Abstract
Synovial sarcoma (SS) is a malignant tumor of soft tissue and is noted for late local recurrence and metastasis. Aberrant epithelial-mesenchymal transition (EMT) has been implicated in the pathogenesis of diverse human malignancies. Immunohistochemical techniques were used to assess EMT-related proteins (E-cadherin, N-cadherin, β-catenin, Snail, and Slug) and the TGF-β1 pathway (TGF-β1 and Smad2/3) proteins expression in different histological subtypes and epithelial mesenchymal compositions of SS. The expression of cell-surface (E-cadherin) and cytoskeletal proteins (β-catenin) were higher significantly in biphasic SSs (BSSs) (70.4%, 51.9%) than MFSSs (both for 10%). Among monophasic fibrous SSs (MFSSs) samples, E-cadherin protein expression was negatively correlated with expression Snail, Slug, TGF-β1, and Smad2/3. The expression levels of Snail and Smad2/3 were correlated with the pTNM stage (I-II vs. III-IV; P=0.047, P=0.021) and TGF-β1 exhibited a tendency toward a positive correlation with pTNM stage (I-II vs. III-IV; P=0.052), but did not correlate with the histological grade (p>0.05). Interestingly, our data showed that expression of E-cadherin protein correlated with greater survival in SS patients. Overexpression of Snail, and TGF-β1 is associated with suppressed expression of E-cadherin in MFSSs, which supports the hypothesis that the MFSS subtype may have developed via neoplastic EMT.
Keywords: Synovial sarcomas, epithelial-mesenchymal transition, TGF-β1 signaling pathway, immunohistochemistry
Introduction
Synovial sarcoma (SS) is a malignant tumor of soft tissue. It is characterized by uncertain histological origin and exhibits a biphasic morphology involving both the mesenchymal and epithelium. The 2 main SS types are the biphasic synovial sarcomas (BSSs), which display glandular epithelial differentiation architecture in a background of spindle cells, and the monophasic fibrous synovial sarcomas (MFSSs), which show predominant spindle cells morphology. SS clinical outcomes are very poor and approximately 90% of patients die from metastatic disease [1].
In recent years, there has been increased interest in understanding the role of epithelial-mesenchymal transition (EMT) in cancer invasion and metastasis. EMT can be defined as a phenomenon whereby epithelial cells dissemble their junctional structures, initiate expression of mesenchymal proteins, remodel their extracellular matrix, and become migratory. After invasion, tumor cells can transition back to the epithelial morphology (mesenchymal-epithelial transition, MET) to proliferate and generate tumors at distant sites. Therefore, EMT/MET is closely associated with the invasion and metastasis of cancer and is involved in the regulation of the TGF-β1, Wnt, Ras, Hedgehog, and Notch signaling pathways and transcription factors [2,3]. Indeed, TGF-β is a major inducer of EMT during development and is overexpressed in many types of human cancer. This suggests that TGF-β might act as an inducer of EMT in tumors that develop into cancers (e.g., breast carcinoma [4] and head and neck squamous cell carcinoma [5]). Importantly, the loss of E-cadherin involves several transcriptional repressors such as Snail, Slug, and Zeb, and the de novo expression of N-cadherin or P-cadherin is also known to be associated with EMT in invasive and metastatic cancer [6]. However, the role of EMT/MET in sarcoma progression is considerably less well understood.
The formation of a mesenchymal tumor cell phenotype is considered to be a complex process regulated by a variety of growth factors like TGF-β1 [7], which is highly expressed in many tumors. Therefore, we hypothesized that the TGF-β signaling pathway plays an important role in sarcoma tumorigenesis. The biphasic morphology and high invasiveness of SS make it a good model for investigating the relationship between TGF-β1-induced EMT/MET for mesenchymal tumors. Therefore, understanding the process by which tumor cells can cause EMT/MET is essential for the advancement of SS treatment strategies and improving survival.
Herein, we ① analyze the expression of cell-surface proteins (E-cadherin and N-cadherin), cytoskeletal proteins (β-catenin), transcription factors (Snail and Slug), and TGF-β1 signal pathway-related proteins (TGF-β1 and smad2/3) in BSS and MFSS; ② evaluate the difference of E-cadherin expression between the glandular and spindle cell components in SS; ③ assess the clinical significance of E-cadherin state in synovial. Taken together, these studies suggest that EMT mediates SS progression.
Materials and methods
Specimens
Thirty-seven fusion gene status–confirmed and paraffin-embedded SS samples (27 BSSs and 10 MFSSs); the patients from whom these samples had been obtained had been treated at the Department of Pathology, the First Affiliated Hospital, Shihezi University, School of Medicine, and the First Affiliated Hospital of Xinjiang Medical University Department of Pathology, between 1968 and 2011. The clinical and demographic data were obtained from the medical charts (Table 1). The diagnosis of SS was confirmed by histological and immunohistochemical analyses and SYT-SSX fusion gene detection by RT-PCR. The histopathological grading system of SS is based on FNCLCC and clinical stage according to the 2012 NCCN guidelines for Soft Tissue Tumors. This study was approved by the internal review board of the Shihezi University School of Medicine.
Table 1.
Clinical features in 37 cases of synovial sarcomas
| Case | sex/age | Site | Size (cm) | Diagnosis | FNCLCC | pTNM | Metastases | Status at last follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | F/32 | Left groin | NA | BSS | 3 | IIA | DOD | |
| 2 | F/38 | Left forearm | 9 | BSS | 3 | IIB | AWD 56 months after presentation | |
| 3 | F/15 | Right neck | 4 | BSS | 3 | IIA | AWD 78 months after presentation | |
| 4 | F/43 | Left hip | 13 | MFSS | 3 | III | DOD | |
| 5 | F/40 | Left thigh | 2.8 | BSS | 3 | IIA | NA | |
| 6 | F/22 | Left heel | 3 | BSS | 3 | IIA | DOD | |
| 7 | M/19 | Left ankle | 8.5 | BSS | 3 | IIB | DOD | |
| 8 | F/10 | Right elbow | 5 | BSS | 3 | IV | Bone marrow cavity | NA |
| 9 | M/36 | Left thigh | 5 | BSS | 3 | IIA | DOD | |
| 10 | M/36 | Right elbow | 11 | BSS | 3 | III | NA | |
| 11 | F/37 | Right tibia | 6; 5 | BSS | 3 | III | DOD | |
| 12 | M/33 | Pleura | 4 | BSS | 2 | IV | Lung | DOD |
| 13 | F/25 | Left Knee | 3 | BSS | 2 | IIA | NA | |
| 14 | M/26 | Left popliteal fossa | 1.4 | BSS | 2 | IIA | AWD 47 months after presentation | |
| 15 | F/37 | left neck | 5.5 | BSS | 2 | III | lung | DOD |
| 16 | F/28 | Right parotid gland | 7 | BSS | 2 | III | recuurence | AWD 36 months after presentation |
| 17 | M/23 | Right upper quadrant | 10.5 | BSS | 2 | III | AWD 26 months after presentation | |
| 18 | M/61 | lumbar vertebrae | NA | BSS | 2 | IV | Bone marrow cavity | AWD 10 months after presentation |
| 19 | F/70 | Right leg | 15 | BSS | 2 | III | DOD | |
| 20 | F/15 | Left toe | 6.5 | BSS | 2 | IIB | DOD | |
| 21 | M/70 | Left hip | 11.5 | BSS | 3 | IV | lung | DOD |
| 22 | M/39 | Left hand and forearm | 6 | BSS | 3 | I | liver | DOD |
| 23 | F/21 | Right thigh | 7.5 | BSS | 3 | I | AWD 27 months after presentation | |
| 24 | M/55 | Right foot | 5.2 | BSS | 3 | I | lung | DOD |
| 25 | M/14 | Left elbow fossa | 6 | MFSS | 3 | IIB | DOD | |
| 26 | F/55 | left ilium | 5 | MFSS | 3 | IV | lung | DOD |
| 27 | F/29 | back | 16 | MFSS | 2 | IIB | lung | DOD |
| 28 | M/37 | Right knee | 2 | MFSS | 2 | III | NA | |
| 29 | M/70 | Right leg | 7.0 | MFSS | 2 | IV | lung | DOD |
| 30 | M/40 | Right groin | 22 | MFSS | 2 | IV | liver | AWD 9 months after presentation |
| 31 | M/35 | Right distal femur | 5 | MFSS | 2 | I | DOD | |
| 32 | F/19 | Left thigh | 7.5 | MFSS | 3 | III | DOD | |
| 33 | M/47 | Left leg | 5 | MFSS | 3 | IV | lung | DOD |
| 34 | M/32 | Oral | 3 | MFSS | 3 | I | lung | DOD |
| 35 | F/38 | Left scapula | 5 | BSS | 2 | I | DOD | |
| 36 | M/24 | Right thumb | 5 | BSS | 2 | II | AWD 6 months after presentation | |
| 37 | M/74 | Left hip | 5 | BSS | 2 | III | AWD 35 months after presentation |
BSS, biphasic synovial sarcoma; MFSS, monophasic fibrous synovial sarcoma; AWD, alive with disease; DOD, died of disease; F, female; M, male; NA, not available.
Immunohistochemical staining
IHC staining was performed on formalin-fixed, paraffin-embedded tissue sections, by using steam heat-induced epitope retrieval or pepsin digestion (Dako Envision Detection System; DAKO, Carpinteria, CA), according to the manufacturer’s instructions. Table 2 details the technical characteristics of the different immunohistochemical markers with respect to their commercial source, working dilution, antigen-retrieval method, primary antibody incubation time, and the method for detection of antigen-antibody complexes. Positive controls for E-cadherin consisted of staining patterns in the basal cells of skin from healthy patients. Breast cancer tissue was used as the positive control for N-cadherin, Slug, and TGF-β1. Colon carcinoma tissue and normal kidney tissue were used as the positive controls for Smad and Snail. Substitution of a primary antibody with PBS buffer constituted the negative control.
Table 2.
Antibodies used of the immunohistochemical examination
| Antibody | Clone | Dilution | Source | Location |
|---|---|---|---|---|
| E-cadherin | Mouse mAb | 1:200 | DAKO | cell membrane |
| Vimentin | Mouse mAb | 1:600 | DAKO | cytoplasm |
| N-cadherin | Rabbit pAb | Use antibody | Abcam | cell membrane or cytoplasm |
| Snail | Goat pAb | 1:800 | Abcam | cell membrane or cytoplasm |
| Slug | Rabbit pAb | 1:200 | Abcam | cell membrane or cytoplasm |
| β-catenin | Mouse mAb | 1:400 | Abcam | cell membrane |
| TGF-β1 | Mouse mAb | 1:25 | Abcam | Extracellular Matrix |
| Smad2/3 | Rabbit pAb | 1:200 | Abcam | cytoplasm |
pAb, polyclonal antibody; mAb, monoclonal antibody.
Scoring of the immunohistochemical staining
The evaluation of E-cadherin, N-cadherin, β-catenin, Snail, Slug, TGF-β1, and Smad2/3 were semiquantitatively scored as described by Martin [8] and Subramaniam [9]. Cellular localization was also noted. EMT-related protein immunoreactivity scoring included E-cadherin, N-cadherin, β-catenin, Snail, and Slug by using the following system: 0, no reactivity or less than 1% positive tumor cells; 1+, 1-10% positive tumor cells; 2+, 11-50% positive tumor cells; and 3+, >50% positive tumor cells. The TGF-β signaling pathway-related proteins analysis included analysis of TGF-β1 and Smad2/3 immunoreactivity with the following scoring system: 0-10% (0), 10-25% (1+, focal), 25-50% (2+), and >50% (3+, diffuse). For β-catenin, only membranous expression was considered to be positive, whereas aberrant nuclear and cytoplasmic accumulation was scored as negative. For BSSs, epithelial and spindle cell components were scored separately, and when the expression was counted in 1 of the components, it was scored accordingly. The signal intensity of cells was indicated as mild, moderate, or intense. The IHC score was calculated independently by 2 pathologists. Cases with discrepant scores were reviewed with the use of a 5-headed microscope and then scored according to the consensus.
Statistical analysis
We evaluated all data based on the all gene expression pattern by SPSS Software 12.0, and using the following two statistical methods. For the frequencies of protein expression between presence and absence of epithelial cells in SSs, as well as correlation of the expression of E-cadherin with other markers in these tumors were performed using the χ2 test. For small sample sizes, the Fisher exact test was used to compare the relationship between the indicators, histological grade, and clinical stage.
Results
Clinical findings
The ratio of patients was about 1:1 [male 18, female 19], with the age at diagnosis ranging from 10 to 74 years (mean, 36 years). The tumors arose with a wide anatomical distribution; however, most arose in the extremities (24, 65%), trunk (8, 22%). Four tumors (11%) arose in the head and neck, and one tumor (3%) in the lung.
Clinical follow-up was available for 32 cases (nine of MFSSs and 23 of BSSs). The mortality rate is very high up 60% (22 of 32 patients), involved 14 of BSS, 9 of MFSS died of disease or metastasis. Parts of surviving patients present local recurrences or liver and lung metastasis. The data are listed in Table 1.
Pathological features
Tumor size ranged from 14 mm to 220 mm (mean, 68 mm). Analysis of the gross appearance of the tumors showed that they were gray-white with soft or rubbery nodules. Areas of necrosis, hemorrhage, and capsular space were common. Histologically, 2 main patterns were observed in the BSS cases, which are mixed between nested epithelial cells and spindle cells with transitional areas that can be seen. The epithelial cells seen included irregular glands (Figure 1A, 1C) and nested epithelial lager cells (Figure 1B). Such areas showed a population of oval or rounded cells with eosinophilic cytoplasm (Figure 1D). Some samples contained partly myxoid-to-sclerosing stroma. In MFSS, Spindle cells showed uniform spindles to ovoid cells with relatively bland nuclear features that form dense cellular sheets and vague fascicles; some areas are similar to fibrosarcoma (Figure 1E, 1F).
Figure 1.
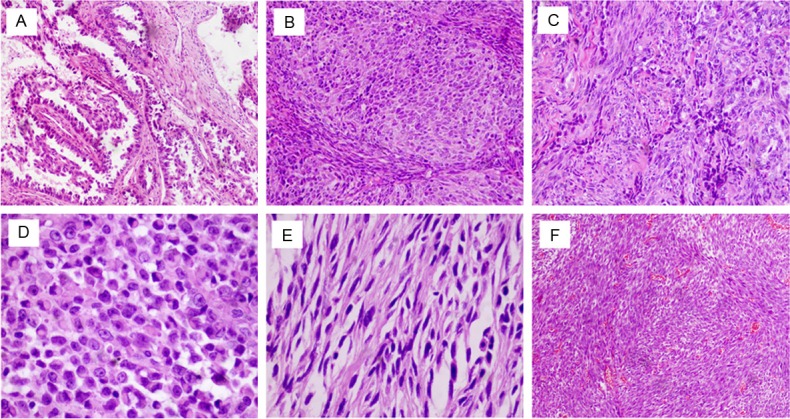
Major histologic feature of BSSs and MFSSs. BSSs displaying glandular structure (A: hematoxylin and eosin (H&E)×200) or epithelial nested architecture (B: H&E×200) in a background of spindle cells (C: H&E×200). Some tumor cells show hyperchromatic, eccentrically placed nuclei and ample, brightly eosinophilic cytoplasm (D: H&E×400). MFSSs composed of highly cellular short fascicles of uniform spindle cells (E: H&E×400, F: H&E×200).
Differential expression of TGF-beta signaling and EMT-related proteins between BSS and MFSS
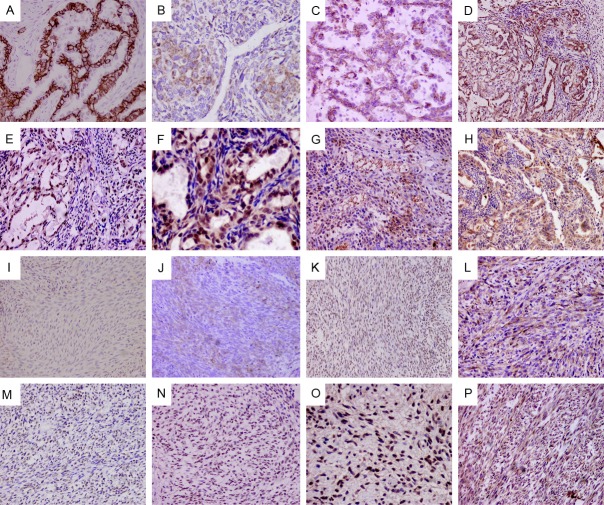
The immunohistochemical staining results are summarized in Tables 3, 4, and representative images are shown in Figure 2 (A though H are BSSs; I through P are MFSSs). TGF-β1 and Smad2/3 expression was high both in BSSs and MFSSs (p>0.05). The expression rates of E-cadherin and β-catenin in BSSs were 70.4% (19/27) and 51.9% (14/27), respectively. In contrast, MFSSs showed significantly downregulated expression of E-cadherin and β-catenin (1 of 10, 10% for both, p<0.05). The expression of N-cadherin and Slug in MFSSs was higher than that in BSSs, but this difference was not statistically significant (p>0.05). However, the expression rate of Snail in the MFSSs was 8 of 10 (80%) higher than BSS (8 of 27, 29.6%), and the difference was statistically significant (p<0.05), while the expression of N-cadherin was high. This suggests that a significant inverse correlation exists between E-cadherin and N-cadherin (p<0.001).
Table 3.
Expression frequencies of TGF-β1 signaling and EMT Proteins in BSSs and MFSSs
| (BSS; n=27) | (MFSSs; n=10) | P Value | BSS Positive cases No. (%) | P Value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No. (%) | No. (%) | Epithelial cells | Spindle cells | |||
| E-cadherin | ||||||
| + | 19 (70.4) | 1 (10) | 0.002* | 19 (100) | 3 (15.8) | <0.001*** |
| - | 8 (29.6) | 9 (90) | 0 (0) | 16 (84.2) | ||
| N-cadherin | ||||||
| + | 19 (70.4) | 9 (90) | 0.393 | 19 (100) | 16 (84.2) | 0.23 |
| - | 8 (29.6) | 1 (10) | 0 (0) | 3 (15.8) | ||
| β-catenin | ||||||
| + | 14 (51.9) | 1 (10) | 0.028* | 14 (100) | 2 (14.3) | <0.001*** |
| - | 13 (48.1) | 9 (90) | 0 (0) | 12 (85.7) | ||
| Snail | ||||||
| + | 8 (29.6) | 8 (80) | 0.009** | 8 (100) | 7 (87.5) | >0.99 |
| - | 19 (70.4) | 2 (20) | 0 (0) | 1 (12.5) | ||
| Slug | ||||||
| + | 11 (40.7) | 7 (70) | 0.151 | 11 (100) | 11 (100) | >0.99 |
| - | 16 (59.3) | 3 (30) | 0 (0) | 0 (0) | ||
| TGF-β1 | ||||||
| + | 20 (74.1) | 9 (90) | 0.404 | 15 (75) | 19 (95) | 0.182 |
| - | 7 (25.9) | 1 (10) | 5 (25) | 1 (5) | ||
| Smad2/3 | ||||||
| + | 20 (74.1) | 10 (100) | 0.155 | 20 (100) | 16 (80) | 0.106 |
| - | 7 (25.9) | 0 (0) | 0 (0) | 4 (20) | ||
Positive expression of E-cadherin, β-catenin correlated significantly with glandularity (BSS).
Positive expression of Snail correlated significantly with lack of glandularity (MFSS).
Both E-cadherin and β-catenin had significant inverse correlation between epithelial component and spindle cell component.
Table 4.
Correlation of E-cadherin expression with TGF-β1 and Other EMT-Related Proteins in epithelial and spindle cells of BSS and MFSS
| Proteins | epithelial cells componentof BSS | spindle cells componentof BSS | spindle cells componentof MFSS | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| E-cadherin | E-cadherin | E-cadherin | |||||||
|
|
|
|
|||||||
| + | - | P Value | + | - | P Value | + | - | P Value | |
|
|
|
|
|||||||
| (n=19) | (n=8) | (n=3) | (n=24) | (n=1) | (n=9) | ||||
| N-cadherin | |||||||||
| + | 16 (84.2) | 5 (62.5) | 0.027* | 2 (66.7) | 15 (62.5) | 0.888 | 1 (100) | 8 (88.9) | 0.725 |
| - | 3 (15.8) | 3 (37.5) | 1 (33.3) | 9 (37.5) | 0 (0) | 1 (11.1) | |||
| β-catenin | |||||||||
| + | 14 (73.7) | 2 (25) | 0.019** | 2 (66.7) | 2 (8.3) | 0.007** | 1 (100) | 0 (0) | 0.002*** |
| - | 5 (26.3) | 6 (75) | 1 (33.3) | 22 (91.7) | 0 (0) | 9 (100) | |||
| Snail | |||||||||
| + | 8 (42.1) | 2 (25) | 0.666 | 0 (0) | 8 (33.3) | 0.233 | 0 (0) | 8 (88.9) | 0.035**** |
| - | 11 (57.9) | 6 (75) | 3 (100) | 16 (66.7) | 1 (100) | 1 (11.1) | |||
| Slug | |||||||||
| + | 8 (42.1) | 3 (37.5) | 0.824 | 0 (0) | 11 (45.8) | 0.128 | 0 (0) | 8 (88.9) | 0.035**** |
| - | 11 (57.9) | 5 (62.5) | 3 (100) | 13 (54.2) | 1 (100) | 1 (11.1) | |||
| TGF-β1 | |||||||||
| + | 12 (63.2) | 4 (50) | 0.525 | 1 (33.3) | 15 (62.5) | 0.332 | 0 (0) | 8 (88.9) | 0.035**** |
| - | 7 (36.8) | 4 (50) | 2 (66.7) | 9 (37.5) | 1 (100) | 1 (11.1) | |||
| Smad2/3 | |||||||||
| + | 14 (73.7) | 4 (50) | 0.233 | 2 (66.7) | 14 (58.3) | 0.782 | 0 (0) | 9 (100) | 0.002**** |
| - | 5 (26.3) | 4 (50) | 1 (33.3) | 10 (41.7) | 1 (100) | 0 (0) | |||
N-cadherin and E-cadherin had significant positive correlation in epithelial cells component;
β-catenin and E-cadherin had significant positive correlation both in epithelial cells and spindle cells component;
β-catenin was significantly down-regulated expression in the MFSSs;
The expression of Snail, Slug, TGF-β1 and Smad2/3 were significantly related to the loss of expression of E-cadherin.
Figure 2.
Immunohistochemical staining of differentially expressed proteins in the BSS and MFSS tissues. A though H, In BSSs, Frequent presented membranous expression of E-cadherin, both glandular (A: ×400) and nested epithelial cells (B: ×400) show definitely positive, with focal and sporadic expression in the spindle cells; β-catenin (C: ×400) show positive membranous expression in most gland epithelial cells; N-cadherin (D: ×200) present positive cytoplastic expression in most gland epithelial cells; snail (E: ×400), slug (F: ×400) are more nuclear expression in glandular than spindle cell components; TGF-β1 (G: ×200) and Smad2/3 (H: ×200) are cytoplastic expression both glandular and spindle cell components. I through P, In MFSSs, E-cadherin (I: ×400) and β-catenin (K: ×400) expression was generally negative, focal membranous expression was seen in few cases (J: ×200); a significant proportion of the MFSSs showed cytoplasm staining N-cadherin (L: ×200) more than E-cadherin; high levels of nuclear Snail (M: ×200) and Slug (N: ×200) were frequently detected; TGF-β1 (O: ×400) and smad2/3 (P: ×200) proteins were intensely expressed and were associated with down-regulation of E-cadherin expression.
Immunohistochemical expression of protein components was obviously different between the glandular and spindle cell components in BSSs. Aberrant β-catenin expression in the form of nuclear and cytoplasmic accumulation was more frequently detected in MFSSs (7 of 10; 70%) than in BSSs (7 of 27; 25.9%). E-cadherin and β-catenin expression in the epithelial component were higher (19 of 19 and 14 of 14, respectively), but lower in the spindle component (3 of 19 and 2 of 14, respectively), thus suggesting a significant inverse correlation between epithelial and spindle cell components for E-cadherin and β-catenin expression (p<0.001). In addition, the mesenchymal marker, N-cadherin, was also expressed at high levels similar to those of E-cadherin in the epithelial component. The expression of other related proteins (Snail, Slug, TGF-β1, and Smad2/3) did not show statistically significant expression differences.
Correlation between positive-E-cadherin expression and other EMT-related proteins in epithelial and spindle cells component of BSS and MFSS
Since E-cadherin expression is a critical component of EMT, we correlated the expression of all other EMT-markers with E-cadherin expression in the epithelial component and spindle cell component, respectively (Table 4, Figure 3A-H).
Figure 3.
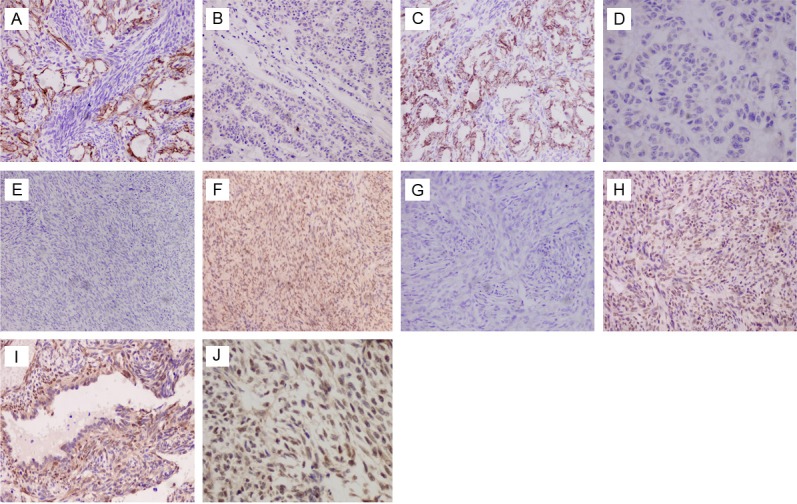
Representative images depicting correlation between E-cadherin and snail and slug in synovial sarcoma (SS), and β-catenin nuclear staining was observed in some cases. Each pair of images (A-H), representing the correlation between E-cadherin (left column) and snail or slug (right column), are taken from the same tumor sample. A through D, In BSSs, positive E-cadherin expression (A and C: ×200) was significantly associated with down-regulated Snail (B: ×200) and slug (D: ×400) expression. E through H, In MFSSs, lack E-cadherin expression (E and G: ×100) was significantly correlated with positive expression of Snail (F: ×200) and slug (H: ×200). Tumor cells frequently show weak and ⁄ or strong (I, J: ×400) nuclear expression of β-catenin in spindle cells no matter MFSSs or BSSs, but negative in glandular cells (J: ×400).
In the E-cadherin-positive epithelial component
Most BSSs showed higher expression of N-cadherin (16 of 19) in E-cadherin-positive cases and, therefore, a significant positive correlation between E-cadherin and N-cadherin expression (p<0.05). The expression rate of Snail, Slug, TGF-β1, Smad2/3, and β-catenin was 42.1% (8 of 19), 42.1% (8 of 19), 63.2% (12 of 19), 73.7% (14 of 19), and 73.7% (14 of 19) in E-cadherin-positive cases, and did not show statistically significant expression differences.
In the E-cadherin-positive spindle cells component
In contrast to the epithelial component, the spindle cells of BSS exhibited fewer cases of preserved membranous expression of E-cadherin (3 of 27; 11.1%) and β-catenin (3 of 27; 11.1%), but expression of N-cadherin, TGF-β1, and Smad2/3 was high in both E-cadherin-negative and E-cadherin-positive samples. The expression rate of Snail and Slug was similar for E-cadherin-negative and E-cadherin-positive samples.
In the E-cadherin-negative MFSSs, the expression rate of Snail, Slug, TGF-β1, and Smad2/3 was 80% (8/10), 80% (8/10), 80% (8/10), and 90% (9/10), respectively, and was significantly related to the loss of E-cadherin expression (p<0.05).
Interestingly, in some BSS cases (7 of 27; 25.9%) showed weak and⁄or strong expression of nuclear β-catenin in spindle cells but no nuclear β-catenin expression in glandular cells (Figure 3I). Aberrant β-catenin expression in the form of nuclear and cytoplasmic accumulation was more frequently detected in MFSSs (7 of 10; 70%) (Figure 3J).
Correlation between TGF-β1, EMT-related proteins and histological grade, and clinical stage of SSs
Increased Snail and smad2/3 protein expression was related to worse clinical stage (p=0.047 and p=0.021, respectively). TGF-β1 exhibited a tendency toward a positive correlation with the pTNM stage (I-II vs. III-IV, P=0.052), but no correlation with the histological grade (p>0.05). In contrast, E-cadherin, N-cadherin, and Slug did not show a statistically significant correlation with the histological differentiation and clinical stage (data not shown).
E-cadherin status and clinical outcome
Kaplan-Meier curves for overall survival (OS) in relation with clinical stage and E-cadherin status are shown in Figure 4. Clinical stages III-IV were related to significantly poorer prognosis than clinical stages I-II. E-cadherin is a key point for EMT/MET; therefore, the patients were stratified based on the presence or absence of reactivity even focal staining of E-cadherin. A highly significant difference was observed. As shown in Figure 4D, E-cadherin-negative patients had a worse event-free survival than E-cadherin-positive patients.
Figure 4.
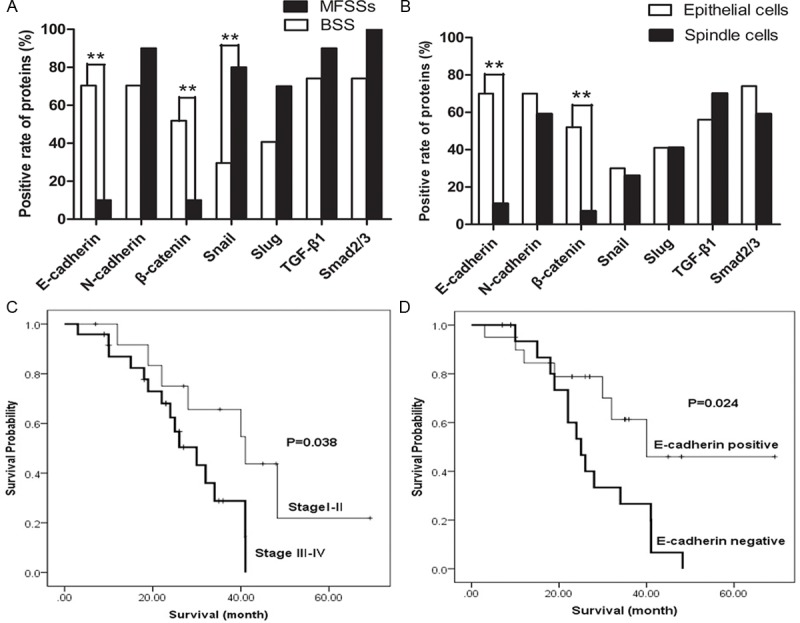
The expression of E-cadherin and other proteins in glandular and spindle cells involved of MFSSs, and the relationship between E-cadherin Status and clinical outcome. The expression of E-cadherin and β-catenin in BSS were significantly higher than MFSS (A). In BSSs, The expression of E-cadherin and β-catenin in glandular cells were also significantly higher than spindle cells (B). However, Snail expression in the MFSSs was higher than BSS (A). Clinical stage III-IV were significantly had poor prognosis than clinical stage I-II (C). Patients who E-cadherin negative had a worse event-free survival than patients E-cadherin positive (D).
Discussion
SS is a malignant soft-tissue neoplasm traditionally known for its biphasic histological pattern and accounts for approximately 5-10% of all soft-tissue sarcomas. Although the incidence rate of SS peaks in the third decade of life [8], 31% of the cases occur in adolescents younger than 20 years [10]. The clinical outcomes of SS are very poor and approximately 90% of patients die as a result of metastasis. Understanding the process by which tumor cells destroy the basement membrane, invade, and metastasize is essential for the advancement of SS treatment strategies and improving survival. EMT plays a significant role in tumor progression and invasion [11]. EMT-related signaling pathways are wide reaching and include pathways such as the TGF-β, NF-κB, Wnt, and Notch pathways [12]. TGF-β is a major inducer of EMT during development and is overexpressed in many types of human cancers, thus indicating a role for TGF-β as an inducer of EMT in tumors through a Smad-dependent transcriptional pathway and a Smad-independent transcriptional pathway [13]. Furthermore, previous studies have demonstrated that the TGF-β signaling pathway is also a key player in promoting tumor progression and metastasis [2,3]. Notably, the role of TGF-β in SS cells is not well understood.
In this study, we primarily focused on E-cadherin expression, the relationship between the TGF-β1/Smad-dependent transcriptional pathway, tumor histology, and progress on SSs. Both epithelial cells and spindle cells in BSSs involved of MFSSs the expression of TGF-β1 and smad2/3 are overexpression, which are inversely correlated with loss of E-cadherin expression for MFSSs (p=0.035, p=0.002). Moreover, for pTNM stage, smad2/3 levels are significantly correlated with clinical stages III and IV (p=0.021), TGF-β1 exhibited a tendency toward a positive correlation with stage III-IV, (p=0.052). Taken together, it appears that TGF-β1/smad play an important role in the EMT of SSs. Moreover, they also appear to be associated with more advanced stages and a poor prognosis. Chiyoda et al [14] characterized the gene expression profile of uterine carcinosarcoma and found that it resembles that of uterine sarcoma more than that of uterine endometrial carcinoma. Interestingly, EMT-acquired markers are upregulated and TGF-beta signaling is activated in uterine carcinosarcoma. In addition, Chiyoda [14] also identified chromosomal amplification and high gene expression of 19q13, including that of the TGF-β locus, suggesting that this may contribute to high expression of TGF-β, therefore, the EMT phenotype of carcinosarcoma. Bostrom et al [15] suggested that sarcomatoid clear cell renal cell carcinoma morphologically and immunohistochemically may represent a completed EMT and that TGF-β1 could be an important driving force during the sarcomatoid transdifferentiation of clear cell renal cell carcinoma.
E-cadherin is anchored to the cytoskeleton via β-catenin, a cytoplasmic plaque protein [16], and functions as a downstream transcriptional activator of the Wnt signaling pathway. In our study, preserved expression of E-cadherin (70.4%) and β-catenin (51.9%) was noted in the glandular component in most cases of BSSs. In contrast, significant loss of E-cadherin and β-catenin expression was noted in the spindle cell component of BSSs as well as in the spindle tumor cells of MFSSs (10% for both). However, the mesenchymal marker, N-cadherin, was highly expressed in both the glandular component of BSSs (70.4%) and in the spindle cell component of BSSs (59.3%), and particularly in MFSSs (90%). In contrast, most carcinomas show cadherin switching [17] (high expression of N-cadherin and low expression of E-cadherin) and are correlated with invasion and lymph node metastasis, as well as EMT features. Interestingly, there is no cadherin switching and the expression of N-cadherin and E-cadherin is as high as that for SS. Our data may imply that a glandular component with biphasic histological features of BSSs involved some degree of MET, and expression of E-cadherin protein correlated with greater survival in SS patients. Matthew et al [18] observed that chondrosarcoma cells begin to express 4 epithelial markers, that is, E-cadherin, desmocollin3, maspin, and 14-3-3σ, all of which are associated with an MET-like phenomenon that accompanies chondrosarcoma progression. These lineage transitions perhaps have important consequences for cell morphology, cell-to-cell adhesion, cell motility, and the extracellular matrix. Interestingly, our data showed that E-cadherin protein expression was correlated with better survival in SS patients. Yang, Thiery and Subramaniam [9,19,20] also proposed that regulating the activity of E-cadherin repressors may be an obvious strategy to suppress sarcoma progression.
The zinc finger proteins Snail and Slug are important mediators and transcription factors of EMT in epithelial tumor cells [21,22]. Snail binds to the E-boxes of the E-cadherin promoter and can recruit the histone deacetylase HDAC1 and DNA methyltransferase DNMT1 to aid in the epigenetic silencing of E-cadherin [23]. Snail triggers EMT by repressing the expression of epithelial markers and induces the expression of mesenchymal markers. This critical step is required for EMT. For BSSs, our data showed that Snail expression significantly decreased in the glandular and spindle cell components. More than 70% of BSSs with preserved E-cadherin expression showed decreased Snail expression, indicating an inverse correlation (p=0.009). However, the expression of Slug was not reduced to the same extent as that of Snail and did not correlate with E-cadherin expression, possibly because Slug is a weaker E-cadherin repressor than Snail [20]. In MFSSs, Snail (80%) and Slug (70%) expression was detected more frequently and was associated with significant downregulation of E-cadherin (p=0.035), also signifying an inverse correlation. This inverse correlation between Ecadherin and Snail expression has been documented previously in SSs [9,23,24] as well as in cervical cancer [25,26], breast [27,28] and gastric cancers [29].
TGF-β1 plays an inhibitory role, but tumor cells at advanced stages can evade anti-proliferative control and undergo tumorigenic progression in response to TGF-β [30]. The regulatory mechanisms of TGF-β signaling in EMT are also complicated. Several pathways induced by TGF-β1 contribute to EMT regulation. Moreover, TGF-β1 often cooperates with Notch, Wnt [31,32], FGF, and other factors during EMT [33]. In this study, we detected that the expression of TGF-β1 and Smad2/3 noted high both in the epithelial cells and spindle cells in BSSs involved of MFSSs. Loss of β-catenin expression in spindle cells, whether in BSSs or MFSSs, shows a pattern similar to that seen with E-cadherin. Additionally, some samples did exhibit nuclear β-catenin. The expression of E-cadherin and β-catenin is correlated with cell differentiation. Reduced expression of E-cadherin and β-catenin may indicate a high potential of recurrence or metastasis and a poor prognosis, while nuclear β-catenin expression is correlated with a poor prognosis in SSs with metastasis [34]. In cells undergoing EMT, β-catenin is located in the cytoplasm. This cytosolic β-catenin translocates to the nucleus to promote the transcription of genes that induce EMT. Nuclear β-catenin is a transcriptional coactivator with T-cell factor/lymphoid enhancer-binding factor, which controls the transcription of Snail [34]. Therefore, we suspect that the zinc finger proteins Snail and Slug interact with each other or others to provide crosstalk between the TGF-β and Wnt signaling pathways.
In conclusion, our results suggest that suppressed expression of E-cadherin is correlated with overexpression of Snail and Slug in spindle tumor cells of MFSSs and the activation of the TGF-β1 signaling pathway in SSs. These data perhaps support the hypothesis that neoplastic EMT contributes to the transition between spindle cell and epithelial morphology of synovial sarcoma. While our data also showed that expression of E-cadherin protein correlated with greater survival in SS patients. These results may provide a foundation and a new target for the treatment and prognosis of SS. Therefore, our observations here may warrant further molecular experiments to investigate the role of TGF-β1-regulated EMT in SS.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (no. 81202120).
Disclosure of conflict of interest
None.
References
- 1.Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors. gastrointestinal stromal tumors. Cancer Genet Cytogenet. 2002 May;135:1–22. doi: 10.1016/s0165-4608(02)00546-0. [DOI] [PubMed] [Google Scholar]
- 2.Massague J. TGF-beta in Cancer. Cell. 2008;134:215–230. [Google Scholar]
- 3.Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFbeta in cancer. FEBS Lett. 2012;586:1959–1970. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Lv ZD, Kong B, Li JG, Qu HL, Wang XG, Cao WH, Liu XY, Wang Y, Yang ZC, Xu HM, Wang HB. Transforming growth factor-beta 1 enhances the invasiveness of breast cancer cells by inducing a Smad2-dependent epithelial-to-mesenchymal transition. Oncol Rep. 2013 Jan;29:219–25. doi: 10.3892/or.2012.2111. [DOI] [PubMed] [Google Scholar]
- 5.Scanlon CS, Van Tubergen EA, Inglehart RC, D’Silva NJ. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res. 2013;92:114–121. doi: 10.1177/0022034512467352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulte J, Weidig M, Balzer P, Richter P, Franz M, Junker K, Gajda M, Friedrich K, Wunderlich H, Ostman A, Petersen I, Berndt A. Expression of the E-cadherin repressors Snail, Slug and Zeb1 in urothelial carcinoma of the urinary bladder: relation to stromal fibroblast activation and invasive behaviour of carcinoma cells. Histochem Cell Biol. 2012;138:847–860. doi: 10.1007/s00418-012-0998-0. [DOI] [PubMed] [Google Scholar]
- 7.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 8.Wolden SL, Alektiar KM. Sarcomas across the age spectrum. Semin Radiat Oncol. 2010;20:45–51. doi: 10.1016/j.semradonc.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Subramaniam MM, Navarro S, Llombart-Bosch A. Immunohistochemical study of correlation between histologic subtype and expression of epithelial-mesenchymal transition-related proteins in synovial sarcomas. Arch Pathol Lab Med. 2011;135:1001–1009. doi: 10.5858/2010-0071-OAR1. [DOI] [PubMed] [Google Scholar]
- 10.Okcu MF, Munsell M, Treuner J, Mattke A, Pappo A, Cain A, Ferrari A, Casanova M, Ozkan A, Raney B. Synovial sarcoma of childhood and adolescence: a multicenter, multivariate analysis of outcome. J Clin Oncol. 2003;21:1602–1611. doi: 10.1200/JCO.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Celia-Terrassa T, Meca-Cortes O, Mateo F, de Paz AM, Rubio N, Arnal-Estape A, Ell BJ, Bermudo R, Diaz A, Guerra-Rebollo M, Lozano JJ, Estaras C, Ulloa C, Alvarez-Simon D, Mila J, Vilella R, Paciucci R, Martinez-Balbas M, de Herreros AG, Gomis RR, Kang Y, Blanco J, Fernandez PL, Thomson TM. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest. 2012;122:1849–1868. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jing Y, Han Z, Zhang S, Liu Y, Wei L. Epithelial-Mesenchymal Transition in tumor microenvironment. Cell Biosci. 2011;1:29. doi: 10.1186/2045-3701-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGF-beta and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–2374. doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- 14.Chiyoda T, Tsuda H, Tanaka H, Kataoka F, Nomura H, Nishimura S, Takano M, Susumu N, Saya H, Aoki D. Expression profiles of carcinosarcoma of the uterine corpus-are these similar to carcinoma or sarcoma? Genes Chromosomes Cancer. 2012;51:229–239. doi: 10.1002/gcc.20947. [DOI] [PubMed] [Google Scholar]
- 15.Bostrom AK, Moller C, Nilsson E, Elfving P, Axelson H, Johansson ME. Sarcomatoid conversion of clear cell renal cell carcinoma in relation to epithelial-to-mesenchymal transition. Hum Pathol. 2012;43:708–719. doi: 10.1016/j.humpath.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Wahl JK 3rd, Kim YJ, Cullen JM, Johnson KR, Wheelock MJ. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J Biol Chem. 2003;278:17269–17276. doi: 10.1074/jbc.M211452200. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen PT, Kudo Y, Yoshida M, Kamata N, Ogawa I, Takata T. N-cadherin expression is involved in malignant behavior of head and neck cancer in relation to epithelial-mesenchymal transition. Histol Histopathol. 2011;26:147–156. doi: 10.14670/HH-26.147. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald MP, Gourronc F, Teoh ML, Provenzano MJ, Case AJ, Martin JA, Domann FE. Human Chondrosarcoma Cells Acquire an Epithelial-Like Gene Expression Pattern via an Epigenetic Switch: Evidence for Mesenchymal-Epithelial Transition during Sarcomagenesis. Sarcoma. 2011;2011:598218. doi: 10.1155/2011/598218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Eddy JA, Pan Y, Hategan A, Tabus I, Wang Y, Cogdell D, Price ND, Pollock RE, Lazar AJ, Hunt KK, Trent JC, Zhang W. Integrated proteomics and genomics analysis reveals a novel mesenchymal to epithelial reverting transition in leiomyosarcoma through regulation of slug. Mol Cell Proteomics. 2010;9:2405–2413. doi: 10.1074/mcp.M110.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 22.Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- 23.Saito T, Nagai M, Ladanyi M. SYT-SSX1 and SYT-SSX2 interfere with repression of E-cadherin by snail and slug: a potential mechanism for aberrant mesenchymal to epithelial transition in human synovial sarcoma. Cancer Res. 2006;66:6919–6927. doi: 10.1158/0008-5472.CAN-05-3697. [DOI] [PubMed] [Google Scholar]
- 24.Saito T, Oda Y, Kawaguchi K, Sugimachi K, Yamamoto H, Tateishi N, Tanaka K, Matsuda S, Iwamoto Y, Ladanyi M, Tsuneyoshi M. E-cadherin mutation and Snail overexpression as alternative mechanisms of E-cadherin inactivation in synovial sarcoma. Oncogene. 2004;23:8629–8638. doi: 10.1038/sj.onc.1207960. [DOI] [PubMed] [Google Scholar]
- 25.Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res. 2008;14:4743–4750. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 26.Lee MY, Shen MR. Epithelial-mesenchymal transition in cervical carcinoma. Am J Transl Res. 2012;4:1–13. [PMC free article] [PubMed] [Google Scholar]
- 27.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–496. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Zu X, Zhang Q, Cao R, Liu J, Zhong J, Wen G, Cao D. Transforming growth factor-beta signaling in tumor initiation, progression and therapy in breast cancer: an update. Cell Tissue Res. 2012;347:73–84. doi: 10.1007/s00441-011-1225-3. [DOI] [PubMed] [Google Scholar]
- 29.Shin NR, Jeong EH, Choi CI, Moon HJ, Kwon CH, Chu IS, Kim GH, Jeon TY, Kim DH, Lee JH, Park DY. Overexpression of Snail is associated with lymph node metastasis and poor prognosis in patients with gastric cancer. BMC Cancer. 2012;12:521. doi: 10.1186/1471-2407-12-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inman GJ. Switching TGFbeta from a tumor suppressor to a tumor promoter. Curr Opin Genet Dev. 2011;21:93–99. doi: 10.1016/j.gde.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad A, Sarkar SH, Bitar B, Ali S, Aboukameel A, Sethi S, Li Y, Bao B, Kong D, Banerjee S, Padhye SB, Sarkar FH. Garcinol regulates EMT and Wnt signaling pathways in vitro and in vivo, leading to anticancer activity against breast cancer cells. Mol Cancer Ther. 2012;11:2193–2201. doi: 10.1158/1535-7163.MCT-12-0232-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacroix-Triki M, Geyer FC, Lambros MB, Savage K, Ellis IO, Lee AH, Reis-Filho JS. beta-catenin/Wnt signalling pathway in fibromatosis, metaplastic carcinomas and phyllodes tumours of the breast. Mod Pathol. 2010;23:1438–1448. doi: 10.1038/modpathol.2010.141. [DOI] [PubMed] [Google Scholar]
- 33.Javelaud D, Pierrat MJ, Mauviel A. Crosstalk between TGF-beta and hedgehog signaling in cancer. FEBS Lett. 2012;586:2016–2025. doi: 10.1016/j.febslet.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]