cells also contribute autoimmune diseases through their function as cellular adjuvants for CD4+ T cell activation and differentiation. Additionally, B cells secrete many cytokines that also regulate T cell function and inflammation. Thus, B cells regulate multiple aspects of humoral and cellular function in the immune system and autoimmune diseases.
Treg cells control B cells and autoantibodies
The major target for Treg cells is T cells, therefore, Treg cells play an important role in controlling T cell-mediated diseases (Figure 1). Nonetheless, recent studies have also demonstrated that Treg cells also exert their effects on other cells including DCs, macrophages, mast, B cells and osteoclast [35,47-52].
Figure 1.
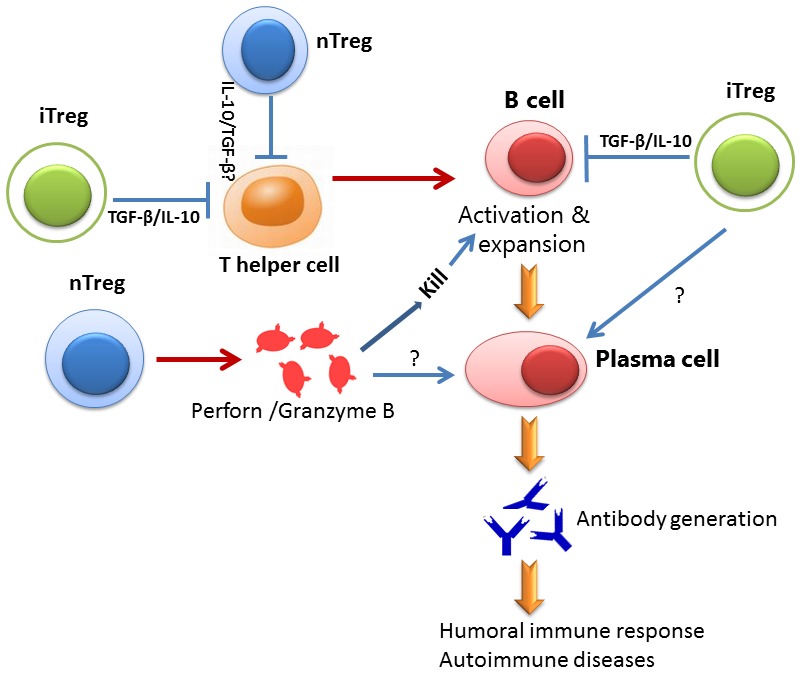
Schematic diagram for the roles of Treg subsets on B cell. Both nTreg and iTregs suppress Th cell response that is important for B cell activation. However, both Tregs also directly suppress B cell response through different mechanisms. While nTreg cells kill B cell through the secretion of Perforin and Granzyme B, iTreg cells suppress B cell response through immune suppressive cytokines including TGF-β and IL-10. Both Treg may have synergetic role on B cells to regulate the production of antibodies. It is unclear so far whether both Treg cell directly suppress plasma cells.
Treg cells can suppress B cell responses and B cell-mediated antibody production. It has been reported that that the depletion of Tregs in rodents can lead to dysregulated Ab production [53] and the transfer of Tregs into autoimmune animals can reduce Ab responses [54]. Further evidence is provided through studying the patients with IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) patients who lack Treg cells. Multiple and diverse autoantibodies are commonly identified in the sera of IPEX patients, suggesting that Tregs represent a key regulator for autoreactive B cells [55]. However, current studies are still less clear whether Treg cells directly act on B cells, since Treg cells can affect T cells first and then indirectly act on B cells.
To determine the impact of Tregs on the establishment of human early B-cell tolerance checkpoints, Kinnunen et al have recently cloned and expressed in vitro recombinant antibodies from single B cells from IPEX patients who lack Foxp3+ Treg cells, and compared their reactivity to those derived from healthy donors. They observed that characteristics and reactivity of antibodies expressed by new emigrant/transitional B cells from IPEX patients were similar to those from healthy donors, demonstrating that defective Treg function does not impact central B-cell tolerance. Conversely, they also observed that FOXP3 deficiency resulted in the accumulation of autoreactive clones in the mature naive B-cell compartment of IPEX patients, providing direct evidence for the role of Tregs in maintaining peripheral B-cell tolerance in humans [56].
Lim et al first identified that Treg cells locate on B cell-rich area. Using immunohistochemical staining combining anti-Foxp3, anti-CD4 and anti-IgD antibodies, and three-color confocal microscope technique in human tonsils, they found while most human Foxp3+ cells locate on T cell zone, they also observed that significant numbers of Foxp3+ cells distribute on T-B border area including mantle zones and germinal center areas. These Foxp3+ cells are T cells but not B cells. They further demonstrated that these Foxp3+ Treg cells suppress B cell activities, including B cell activation, proliferation, IgG production and switch. Moreover, the inhibitory effect of Treg on B cells is independent upon T cells since it still works in T cell-free condition [57].
Using transgenic mice expressing model antigens in the kidney, Gotot et al demonstrated that Treg cells are essential to suppress autoreactive B cells in an antigen-specific manner and to prevent them from producing autoantibodies. This suppression requires PD-1 expression on autoreactive B cells and expression of the two PD-1 ligands on Treg cells. These findings demonstrate in vivo that Treg cells use PD-1 ligands to directly suppress autoreactive B cells [58].
Zhao et al used an in vitro culture system to demonstrate that mice Treg cells can directly suppress B cell activation and proliferation [49]. This suppression needs cell to cell contact but not suppressive cytokines. They further found that activated T cells kill B cells through the emancipation of perforin and granzyme B by Treg cells. Iikuni et al further found that Treg cells also can suppress B cell activity and Ig production from SLE patients. Moreover, they also conducted an in vivo experiment to document Treg can suppress B cell immune responses. Similarly, they confirmed that perforn and granzyme B released from Treg cells are responsible for B cell killing and suppression in patient with SLE [59].
Given the significant differences between natural and induced Treg cells exist, we also studied whether induced Treg cells suppress B cell immune responses. Like natural Treg cells, induced Treg cells also directly suppress B cell activities in vitro and in vivo. Unlike natural Treg cells, induced Treg cells suppress B cells not through cell killing but suppressive cytokines. Using granzyme B and perforin deficient mice, we found that induced Treg cells lacking these killing molecules still suppressed B cell responses [Liu and Zheng, manuscript under review]. The significance of the mechanism’s difference between natural and induced Treg cells on B cell suppression needs further investigation.
Acknowledgements
This work is supported in part by grants from the NIH (AR059103 and AI084359), ACR Within Our Reach Fund, Arthritis Foundation and Wright Foundation.
Disclosure of conflict of interest
None.
References
- 1.Horwitz DA, Zheng SG, Gray JD. Natural and tgf-beta-induced foxp3(+)cd4(+) cd25(+) regulatory t cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. Cd4+ cd25+ suppressor t cells: More questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory t cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Lan Q, Fan H, Quesniaux V, Ryffel B, Liu Z, Zheng SG. Induced foxp3(+) regulatory t cells: A potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol. 2012;4:22–28. doi: 10.1093/jmcb/mjr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou X, Kong N, Zou H, Brand D, Li X, Liu Z, Zheng SG. Therapeutic potential of tgf-β-induced cd4(+) foxp3(+) regulatory t cells in autoimmune diseases. Autoimmunity. 2011;44:43–50. doi: 10.3109/08916931003782163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated t cells expressing il-2 receptor alpha-chains (cd25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 7.Tran DQ. Tgf-β: The sword, the wand, and the shield of foxp3(+) regulatory t cells. J Mol Cell Biol. 2012;4:29–37. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- 8.Tang Q, Bluestone JA, Kang SM. Cd4(+)foxp3(+) regulatory t cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of cd4+cd25+ regulatory t cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 10.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for scurfin in cd4+cd25+ t regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 11.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and x-linked inheritance (ipex), a syndrome of systemic autoimmunity caused by mutations of foxp3, a critical regulator of t-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced foxp3 in human t effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. Epub 2007 Feb 27. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Lan Q, Lu L, Chen M, Xia Z, Ma J, Wang J, Fan H, Shen Y, Ryffel B, Brand D, Quismorio F, Liu Z, Horwitz DA, Xu A, Zheng SG. Phenotypic and functional characteristic of a newly identified cd8+foxp3-cd103+ regulatory t cells. J Mol Cell Biol. 2013 Sep 7; doi: 10.1093/jmcb/mjt026. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vlad G, Suciu-Foca N. Resurgence or emergence of cd8+ ts. Hum Immunol. 2008;69:679–680. doi: 10.1016/j.humimm.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Zheng SG, Wang JH, Koss MN, Quismorio F, Gray JD, Horwitz DA. Cd4+ and cd8+ regulatory t cells generated ex vivo with il-2 and tgf-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 16.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory t cells among regulatory t cell subsets in vivo. J Exp Med. 2012 Sep 24;209:1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of helios, an ikaros transcription factor family member, differentiates thymic-derived from peripherally induced foxp3+ t regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin X, Chen M, Liu Y, Guo Z, He X, Brand D, Zheng SG. Advances in distinguishing natural from induced foxp3(+) regulatory t cells. Int J Clin Exp Pathol. 2013;6:116–123. [PMC free article] [PubMed] [Google Scholar]
- 19.Serre K, Benezech C, Desanti G, Bobat S, Toellner KM, Bird R, Chan S, Kastner P, Cunningham AF, Maclennan IC, Mohr E. Helios is associated with cd4 t cells differentiating to t helper 2 and follicular helper t cells in vivo independently of foxp3 expression. PLoS One. 2011;6:e20731. doi: 10.1371/journal.pone.0020731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. Tgf-beta requires ctla-4 early after t cell activation to induce foxp3 and generate adaptive cd4+cd25+ regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 21.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced cd4+cd25+ cells educate cd4+cd25- cells to develop suppressive activity: The role of il-2, tgf-beta, and il-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 22.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of tgf-beta-producing regulatory t cells from cd4+cd25- precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 23.Li CR, Baaten BJ, Bradley LM. Harnessing memory adaptive regulatory t cells to control autoimmunity in type 1 diabetes. J Mol Cell Biol. 2012;4:38–47. doi: 10.1093/jmcb/mjr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L, Ma J, Wang X, Wang J, Zhang F, Yu J, He G, Xu B, Brand DD, Horwitz DA, Shi W, Zheng SG. Synergistic effect of tgf-beta superfamily members on the induction of foxp3+ treg. Eur J Immunol. 2010;40:142–152. doi: 10.1002/eji.200939618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X, Horwitz DA, Shi W, Zheng SG. Role of smad and non-smad signals in the development of th17 and regulatory t cells. J Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Wang J, Shi W, Brand DD, Liu Z, Fan H, Zheng SG. Isolation of purified and live foxp3+ regulatory t cells using facs sorting on scatter plot. J Mol Cell Biol. 2010;2:164–169. doi: 10.1093/jmcb/mjq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. Il-2 is essential for tgf-beta to convert naive cd4+cd25- cells to cd25+foxp3+ regulatory t cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 28.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting edge: Il-2 is essential for tgf-beta-mediated induction of foxp3+ t regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 29.Lu L, Ma J, Li Z, Lan Q, Chen M, Liu Y, Xia Z, Wang J, Han Y, Shi W, Quesniaux V, Ryffel B, Brand D, Li B, Liu Z, Zheng SG. All-trans retinoic acid promotes tgf-β-induced tregs via histone modification but not dna demethylation on foxp3 gene locus. PLoS One. 2011;6:e24590. doi: 10.1371/journal.pone.0024590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M, Noelle RJ, Cheroutre H. Retinoic acid can directly promote tgf-beta-mediated foxp3(+) treg cell conversion of naive t cells. Immunity. 2009;30:471–472. doi: 10.1016/j.immuni.2009.03.008. author reply 472-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ. Retinoic acid inhibits th17 polarization and enhances foxp3 expression through a stat-3/stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Q, Cao H, Liu J, Zhou X, Lan Q, Zheng S, Liu Z, Li Q, Fan H. Cd4+ foxp3+ regulatory t cells induced by tgf-beta, il-2 and all-trans retinoic acid attenuate obliterative bronchiolitis in rat trachea transplantation. Int Immunopharmacol. 2011;11:1887–1894. doi: 10.1016/j.intimp.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+cd4+cd25+ regulatory t cells induced by il-2 and tgf-beta are resistant to th17 conversion by il-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, Zheng SG. Cutting edge: All-trans retinoic acid sustains the stability and function of natural regulatory t cells in an inflammatory milieu. J Immunol. 2010;185:2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong N, Lan Q, Chen M, Zheng T, Su W, Wang J, Yang Z, Park R, Dagliyan G, Conti PS, Brand D, Liu Z, Zou H, Stohl W, Zheng SG. Induced t regulatory cells suppress osteoclastogenesis and bone erosion in collagen-induced arthritis better than natural t regulatory cells. Ann Rheum Dis. 2012;71:1567–1572. doi: 10.1136/annrheumdis-2011-201052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong N, Lan Q, Chen M, Wang J, Shi W, Horwitz DA, Quesniaux V, Ryffel B, Liu Z, Brand D, Zou H, Zheng SG. Antigen-specific transforming growth factor β-induced treg cells, but not natural treg cells, ameliorate autoimmune arthritis in mice by shifting the th17/treg cell balance from th17 predominance to treg cell predominance. Arthritis Rheum. 2012;64:2548–2558. doi: 10.1002/art.34513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu W, Lan Q, Chen M, Chen H, Zhu N, Zhou X, Wang J, Fan H, Yan CS, Kuang JL, Warburton D, Togbe D, Ryffel B, Zheng SG, Shi W. Adoptive transfer of induced-treg cells effectively attenuates murine airway allergic inflammation. PLoS One. 2012;7:e40314. doi: 10.1371/journal.pone.0040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puri KD, Di Paolo JA, Gold MR. B-cell receptor signaling inhibitors for treatment of autoimmune inflammatory diseases and b-cell malignancies. Int Rev Immunol. 2013;32:397–427. doi: 10.3109/08830185.2013.818140. [DOI] [PubMed] [Google Scholar]
- 39.Peng SL. Signaling in b cells via toll-like receptors. Curr Opin Immunol. 2005;17:230–236. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–99. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham-Rundles C, Ponda PP. Molecular defects in t- and b-cell primary immunodeficiency diseases. Nat Rev Immunol. 2005;5:880–892. doi: 10.1038/nri1713. [DOI] [PubMed] [Google Scholar]
- 42.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 43.Tuscano JM, Harris GS, Tedder TF. B lymphocytes contribute to autoimmune disease pathogenesis: Current trends and clinical implications. Autoimmun Rev. 2003;2:101–108. doi: 10.1016/s1568-9972(02)00148-9. [DOI] [PubMed] [Google Scholar]
- 44.Vaux DL, Flavell RA. Apoptosis genes and autoimmunity. Curr Opin Immunol. 2000;12:719–724. doi: 10.1016/s0952-7915(00)00168-0. [DOI] [PubMed] [Google Scholar]
- 45.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of b cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertsias GK, Pamfil C, Fanouriakis A, Boumpas DT. Diagnostic criteria for systemic lupus erythematosus: Has the time come? Nat Rev Rheumatol. 2013;9:687–94. doi: 10.1038/nrrheum.2013.103. [DOI] [PubMed] [Google Scholar]
- 47.Lan Q, Zhou X, Fan H, Chen M, Wang J, Ryffel B, Brand D, Ramalingam R, Kiela PR, Horwitz DA, Liu Z, Zheng SG. Polyclonal cd4+foxp3+ treg cells induce tgfβ-dependent tolerogenic dendritic cells that suppress the murine lupus-like syndrome. J Mol Cell Biol. 2012;4:409–419. doi: 10.1093/jmcb/mjs040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su W, Fan H, Chen M, Wang J, Brand D, He X, Quesniaux V, Ryffel B, Zhu L, Liang D, Zheng SG. Induced cd4+ forkhead box protein-positive t cells inhibit mast cell function and established contact hypersensitivity through tgf-β1. J Allergy Clin Immunol. 2012;130:444–452. e447. doi: 10.1016/j.jaci.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated cd4+cd25+ t cells selectively kill b lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaiss MM, Axmann R, Zwerina J, Polzer K, Guckel E, Skapenko A, Schulze-Koops H, Horwood N, Cope A, Schett G. Treg cells suppress osteoclast formation: A new link between the immune system and bone. Arthritis Rheum. 2007;56:4104–4112. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]
- 51.Galani IE, Wendel M, Stojanovic A, Jesiak M, Muller MM, Schellack C, Suri-Payer E, Cerwenka A. Regulatory t cells control macrophage accumulation and activation in lymphoma. Int J Cancer. 2010;127:1131–1140. doi: 10.1002/ijc.25132. doi: 1110.1002/ijc.25132. [DOI] [PubMed] [Google Scholar]
- 52.Tonkin DR, Haskins K. Regulatory t cells enter the pancreas during suppression of type 1 diabetes and inhibit effector t cells and macrophages in a tgf-beta-dependent manner. Eur J Immunol. 2009;39:1313–1322. doi: 10.1002/eji.200838916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. Cd25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 54.Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, Noelle RJ, Turka LA, Finkelman FD, Caton AJ, Erikson J. The impact of t helper and t regulatory cells on the regulation of anti-double-stranded dna b cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 55.Tsuda M, Torgerson TR, Selmi C, Gambineri E, Carneiro-Sampaio M, Mannurita SC, Leung PS, Norman GL, Gershwin ME. The spectrum of autoantibodies in ipex syndrome is broad and includes anti-mitochondrial autoantibodies. J Autoimmun. 2010;35:265–268. doi: 10.1016/j.jaut.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Kinnunen T, Chamberlain N, Morbach H, Choi J, Kim S, Craft J, Mayer L, Cancrini C, Passerini L, Bacchetta R, Ochs HD, Torgerson TR, Meffre E. Accumulation of peripheral autoreactive b cells in the absence of functional human regulatory t cells. Blood. 2013;121:1595–1603. doi: 10.1182/blood-2012-09-457465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: Direct suppression of b cells by cd4+ cd25+ regulatory t cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 58.Gotot J, Gottschalk C, Leopold S, Knolle PA, Yagita H, Kurts C, Ludwig-Portugall I. Regulatory t cells use programmed death 1 ligands to directly suppress autoreactive b cells in vivo. Proc Natl Acad Sci U S A. 2012;109:10468–10473. doi: 10.1073/pnas.1201131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iikuni N, Lourenço EV, Hahn BH, La Cava A. Cutting edge: Regulatory t cells directly suppress b cells in systemic lupus erythematosus. J Immunol. 2009;183:1518–1522. doi: 10.4049/jimmunol.0901163. [DOI] [PMC free article] [PubMed] [Google Scholar]


