Abstract
Objective: This study aimed to investigate the development of neural stem cells (NSCs) in fetal brain, which may provide experimental evidence for the clinical treatment of brain injury in children. Methods: A total of 60 fetuses were collected after labor induction and divided into 6 groups according to the gestational age (16 w, 20 w, 24 w, 28 w, 32 w and 36 w; n=10 per group). The hippocampus, striatum, subventricular zone, frontal lobe, temporal lobe, occipital lobe and parietal lobe were harvested. In situ hybridization, immunohistochemistry and light microscopy were done to determine the morphology and quantity of NSCs. Results: NSCs were identified in the brain of fetuses with different gestational age. NSCs were round, oval, spindle-shaped, starlike, triangular or polygonal. NSC colony was also observed with symmetrical or asymmetrical division. Single NSC, group-like NSCs and cluster-like NSCs were found in the different sites of fetal brain, and NCSs interacted with each other via synapses. However, the distribution, morphology, growth and quantity of NSCs were different in the brain of fetuses with different gestational age. The number of NSCs reduced with the increase in gestational age, but they were always observed. Conclusion: The morphology of NSCs in fetal brain is variable and they are widely distributed in the hippocampus, subventricular zone, striatum and cortex. The number of NSCs reduced with the increase of gestational age.
Keywords: Human, fetal brain, nestin
Introduction
Neural stem cells (NSCs) are multipotent cells that can give rise to various cell types in the central nervous system (CNS), including neurons, astrocytes, and oligodendrocytes [1]. NSCs can be generated from either the fetus, the adult, embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) [2]. In adult mammals, neurogenesis is limited to two brain regions; the subventricular zone (SVZ) by the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus. The microenvironment of SVZ and SGZ is beneficial for the survival of NSCs [3-6]. To date, NSCs have been identified in the cortex, hippocampus, striatum, olfactory bulb, and tissues along the cerebral ventricle (lateral ventricle, third ventricle and fourth ventricle), diencephalon, midbrain, cerebellum, spinal cord and retina [7,8].
NSCs from specific brain region and those from precursors have been found to exert therapeutic effects on degenerative diseases of the nervous system after transplantation of exogenous NSCs, pharmacological regulation of endogenous NSCs or NSC transplantation induced regulation of endogenous NSCs, which has been attributed to the “bypass effect”. The optimal amount of NSCs for treatment, the way in which NSCs are administered, the time of NSC transplantation, the interaction between transplanted NSCs and immune system and the therapeutic efficacy of NSC transplantation in combination of nerve repair surgery are still being studied. To timely and completely understand the therapeutic mechanisms, effectiveness and safety of NSC transplantation and the clearance of transplanted NSCs is crucial for the treatment of degenerative diseases of nervous system with NSCs. Thus, it is cautious to treat diseases with NSCs in animals and humans [9]. As compared to transplantation of NSCs for the treatment of diseases of nervous system, endogenous NSCs/precursor cells are activated to repair the injury, which avoids the confounding cells in transplanted NSCs and the tumorigenesis and immune rejection after NSC transplantation. In addition, in diseases with diffused lesions in nervous system, cell transplantation is usually performed repeatedly, and thus, the therapeutic efficacy is often poor. On the basis of above findings, auto-repair of nervous system seems to be more feasible [10,11]. Hypoxic-ischemic brain damage (HIBD) is a disease of nervous system with multiple lesions. Neonates are a continuation of fetal life. We speculate that NSCs are more widely distributed in neonates than in adults. Thus, to induce the proliferation and differentiation of endogenous NSCs has better prospective in the treatment of HIBD in neonates.
This study aimed to investigate the distribution, morphology, growth and quantity of NSCs in fetal brain. Our findings may provide an experimental and theoretical basis for in vivo and in vitro regulation of proliferation and differentiation of NSCs and the clinical treatment of degenerative disease of nervous system with NSCs.
Materials and methods
Samples
A total of 60 fetuses were collected after labor induction and divided into 6 groups according to the gestational ages (16 w, 20 w, 24 w, 28 w, 32 w and 36 w; n=10 per group). The hippocampus, striatum, subventricular zone, frontal lobe, temporal lobe, occipital lobe and parietal lobe were harvested from each fetus. Prenatal ultrasonography showed refractory congenital heart diseases in these fetuses and induced labor was required by the mother and her relatives. These refractory congenital heart diseases included persistent truncus arteriosus (PTA), total abnormal pulmonary venous drainage (TAPVD), critical tetralogy of Fallot (TOF), critical aortic stenosis and critical pulmonary stenosis. Physical examination showed the mothers were healthy. Informed consent was obtained from the mothers and this study protocol was approved by the Ethics Committee of General Hospital of Beijing Military Region.
Main reagents
Rabbit anti-human Nestin polyclonal antibody (Chemicon, USA), in situ hybridization kit for Nestin, DAB kit, SABC kit for immunohistochemistry (Zhongshan Company, China), mRNA targeting human Nestin (Shanghai Sangon Biotech Co., Ltd) and digoxin labeled NESTIN oligonucleotide probes ([1] 5’-ATCTT TTCAG ATGTG GGAGC TCAAT CGACG-3’; [2] 5’-CCTCC TGGAG AGCCC GAGCC GATGA CGAGC-3’; [3] 5’-AAGCA AGGTC TACAG AGTCA GATCG CTCAG-3’) were used in the present study. Other reagents were domestic and analytically pure.
Immunohistochemistry for Nestin at 7 sites of fetal brain
Immunohistochemistry was done with SABC method. Tissue was harvested from embryos, fixed in 4% paraformaldehyde (PFA), equilibrated in 30% sucrose at 4°C and frozen in Tissue-Tek OCT. Cryosections (20 μm) were blocked with 10% goat serum in 0.1% Triton X-100 at room temperature and primary antibodies (rabbit anti-human nestin polyclonal antibody) was applied overnight at 4°C. Secondary antibodies included biotin conjugated goat anti-rabbit IgG. Incubation was done at 37°C for 30 min. After addition of SABC, incubation was done at 37°C for 30 min, and visualization was done with DAB for 5 min, followed by counterstaining with hematoxylin. After washing in water for 1 min, observation was done under a light microscope and representative photographs were captured. In negative control group, the primary antibody was replaced with 0.01 mol/L KPBS and the secondary antibody with normal goat serum.
In situ hybridization for Nestin mRNA at 7 sites of fetal brain
Tissue was harvested from embryos, fixed in 4% paraformaldehyde (PFA), equilibrated in 30% sucrose at 4°C and frozen in Tissue-Tek OCT. Cryosections (15 μm) were blocked with 10% goat serum in 0.1% Triton X-100 at room temperature. Digestion was done with pepsin in 3% citric acid at 37°C for 100 s, followed by fixation in 1% paraformaldehyde in /0.1 M PBS (pH 7.2-7.6) at room temperature for 10 min. After addition of prehybridization solution, prehybridization was done at 42°C for 4 h. The digoxin labeled probe in in situ hybridization solution was added followed by hybridization at 42°C for 20 h. After washing in series of SSC solution (2×, 0.5×, 0.2×) at 37°C, blocking solution was added followed by addition of biotinylated anti-digoxin antibody and subsequent incubation at 37°C for 90 min. Incubation was performed with SABC at 37°C for 30 min and then with biotinylated peroxidase at 37°C for 30 min. Visualization was done with DAB, followed by counterstaining with hematoxylin for 1 min. After washing in water for 1 min, observation was done under a microscope and representative photographs were captured. In negative control group, probes or antibody were not used.
Quantification and qualification of NSCs in fetal brain
Cells positive for Nestin protein or Nestin mRNA had brown granules in the cytoplasm. At 400×, cells were counted, and a total of 100 grids were included in one field. At different sites, observation was done in 3 sections, and 10 fields were randomly selected from each section. Thus, a total of 300 fields were used for the detection of total cells and positive cells in each group. The proportion of positive cells to total cells was calculated at different sites in each group, which was used as the proportion of Nestin positive cells at different sites.
Statistical analysis
Statistical analysis was done with SPSS version 13.0. The proportion of NSCs positive for Nestin protein and mRNA at different sites was compared with chi square test. A value of P<0.05 was considered statistically significant.
Results
NSCs morphology from fetus brain
In the hippocampus, NSCs were round, oval, triangular or star-like. Most of NSCs were round and single cell was observed. Symmetrical division, colony formed by 2-8 cells and cluster-like distribution were found. One NSC usually had 0-3 processes. Some NSCs interact with other neurons via synapses (Figure 1). In the subventricular zone, NSCs were star-like, round, oval or spindle-shaped, and most of them were star-like. NSCs had scattered distributed in astrocytes and single cell was frequently observed. Most NSCs had 1-4 nucleoli. Symmetrical and asymmetrical division was found in these NSCs which had 0-2 processes. Some NSCs interact with other neurons via synapses (Figure 2). In the striatum, NSCs were round, oval, polygonal or pleomorphic, and most cells were oval. The nucleus was vacuole-like and had 1-4 nucleoli. One NSC had 0-3 processes and single NSC was found among neurons. Symmetrical division was occasionally observed in NSCs with colony-like distribution (Figure 3). In the cortex, NSCs were round or oval, or occasionally polygonal. Most NSCs were round and had 1-5 nucleoli. NSCs were group-like, had colony-like growth and presented with regional distribution (Figure 4).
Figure 1.
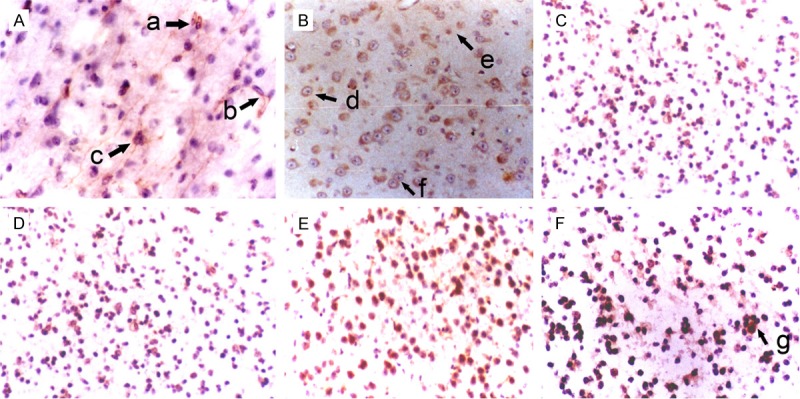
Morphology of NSCs in the hippocampus of fetuses with different gestational age (SABC, DAB staining, ×200). A: 36 weeks; B: 32 weeks; C: 28 weeks; D: 24 weeks; E: 20 weeks; F: 16 weeks. a: Oval NSCs; b: NSCs interacted with each other via synapses; c: Colony formation for 3 NSCs; d: Big and round NSCs; e: Small and round NSCs; f: Symmetrical division of NSCs; g: Colony formation from several NSCs.
Figure 2.
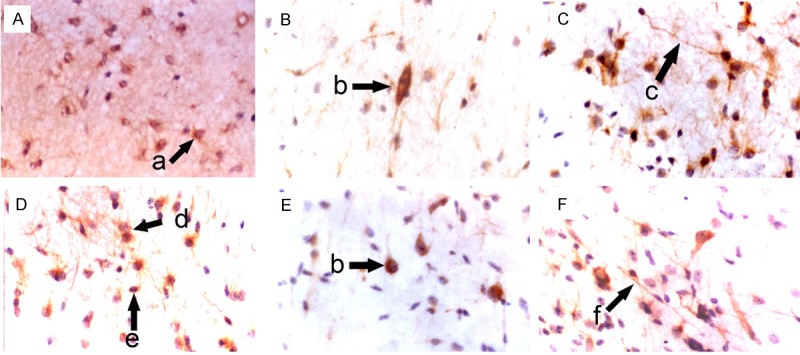
Morphology of NSCs in the subventricular zone of fetuses with different gestational age (SABC, DAB staining, ×400). A: 36 weeks; B: 32 weeks; C: 28 weeks; D: 24 weeks; E: 20 weeks; F: 16 weeks; a: Star-like NSCs; b: Oval NSCs; c: NSCs interacted with each other via synapses; d: Symmetrical division of NSCs; e: Round NSCs; f: Spindle-shaped NSCs.
Figure 3.
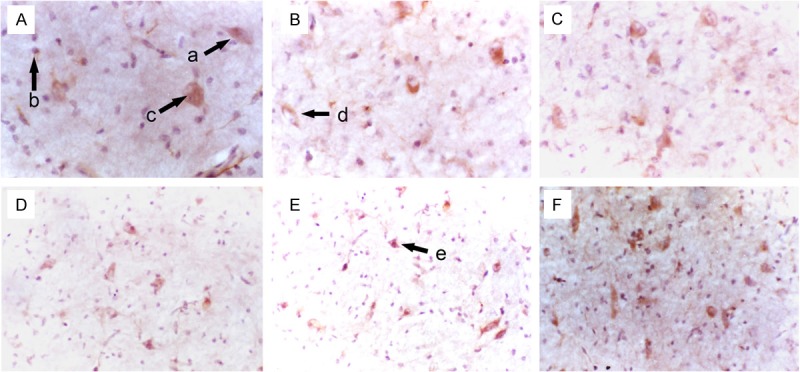
Morphology of NSCs in the striatum of fetuses with different gestational age (SABC, DAB staining, A-C: ×400; D-F: ×200). A: 36 weeks; B: 32 weeks; C: 28 weeks; D: 24 weeks; E: 20 weeks; F: 16 weeks; a: Big and oval NSCs; b: Round NSCs; c: Pleomorphic NSCs; d: Small and oval NSCs; e: Polygonal NSCs.
Figure 4.
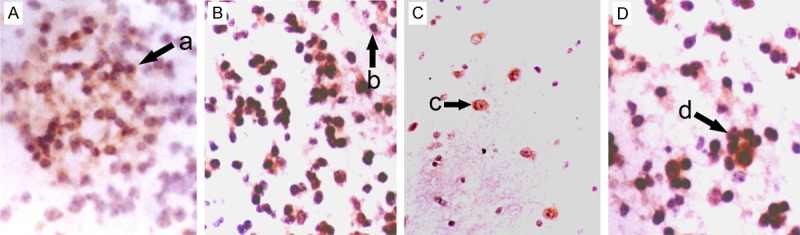
Morphology of NSCs in the cortex of fetuses with different gestational age (SABC, DAB staining, ×200). A: Frontal lobe; B: Temporal lobe; C: Parietal lobe; D: Occipital lobe; a: Group-like growth of NSCs; b: Round NSCs; c: Oval NSCs; d: Colony-like growth of NSCs.
Proportion of nestin positive NSCs at different sites of fetal brain
In the brain of fetuses with specific gestational age, the proportion of nestin positive cells reduced in the following order: hippocampus, subventricular zone, striatum, frontal lobe, temporal lobe, parietal lobe and occipital lobe (P<0.01). At the same site, the proportion of nestin positive cells reduced with the increase in gestational age (P<0.01) (Table 1, Figure 5).
Table 1.
Proportion of Nestin positive cells at different sites of fetal brain (%)
| Group/site | hippocampus | subventricular zone | striatum | frontal lobe | temporal lobe | parietal lobe | occipital lobe | χ2 | P-value |
|---|---|---|---|---|---|---|---|---|---|
| 16 w | 37.5 | 31.35 | 26.93 | 15.59 | 12.25 | 10.09 | 8.31 | 13219.62 | <0.01 |
| 20 w | 31.4 | 28.31 | 23.37 | 13.48 | 10.54 | 7.73 | 6.52 | 10245.49 | <0.01 |
| 24 w | 24.8 | 22.46 | 18.70 | 11.62 | 9.57 | 5.42 | 3.87 | 9210.018 | <0.01 |
| 28 w | 20.2 | 18.92 | 15.98 | 10.52 | 8.73 | 4.13 | 2.58 | 8014.862 | <0.01 |
| 32 w | 17.09 | 14.97 | 12.87 | 9.87 | 7.53 | 3.46 | 1.65 | 6830.853 | <0.01 |
| 36 w | 12.38 | 10.88 | 8.57 | 6.68 | 4.16 | 1.98 | 0.96 | 5693.014 | <0.01 |
| χ2 | 6253.974 | 4644.602 | 4050.503 | 1265.152 | 1448.848 | 2275.611 | 2869.747 | ||
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Figure 5.
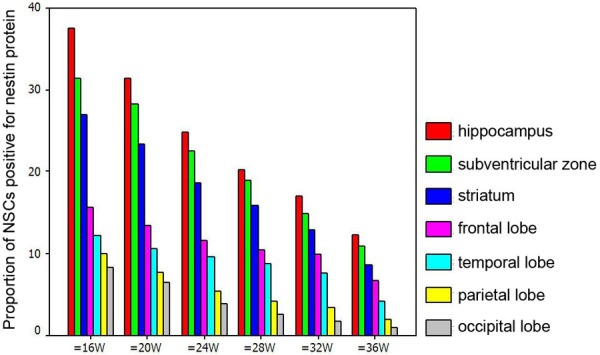
Proportion of Nestin positive cells at different sites of fetal brain.
Nestin mRNA positive NSCs in fetal brain
In the brain of fetuses with specific gestational age, the proportion of nestin mRNA positive NSCs reduced in the following order: hippocampus, subventricular zone, striatum, frontal lobe, temporal lobe, parietal lobe and occipital lobe (P<0.01). At the same site, the proportion of nestin mRNA positive cells reduced with the increase in gestational age (P<0.01) (Table 2, Figure 6).
Table 2.
Proportion of Nestin mRNA positive NSCs at different sites of fetal brain (%)
| Group/site | hippocampus | subventricular zone | striatum | frontal lobe | temporal lobe | parietal lobe | occipital lobe | χ2 | P-value |
|---|---|---|---|---|---|---|---|---|---|
| 16 w | 46.47 | 41.42 | 28.51 | 14.95 | 12.12 | 10.1 | 9.0 | 23644.08 | <0.01 |
| 20 w | 36.5 | 35.5 | 24.83 | 13.76 | 12.57 | 9.68 | 7.97 | 15712.73 | <0.01 |
| 24 w | 30.4 | 27.36 | 20.32 | 12.58 | 11.03 | 7.33 | 6.31 | 10811.92 | <0.01 |
| 28 w | 22.35 | 20.57 | 17.68 | 11.31 | 9.28 | 6.12 | 4.82 | 7108.268 | <0.01 |
| 32 w | 19.36 | 17.38 | 15.31 | 9.97 | 8.22 | 4.98 | 2.88 | 6928.515 | <0.01 |
| 36 w | 15.76 | 13.21 | 10.48 | 7.86 | 5.38 | 4.45 | 1.48 | 6150.322 | <0.01 |
| χ2 | 9855.902 | 8848.345 | 3697.819 | 4336.394 | 1170.125 | 1435.392 | 2156.375 | ||
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Figure 6.
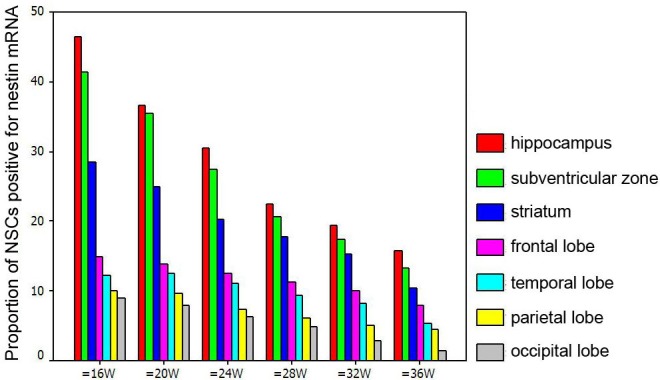
Proportion of Nestin mRNA positive NSCs at different sites of fetal brain.
Discussion
Neuroepithelial stem cell protein (Nestin) is a specific antigen of NSCs and belongs to type VI intermediate filament protein. Nestin is a major intermediate filament protein in the central nervous system (CNS) of embryo and mainly expressed in the developing CNS and skeletal muscles. Nestin expression initiates since the formation of neural plate and becomes disappeared when neurons begin migration and differentiation. Once the differentiation of neurons completes, the nestin expression stops. In the CNS of mammalians, change in intermediate filament protein occurs at the key stage of cell differentiation. In the CNS, NSCs express nestin, and the nestin expression reduces rapidly in the order from proliferating NSCs to neurons. In the nestin gene, an enhancer is found in the intron 2 and can act on NSCs of CNS. This suggests that there is a transcriptional regulation of NSCs in the CNS. Of note, this enhancer in the intron has no influence on peripheral nervous system [12,13]. The introns of human nestin gene are genetically protected, and 2 of 3 introns are shared with neurofilaments. Before the reclassification of neurofilaments, nestin and neurofilament share a precursor [14]. To date, nestin has been widely used as a marker for NSCs in in vivo and in vitro studies and employed to identify NSCs from cells in nervous system [15]. In the present study, in situ hybridization and immunohistochemistry were employed to detect the nestin mRNA and protein, respectively, at 7 sites of human fetal brain, and NSCs positive for nestin protein and mRNA were observed in the fetal brain. In addition, the protein and mRNA expression of nestin showed similar trend in the fetal brain. These findings indicate that nestin is a favorable antigen used to identify NSCs.
NSCs are primitive neural cells with incomplete maturation and can be found in the whole CNS and at different developmental stages of CNS. During the embryonic development of nervous system, the multiplication of NSCs origins from the surface of central canal connected to the nerviduct. The central canal finally forms ventricular system in adults. Some investigators speculate that NSCs divide on the inner surface of nerviduct and migrate from the ependyma and dentate gyrus into specific layer, and these nerve fibers are also NSCs [16]. NSCs are a group of cells with self-renewal and differentiation potential, and in the non-terminal state of differentiation. NSCs can symmetrically and asymmetrically divide into new NSCs or differentiate into small daughter cells which finally differentiate into three types of cells: neurons, astrocytes and oligodendrocytes [17]. In previous studies, NSCs are regarded to origin from the subventricular zone and dentate gyrus [18]. In depth studies reveal that NSCs also exist in the olfactory bulb, ependyma, and subependymal zone of rats, and the amount of NSCs in the brain reduces over gestational age [8]. Subsequently, NSCs are also identified in the hippocampus, cortex, striatum and subependymal zone of humans [19]. However, the alteration of NSCs during the development of embryonic brain has never comprehensively studied.
To date, no studies have been conducted to investigate the changes in the distribution, morphology, growth and amount of NSCs over gestational age. In the present study, in situ hybridization and immunohistochemistry were employed to detect the mRNA and protein expression of nestin (a marker of NSCs) at 7 sites of the brain of fetuses with different gestational ages. Results demonstrated NSCs were found in the brain of fetuses with different gestational age, but the distribution, morphology, growth and amount of NSCs varied among different sites and among fetuses with different gestational ages. Our results showed NSCs were round, oval, spindle-shaped, star-like, triangular or polygonal and had small or large size. NSCs with symmetrical or asymmetrical division were also observed, and 2-8 NSCs could form a colony and presented with group-like or cluster-like growth. The group-like or cluster-like NSCs formed NSC colonies or the center for the generation of new NSCs. Some NSCs had processes extending to surrounding cells and interacted with other cells via synapses. The amount of NSCs reduced with the increase in gestational age. The above characteristics of NSCs have never been reported previously. One may imagine that any thing has its special morphology and functions, and the morphology and structure of cells determine their biological functions. The morphology and growth of NSCs in fetal brain indicate that NSCs with different morphologies, at different sites of the brain or at different gestational age may have distinct biological functions. Our results also revealed that the amount of NSCs at a specific site reduced with the increase in gestational age; in the brain of fetus with a specific gestational age, the amount of NSCs reduced in the following order: hippocampus, subventricular zone, striatum, frontal lobe, temporal lobe, parietal lobe, and occipital lobe, and significant difference was observed between any two sites. These findings indicate that the amount of NSCs in fetal brain is larger than that in adult brain. Neonates are a continuation of fetal life and may develop further into children and finally into adults. Once focal ischemia/hypoxia is present in the brain, NSCs at other sites may proliferate and differentiate to repair this injury. Thus, our results not only uncovered the development of NSCs at different sites of the brain of fetuses with different gestational age, but validated our hypothesis that to induce the proliferation and differentiation of endogenous NSCs is better for the treatment of HIBD in neonates.
Although great progresses have been achieved in studies on NSCs, some problems still exist in the NSC transplantation, such as immune rejection, reconstruction of morphology and cell circuit, functional integration and evaluation of short-term and long-term prognosis. Thus, it has a long way before the transplantation of NSCs is used in clinical practice. Directional induction of NSC differentiation is crucial for the clinical application of NSCs. Different types of neural cells might be required for the repair of lesions of different types or at different sites in nervous system. Although in vitro experiments have confirmed that some substances may induce the directional differentiation of NSCs, the differentiation of NSCs might vary in distinct environments. Thus, it is difficult to collect NSCs with the same ancestry, homogeneity and identical differentiation level. In addition, the brain is an integrant that can auto-regulate according to the pathophysiological conditions, which can not be mimicked in vitro due to its complexity, and the direction of NSC differentiation can not be accurately determined. In addition, whether transplanted NSCs induce immune rejection is still controversial. Thus, the in vivo survival and proliferation of NSCs are required to further investigated. NSCs exist in the whole life of mammalians. They may generate precursor cells and directionally differentiate into cells to maintain the homeostasis. Thus, NSCs may self-renew and stay in a quiescent state. Transplantation of NSCs is a new strategy for the treatment of brain injury, and exert therapeutic effects via multiple mechanisms, such as neural replacement, neuroprotection, inflammation and apoptosis [20]. It is accepted that NSCs can be used to treat nervous system injury and degenerative diseases. However, exogenous NSCs have limitations, and to induce the endogenous NSCs to repair brain injury may be a better strategy. In HIBD of neonates, to induce the proliferation and differentiation of endogenous NSCs may be a preferred strategy. Of note, the time window for the induction of NSC proliferation and differentiation should be paid attention to.
Conclusion
NSCs in fetal brain have distinct morphologies and are widely distributed in the hippocampus, subventricular zone, striatum and cortex. The amount of NSCs reduces over gestational age. To induce the proliferation and differentiation of endogenous NSCs may be a promising and effective strategy for the treatment of degenerative disease of nervous system of children (including neonates).
Acknowledgements
This work was supported by grants from the China Postdoctoral Science Foundation (No. 20070410505).
References
- 1.Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull. 2002;57:751–758. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Jin CY. Stem Cells in Drug Screening for Neurodegenerative Disease. Korean J Physiol Pharmacol. 2012;16:1–9. doi: 10.4196/kjpp.2012.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Carreira BP, Carvalho CM, Araújo IM. Regulation of injury-induced neurogenesis by nitric oxide. Stem Cells Int. 2012;2012:895659. doi: 10.1155/2012/895659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akerblom M, Sachdeva R, Jakobsson J. Functional Studies of microRNAs in Neural Stem Cells: Problems and Perspectives. Front Neurosci. 2012;6:14. doi: 10.3389/fnins.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 8.Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 9.Gincberg G, Arien-Zakay H, Lazarovici P, Lelkes PI. Neural stem cells: therapeutic potential for neurodegenerative diseases. Br Med Bull. 2012;104:7–19. doi: 10.1093/bmb/lds024. [DOI] [PubMed] [Google Scholar]
- 10.Pimentel-Coelho PM, Mendez-Otero R. Cell therapy for neonatal hypoxic-ischemic encephalopathy. Stem Cells Dev. 2010;19:299–310. doi: 10.1089/scd.2009.0403. [DOI] [PubMed] [Google Scholar]
- 11.Velthoven CT, Kavelaars A, Bel F, Heijnen CJ. Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr Res. 2010;68:419–422. doi: 10.1203/PDR.0b013e3181f1c289. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Cheng L, Yan Y, Bian W, Tomooka Y, Shiurba R, Jing N. Mouse nestin cDNA cloning and protein expression in the cytoskeleton of transfected cells. Biochim Biophys Acta. 2001;1520:251–254. doi: 10.1016/s0167-4781(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 14.Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992;103:589–597. doi: 10.1242/jcs.103.2.589. [DOI] [PubMed] [Google Scholar]
- 15.Messam CA, Hou J, Major EO. Coexpression of nestin in neural and glial cells in the developing human CNS defined by a human-specific anti-nestin antibody. Exp Neurol. 2000;161:585–596. doi: 10.1006/exnr.1999.7319. [DOI] [PubMed] [Google Scholar]
- 16.Pincus DW, Goodman RR, Fraser RA, Nedergaard M, Goldman SA. Neural stem and progenitor cells: a strategy for gene therapy and brain repair. Neurosurgery. 1998;42:858–867. doi: 10.1097/00006123-199804000-00103. discussion 867-8. [DOI] [PubMed] [Google Scholar]
- 17.Cataudella T, Conti L, Cattaneo E. Neural stem and progenitor cells: choosing the right Shc. Prog Brain Res. 2004;146:127–133. doi: 10.1016/S0079-6123(03)46009-3. [DOI] [PubMed] [Google Scholar]
- 18.Galli R, Gritti A, Bonfanti L, Vescovi AL. Neural stem cells: an overview. Circ Res. 2003;92:598–608. doi: 10.1161/01.RES.0000065580.02404.F4. [DOI] [PubMed] [Google Scholar]
- 19.Poltavtseva RA, Revishchin AV, Aleksandrova MA, Korochkin LI, Viktorov IV, Sukhikh GT. Neural stem and progenitor cells of human embryos and fetuses as a basis of biomedical new technologies. Ontogenez. 2003;34:211–215. [PubMed] [Google Scholar]
- 20.Cusulin C, Monni E, Ahlenius H, Wood J, Brune JC, Lindvall O, Kokaia Z. Embryonic stem cell-derived neural stem cells fuse with microglia and mature neurons. Stem Cells. 2012;30:2657–2671. doi: 10.1002/stem.1227. [DOI] [PubMed] [Google Scholar]


