Abstract
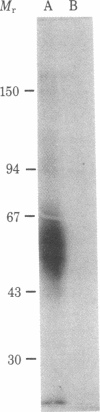
Xenogeneic monoclonal antibodies were prepared to the murine interleukin 2 (IL-2)-dependent HT2 cell line. One rat IgM monoclonal antibody (7D4) was identified that inhibited proliferation of the HT2 cells and of IL-2-dependent CTLL cells in the presence of crude rat IL-2 as well as of purified human IL-2. The level of inhibition was dependent on both antibody and IL-2 concentration. Cell distribution studies using a fluorescence-activated cell sorter showed that the antigen identified by 7D4 is expressed at a high density on HT2 cells and on concanavalin A (Con A)-induced T-cell blasts and at a substantially lower density on lipopolysaccharide-induced B-cell blasts; 7D4 binding was not detected on greater than 95% of nonactivated thymocytes, T cells, or B cells. Competition binding studies indicated that 7D4 fails to inhibit the binding of 3H-labeled human IL-2 to CTLL cells. However, 7D4 specifically immunoprecipitated 3H-labeled human IL-2 from detergent extracts of HT2 cells or Con A-induced T-cell blasts that had been pulsed with [3H]IL-2; in contrast, 7D4 did not react with free [3H]IL-2. Initial biochemical analysis of immunoprecipitates with 7D4 of detergent extracts from surface-iodinated Con A-activated spleen cells showed a major band having apparent molecular weight of 48,000-62,000. Collectively, these results suggest that 7D4 detects an epitope on the IL-2 receptor distal to the ligand binding site or another molecule that physically associates with the receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. E., Gillis S., Smith K. A. Monoclonal cytolytic T-cell lines. J Exp Med. 1979 Jan 1;149(1):273–278. doi: 10.1084/jem.149.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnard G. D., Yasaka K., Jacobson D. Ligand-activated T cell growth factor-induced proliferation: absorption of T cell growth factor by activated T cells. J Immunol. 1979 Dec;123(6):2704–2708. [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Couraud P. O., Delavier-Klutchko C., Durieu-Trautmann O., Strosberg A. D. "Antibodies raised against beta-adrenergic receptors stimulate adenylate cyclase". Biochem Biophys Res Commun. 1981 Apr 30;99(4):1295–1302. doi: 10.1016/0006-291x(81)90760-9. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Larsson E. L., Grönvik K. O., Andersson J. Studies on T lymphocyte activation II. The target cells for concanavalin A-induced growth factors. Eur J Immunol. 1979 Aug;9(8):587–592. doi: 10.1002/eji.1830090803. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Benjamin W. R., Hilfiker M. L., Howard M., Farrar W. L., Fuller-Farrar J. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol Rev. 1982;63:129–166. doi: 10.1111/j.1600-065x.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S., Henney C. S. The biochemical and biological characterization of lymphocyte regulatory molecules. VI. Generation of a B cell hybridoma whose antibody product inhibits interleukin 2 activity. J Immunol. 1981 May;126(5):1978–1984. [PubMed] [Google Scholar]
- Gillis S., Mochizuki D. Y., Conlon P. J., Hefeneider S. H., Ramthun C. A., Gillis A. E., Frank M. B., Henney C. S., Watson J. D. Molecular characterization of interleukin 2. Immunol Rev. 1982;63:167–209. doi: 10.1111/j.1600-065x.1982.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Su Y. F., Cuatrecasas P. A monoclonal antibody to human insulin receptor. Biochem Biophys Res Commun. 1982 Jun 15;106(3):1019–1026. doi: 10.1016/0006-291x(82)91813-7. [DOI] [PubMed] [Google Scholar]
- Kung J. T., Sharrow S. O., Sieckmann D. G., Lieberman R., Paul W. E. A mouse IgM allotypic determinant (Igh-6.5) recognized by a monoclonal rat antibody. J Immunol. 1981 Sep;127(3):873–876. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Gutman G. A., Lewis D. E., Griswold S. T., Warner N. L. Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1982;1(2):125–131. doi: 10.1089/hyb.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. Immunological studies of acetylcholine receptors. J Supramol Struct. 1976;4(3):389–403. doi: 10.1002/jss.400040310. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification of a membrane antigen that is distinct from the interleukin 2 receptor and that may be required for interleukin 2-driven proliferative responses. J Immunol. 1983 Feb;130(2):747–755. [PubMed] [Google Scholar]
- Parker D. C. Induction and suppression of polyclonal antibody responses by anti-Ig reagents and antigen-nonspecific helper factors: a comparison of the effects of anti-Fab, anti-IgM, and anti IgD on murine B cells. Immunol Rev. 1980;52:115–139. doi: 10.1111/j.1600-065x.1980.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Paul W. E., Sredni B., Schwartz R. H. Long-term growth and cloning of non-transformed lymphocytes. Nature. 1981 Dec 24;294(5843):697–699. doi: 10.1038/294697a0. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler B. M., Berenstein E. H., Siraganian R. P., Oppenheim J. J. Monoclonal antibody against human interleukin 2 (IL 2). I. Purification of IL 2 for the production of monoclonal antibodies. J Immunol. 1982 Apr;128(4):1620–1624. [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Dutton R. W. Production of a B cell growth-promoting activity, (DL)BCGF, from a cloned T cell line and its assay on the BCL1 B cell tumor. J Exp Med. 1982 Dec 1;156(6):1821–1834. doi: 10.1084/jem.156.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]