Abstract
MiR-195, which exhibits a proliferation-inhibiting role in different tumors, has been reported to be down-regulated in the ectopic endometrium. The aim of this study was to determine the impact of miR-195 on the biological characteristic of the endometrial stromal cells (ESCs). MiR-195 has been presumed to target the 3’-untranslated regions (3’-UTR) of Fractalkine (FKN), which also plays important roles in endometriosis. Fluorescence reporter assays showed that miR-195 effectively binds to the 3’-UTR of FKN. The normal ESCs showed a significant higher miR-195 expression than that of eutopic and ectopic ESCs associated with endometriosis, while the FKN expression showed opposite results. MiR-195 mimics inhibited proliferation and growth and induced apoptosis of eutopic ESCs, and these effects were abolished by FKN-siRNA. miR-195 could decrease the expression of survivin, matrix metalloproteinase-9 (MMP9) and up-regulate the expression of CD82, tissue inhibitor of metalloproteinase 1 (TIMP1) and TIMP2 of eutopic ESCs by targeting FKN. Our study has demonstrated for the first time that miR-195 plays important roles in regulating the functions of ESCs through targeting FKN. The information may be useful for developing a new therapeutic strategy for endometriosis.
Keywords: MiR-195, endometriosis, endometrial stromal cells, fractalkine
Introduction
The pathogenesis of endometriosis remains controversial despite extensive research. The retrograde displacement of eutopic endometrium into the pelvis and its subsequent implantation on peritoneal surfaces is a leading theory as to the etiology of this condition. A heritable or acquired molecular aberration within the endometrium may impart a selective survival advantage to refluxed endometrial tissue in women predisposed to the development of endometriosis. The identification of molecular differences in the eutopic endometrium of women with endometriosis is an important step toward understanding the pathogenesis of this condition and toward developing novel strategies for the treatment of associated infertility and pain [1].
MicroRNAs (miRNAs) are a large class of endogenous, single-stranded, short, ncRNA of approximately 22-nucleotides in length, that play a key role in regulating gene expression through interaction with mRNA of protein-coding genes [2]. MiRNA expression is tissue- and cell-specific [3-5]. It has been demonstrated that miRNAs are important in developmental processes as well as for other cellular activities involving cell growth, differentiation, and apoptosis [6]. One miRNA expression profiling study identified 48 out of 287 miRNAs that are differently expressed with progressive decline in expression level in endometrium from women without endometriosis (EN), paired eutopic and ectopic endometrium (EU and EC), and ectopic endometrium from women with endometriosis (EE). As a result, gene expression re-programming the endometrial fragments derived from women with endometriosis may have an altered regulatory mechanism that leads to their survival and growth at the ectopic sites when compared with tissues derived from women without endometriosis [7].
MiR-195 has been reported to be downregulated in ectopic endometrium and a variety of different tumor types including hepatocellular carcinoma, gastric cancer and breast cancer [7-10]. In our preliminary study using the software programs TargeScan (http://www.targetscan. org/), and miRBD (http://mirdb.org/miRDB/), FKN was predicted to be target site of miR-195. MiR-195 has been reported to reduce phosphorylation of pRb and downregulate the proliferative marker PCNA. Strikingly, one microRNA has multiple targets to perform its biological function, as found that miR-195 exhibited proliferation-inhibiting role by targeting Cyclin D1, Cyclin E1, E2F3 and CCND3 [11] In this study, we investigated whether miR-195 regulate proliferation, growth and apoptosis of ESCs via targeting FKN.
Materials and methods
Patients
This study was conducted under a protocol approved by the Institutional Review Board of Shanghai ninth hospital affiliated to JiaoTong University School of Medicine, and written informed consent for participation was obtained from all participants.
All the eutopic endometrial and endometriotic tissues were obtained from 19 patients with endometriosis (mean age 39.3 years; range 34-43) by laparoscopy, respectively, in Hospital of Shanghai ninth hospital affiliated to JiaoTong University School of Medicine. Patients had not received any GnRH analog or other hormonal drug in the 6 months prior to the surgical operation. All the samples were obtained in the proliferative phase of the cycle that had been confirmed histologically according to established criteria. Normal endometrium was obtained from 12 disease-free women as healthy control.
Cell culture
The endometrial tissues were collected under sterile conditions and transported to the laboratory on ice in DMEM (Dulbecco’s modified Eagle’s medium)/F-12 (Gibco, USA) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT, USA). The ESCs were isolated according to methods described previously [12]. The endometrial tissues were digested with collagenase type IV (0.1%; Sigma, USA) for 30 min at 37 °C with constant agitation for recovering ESCs. The tissue pieces were filtered through sterile gauzes pads (pore diameter sizes: 200 mesh) to remove cellular debris. Following gentle centrifugation, the supernatant was discarded and the cells were resuspended in DMEM/F-12. The ESCs were separated from epithelial cells by passing them over sterile gauzes pads (pore diameter sizes: 400 mesh). The filtrated suspension was layered over Ficoll and centrifuged at 800 g for 20 min to further remove leukocytes and erythrocytes, and the middle layer was collected and then washed with D-Hanks solution. The ESCs were placed in a culture flask and allowed to adhere for 20 min. The adherent stromal cells were cultured as a monolayer in flasks with DMEM/F-12 supplemented with 10% FCS and 20 mmol/l HEPES and incubated in a humidified incubator with 5% CO2 at 37 °C. This method supplied 95% vimentin positive and cytokeratin-negative ESCs.
Quantitative real-time PCR
The total RNA was extracted from the normal (n=5), eutopic (n=5), and ectopic (n=5) ESCs with Tri reagent (Molecular Research Center, USA). MiR-195 was analyzed using the TaqMan microRNA assay kit (Applied Biosystems, USA). The U6 gene was used as an internal control for normalization. The cDNA was generated with oligo (dT) 18 primers using Revert Aid First, Strand cDNA Synthesis Kit (Fementas Life Science, Glen Burnie, MD, USA). Triplicate samples containing cDNA prepared as mentioned-above. In this study, these genes were chosen for quantitative real-time PCR (q-PCR), and miR-195 and FKN primers were designed and synthesized by TakaRa Biotechnology Co., Ltd (Dalian, China). The primer pairs for cDNA amplification were as follows: 5’-GAATTCGCCTCAAGAGAACAA AGTGGAG-3’ (forward), 5’-AGATCTCCCATGGGGGCTCAGCCCCT-3’ (reverse), for human miR-195; 5-GGGGAGCCAAAAGGGTCATCATCT-3’ (forward) and 5’-GAGGGGCCATCCACAGTCTTCT-3’ (reverse) for human glyceraldehydes-3- phosphate dehydrogenase (GAPDH). The cycling conditions consisted of a denaturation step at 95 °C for 10 min, 40 cycles at 95 °C for 15 sec, a 60-sec annealing step at 62 °C, and finally a holding temperature of 15 °C. To determine the amount of gene product present in the sample, cycle time (Ct) was determined. The average Ct value was calculated from triplicate wells for each sample with each primer set. Most duplicate samples varied by, 0.5 Ct. The relative gene expression was determined by calculating DCt values (DCt) by subtraction of the Ct value for GAPDH primers from the Ct value for target gene primers. The relative fold expression of each gene was determined compared with control in the experiment. The experiments were carried out in triplicate.
MiRNA or siRNA transfection
For miRNA mimics or short interfering RNA (siRNA) transfection, ESCs from eutopic endometrium with endometriosis were seeded in 96-well plates. When cells had reached confluency, medium was changed to OPTIMEM (Invitrogen). The miRNA mimics, negative control RNA mimics (denoted as NC, Table 1) or siRNA oligonucleotides targeting FKN (set of three oligonucleotides; Stealth Select RNAi; Invitrogen) and Lipofectamine 2000 (Invitrogen) were mixed in OPTIMEM, and then added to the cells at room temperature with non-targeting siRNA oligonucleotides as negative control, without any treatment group as blank control. After 6 h incubation, the cells were incubated in DMEM/F-12 for further 72 h in 5% CO2 at 37 °C until the successful gene expression or knockdown was confirmed by in-cell Western and western blot. The sequences for three siRNA (dsRNA oligonucleotides) were as follows: (1) 5’-AUU GGC AGA CUC GCU UCC CUG-3’ (forward) and 5’-GGG AAG CGA GUC UG-3’ (reverse); (2) 5’-UAU UGG CAG ACU CGC UUC CCU-3’ (forward) and 5’-GGA AGC GAG UCU GCC AAU AUU-3’ (reverse); and (3) 5’-AUA UUG GCA GAC UCG CUU CCC-3’ (forward) and 5’-GAA GCG AGU CUG CCA AUA UUG-3’ (reverse). The results showed that the silencing efficiency of the second is best, so in all subsequent experiments, we used this siRNA to silence FKN expression in eutopic ESCs, with non-targeting siRNA as control.
Table 1.
Nucleic acid sequences for miR-195 mimics
| Gene names | Sequences [5’-3’] |
|---|---|
| miR-195 mimics | UAGCAGCACAGAAAUAUUGGC |
| Negative controls | UUCUCCGAACGUGUCACGUTT |
Northern blot
Total RNA extracted from ESC was electrophoresed on a 12% polyacrylamide-urea gel and transferred to a Hybond-N+ membrane (Amersham Biosciences). Membranes were hybridized with miR-195 oligonucleotide probes labeled with γ-32P-ATP at the 5′-ends. U6 snRNA was used as a loading control. Probes for miR-195: GCCAATATTTCTGTGCTG CTA; probes for U6: TTCACGAATTTGCGTGTCAT.
In-cell western
According to the description by Egorina [13], we used a newly set up assay called in-cell Western to determine the in-cell protein level of FKN, CD82, survivin, PCNA, MMP2, MMP9, TIMP1 and TIMP2. The procedure was as follows: 2×104 cells/well normal ESCs (n=6), miRNA-195 transfected ESCs (n=6) or both miR-195 and FKN-siRNA co-transfected ESCs (n=6) in 96-well plate were incubated with or without vehicle for another 24 h, and then cells were immediately fixed with 4% formaldehyde in PBS for 20 min at room temperature. After washing with 0.1% Triton, the cells were blocked by adding 150 ml of LI-COR Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE, USA) for 90 min at room temperature. The cells were incubated with mouse anti-human FKN (20 ug/ml, eBioscience) or mouse anti-human CD82 (20 ug/ml, Santa Cruz Biotechnology,), rabbit anti-human survivin (20 ug/ml, Cell Signaling Technology), mouse anti-human PCNA antibody (1:80, Santa Cruz Biotechnology), mouse anti-human MMP2 (20 mg/ml, R&D Systems), MMP9 (20 mg/ml, R&D Systems), tissue inhibitor of metalloproteinase (TIMP1) (15 mg/ml, R&D Systems) or TIMP2 (15 mg/ml, R&D Systems) antibody. To assess the housekeeping protein actin, goat anti-human actin (Abcam) was added to each well at the same time as an internal control. After overnight treatment at 4 °C, the wells were incubated with corresponding second IRDye 700DX-conjugated affinity purified (red fluorescence) anti-mouse and IRDye 800DX-conjugated affinity purified (green fluorescence) anti-goat. However, for the survivin detection group, the wells were incubated with corresponding second IRDye 700DXconjugated affinity purified (red fluorescence) anti-rabbit and IRDye 800DX-conjugated affinity purified (green fluorescence) anti-goat, fluorescence antibody recommended by the manufacturer (Rockland, Inc., Gilbertsville, PA, USA). This procedure must be carried out in the dark. Images of target gene were obtained using the Odyssey Infrared Imaging System (LI-COR Biosciences). The expression level of the correspondent molecules was calculated as the ratio of the intensity of target gene to actin. The experiments were carried out in triplicate, and repeated three times.
MicroRNA expression system
To construct a plasmid expressing miR-195, we amplified a 160-bp DNA fragment containing a miR-195 precursor from human genomic DNA (293T) and cloned the amplified fragment into a modified pcDNA6.2-GW/EmGFP vector [14]. The FKN-3’-UTR regions of the predicted miR-195 targets were cloned into the psiCHECK-2 vector (Promega) downstream of the Renilla luciferase gene (Xho I/Not I sites) [14]. Mutant plasmids corresponding to wild-type 3′ UTR regions were also constructed.
Luciferase reporter assays
Sequences from the miR-195 target site in the 3’-UTR of FKN and their mutant variants were successfully cloned into the psiCHECK-2 vector (Promega). Briefly, the 3’-UTRs of human FKN and their corresponding mutated 3’-UTRs were amplified by PCR using the primers shown in Table 2. ESCs were seeded into 48-well plates at a density of 2×104. Luciferase activity was measured 48 h post-transfection using the Dual-Luciferase Reporter Assay System, according to the manufacturer’s instructions (Promega). For each sample, Renilla luciferase activity was normalized by the total protein content.
Table 2.
Primers for human FKN 3’-UTR and its mutated fragments
| Gene names | Primers [5’-3’] |
|---|---|
| FKN 3’-UTR | Forward: tgggatccctcatcctcata |
| Reverse: ccagcagcagaggagagttt | |
| FKN 3’-UTR-mut | Forward: ctgagctgggatgattggag |
| Reverse: cagccagtaacggaggagag |
BrdU cell proliferation assay, cell number count and apoptosis assay
The BrdU cell proliferation assay and an annexin V-FITC assay were utilized to evaluate the effects of miR-195 on cell proliferation and apoptosis, respectively, while cell growth was assessed by counting cell numbers. Normal ESCs, eutopic ESCs and ectopic ESCs were resuspended in DMEM/F-12 supplemented with 10% FBS and seeded at a density of 1×104 cells/well in 96-well flat-bottom microplates for the BrdU cell proliferation assay. Thereafter, the cells were starved with DMEM containing 1% FBS for 12 h before treatment. Alternatively, mouse isotype (1 mg/ml; Sino-America Co., Ltd) or vehicle was added as the negative control. Before and after treatment, the cells in 12-well flat-bottom microplates were counted at a magnification of ×100. The results were observed under the microscope (Olympus 1X71; Olympus, Tokyo, Japan) fitted with a digital camera (Olympus DP72; Olympus). The cells were counted in five predetermined fields.
The ability of ESCs to proliferate was assessed using the BrdU cell proliferation assay (Millipore, USA) according to the manufacturer’s instruction. As a marker of apoptosis, phosphatidylserine externalization was quantified by flow cytometry by using a commercially available annexin V-FITC apoptosis detection kit (Invitrogen, USA) according to the manufacturer’s guideline. ESCs were trypsinized and collected. The culture medium was also retained and pooled with the adherent cells. Cells were centrifuged and the supernatant was discarded. The cells were resuspended in PBS, and washed twice and resuspended in the kit-binding buffer (100 ml/pellet) containing annexin V solution (5 ml/pellet) and propidium iodide (2.5 mg/ml). Samples were incubated in the dark for 15 min, and the percent of annexin V-positive cells in ESCs was determined by FACS Caliber flow cytometry. The experiments were performed in triplicate and repeated three times.
Statistical analysis
All values are shown as the mean+SD. Data were analyzed by using one-way analysis of variance and least significant difference (equal variances assumed), or Tamhane’s test (equal variances not assumed) was used post hoc for multiple comparisons by Statistical Package for the Social Sciences software version 11.5. Differences were considered as statistically significant at P<0.05.
Results
MiR-195 expression is downregulated and FKN expression is upregulated in primary ESCs from patients with endometriosis
MiR-195 transcription in the primary ESCs were detected by quantitative real time PCR (Figure 1A), and the results showed that the mRNA level of miR-195 in the normal ESCs without endometriosis (n=6) is higher than that of the eutopic (n=6) and ectopic ESCs (n=6) with endometriosis, respectively (Figure 1A) (P< 0.05 or P<0.01). Consistent with transcriptional level, the normal ESCs showed a significant higher miR-195 expression than that of eutopic ESCs (P<0.05), and the latter was further significantly higher than that of the ectopic ESCs (P<0.01) by northern blot (Figure 1B). These results above suggested that low expression of miR-195 in the eutopic and ectopic ESCs may be involved in the occurrence and development of endometriosis.
Figure 1.

The expression of MiR-195 is decreased and FKN expression is up-regulated in the eutopic and ectopic ESCs associated with endometriosis. A: The expression of miR-195 was determined by real time polymerase chain reaction. B: MiR-195 expression was analyzed by in-cell western blot. C: The expression of FKN in the normal ESCs, eutopic ESCs and ectopic ESCs were analyzed, respectively. Normal ESCs is ESC from endometrium without endometriosis; eutopic ESCs is ESC from the eutopic endometrium with endometriosis. Statistical results from three separate experiments. Results were highly reproducible in three independent experiments. Original magnification ×200. Error bars depict the standard error of the mean. *P <0.05 compared with the normal ESC control. **P<0.01 compared with the normal ESC control.
The results by in-cell western (Figure 1C) showed a lower FKN expression in the normal ESCs than that of eutopic ESCs (P<0.05), and the latter was further lower than that of the ectopic ESCs (P<0.01), which is consistent with the prediction that miR-195 down-regulates FKN by binding to its 3’-UTR.
FKN is identified as a functional target of miR-195 in ESCs
Targetscan showed that the binding site for miR-195 in the 3’-UTR of FKN is ACGACGA-UGCUGCU (Figure 2A). We next carried out experiments to confirm the binding of miR-195 in the FKN 3’-UTR. As shown in Figure 2B, miR-195 significantly suppressed the expression of the Renilla luciferase reporter carrying the wild-type putative target sites of FKN (60% reduction) compared with controls (P<0.01), suggesting that FKN contain effective miR-195 binding site in its 3’-UTRs. We then performed Western blot to determine whether transfection of miR-195 mimics into ESCs reduced the endogenous protein expression of FKN. As shown in Figure 2C, FKN expression was significantly down-regulated by its cognate siRNAs and miR-195 in ESCs, whereas FKN protein levels were highly reduced by miR-195 in ESCs. These data suggest that FKN may be a functional target of miR-195 in ESCs.
Figure 2.
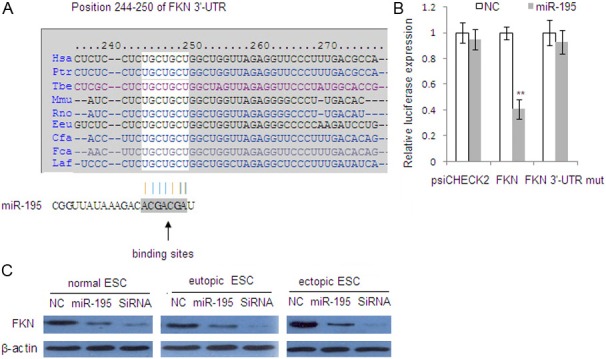
FKN is a direct primary functional target of miR-195. A: FKN possesses binding sites for miR-195 in its 3′-UTRs. B: Luciferase assays indicated that miR-195 directly lowered the expression of FKN in the ectopic ESC. Relative luciferase values were normalized to co-transfection with the psiCHECK-2-control. Data represent the mean±SD from 2 separate determinations performed in triplicate. **P<0.01. C: Western blot shows the endogenous expression levels of FKN after transfection with miR-195 or FKN siRNA in ESC. β-actin served as an internal control.
MiR-195 inhibits the proliferation and growth and induces apoptosis of ESCs by targeting FKN
To clarify the influence of miR-195 on ESC proliferation and growth, we first investigated its effects on proliferation, growth and apoptosis of ESCs. Data presented in Figure 3 demonstrated that miR-195 notably inhibits proliferation (P<0.05 or P<0.01; Figure 3A) and growth (P<0.05 or P<0.01; Figure 3B), and induces apoptosis in normal ESCs (P<0.05 or P<0.01; Figure 3C), eutopic ESCs (P<0.05 or P<0.01; Figure 3D) and ectopic ESCs (P<0.05 or P<0.01; Figure 3E). The decrease in proliferation and growth and the increase in apoptosis of the eutopic ESCs caused by miR-195 could be significantly reversed by FKN. Our results indicate that miR-195 can inhibits proliferation and growth and induces apoptotic behavior of ESCs by targeting FKN.
Figure 3.

MiR-195 inhibits proliferation and growth and induces apoptosis of ESCs by targeting FKN. A: BrdU proliferation assays were used to analyze proliferation of ESCs. Normal, eutopic and ectopic ESCs [1×104 cell/well] from women with endometriosis were treated, respectively, with recombinant human FKN (100 ng/ml) for 24 h, transfected with miR-195 mimics (50 pmol/well), or FKN-siRNA (50 pmol/well) or vehicle as controls for another 24 h. B: Cell number counts were used to analyze growth of ESCs. C: Annexin V-FITC apoptosis detection assays were used to analyze apoptosis of normal ESCs. D: Annexin V-FITC apoptosis detection assays were used to analyze apoptosis of eutopic ESCs associated with endometriosis. E: Annexin V-FITC apoptosis detection assays were used to analyze apoptosis of ectopic ESCs. Results were highly reproducible in three independent experiments. Error bars depict the standard error of the mean. *P<0.05, **P<0.01 compared with the vehicle control. #P<0.05, ##P<0.01 compared with the miR-195 treatment.
MiR-195 inhibits the expression of survivin, MMP9, up-regulates the expression of CD82, TIMP1 and TIMP2 of eutopic ESCs by targeting FKN
To test the effects of miR-195 on the survival-relative and apoptosis-relative molecules of ESCs, in-cell Western was applied to detect the expression of PCNA, survivin, CD82, MMP2, MMP9, TIMP1, and TIMP2. As shown in Figure 4, miR-195 notably inhibited survivin, (Figure 4A; P<0.05 or P<0.01) and MMP9 expression (Figure 4B; P<0.05 or P<0.01) and up-regulated expression of CD82 (Figure 4A; P<0.05 or P<0.01), TIMP1 and TIMP2 (Figure 4B; P<0.05 or P<0.01), and these effects were abolished by FKN-siRNA. Our results have illustrate that miR-195 inhibits the expression of survivin, and MMP9, up-regulates the expression of CD82, TIMP1 and TIMP2 by targeting FKN in ESCs.
Figure 4.

MiR-195 down-regulates the expression of survivin, MMP9, up-regulates the expression of CD82, tissue inhibitor of TIMP1 and TIMP2 of eutopic ESCs by targeting FKN. A: In-cell Western blot was used to analyze the expression of survivin, CD82, PCNA. B: In-cell Western assays were conducted to analyze the expression of MMP2, MMP9, TIMP1, and TIMP2. Survivin, CD82, MMP2, MMP9, TIMP1, and TIMP2: Red; actin: green. These pictures are representatives of three individual experiments. Error bars depict the standard error of the mean. *P<0.05, **P<0.01 compared with the vehicle control. #P<0.05, ##P<0.01 compared with the miR-195 treatment.
Discussion
The pathogenesis of endometriosis is likely multifactorial and several hypotheses have been suggested to explained the presence of ectopic endometrial tissue and stroma, such as retrograde menstrual reflux, immune system defects and involvement of genetic factors [6]. Conventional and recent large-scale gene expression studies have provided further evidence reflecting the molecular environments that differentiate endometrium from disorders affecting this tissue, including endometriosis [7]. The product of these genes have been considered to contribute toward endometrial disorders.
MiR-195 has been reported to be deregulated in certain types of cancer, including upregulation in chronic lymphocytic leukemia and breast cancer but downregulation in hepatocellular carcinoma, adrenocortical carcinoma, and squamous cell carcinoma of the tongue. miR-195 was also suggested to be correlated with lymph node metastasis and poor prognosis in colorectal cancer [15]. Our findings reveal a new biological function of miR-195 and also highlight its key pathophysiological implications.
FKN is a CX3CL1 chemokine and is expressed in neurons, endothelial cells, hepatocytes, and vascular smooth muscle cells [16-20]. FKN is expressed as a membrane-bound protein, which can interact with CX3CR1 on adjacent cells to facilitate cell-cell adhesion and communication [18,20]. The extracellular domain of FKN can be cleaved through the action of the extracellular proteases Adam 10 and 17 to produce a soluble form of FKN [21,22]. Soluble FKN can exert paracrine effects in the extracellular space and can also enter the circulation to potentially cause endocrine effects on distant tissues [23]. This study provides the first evidence of regulation effects of FKN in ESCs from patients with endometriosis.
Analogous to other studies [1], the present study has also demonstrated that the expression of miR-195 in the normal ESCs from women without endometriosis is higher than that in the eutopic ESCs from women with endometriosis; the latter is higher than that in the ectopic ESCs. But the expression of FKN shows the opposite results. Subsequently, we have further found that miR-195 can inhibits proliferation and growth and induces apoptosis of ESCs, and these effects can be inhibited by FKN-siRNA. These findings indicate that the ESC-derived miR-195 regulates the biological functions of ESCs at least partly through binding to FKN.
Endometriosis is a benign gynecological disorder, but appears malignant behaviors including invasion, ectopic implantation and recurrence. Tetraspanin CD82 is a wide- spectrum tumor metastasis suppressor that inhibits motility and invasiveness of cancer cells. Previous studies [24] have found that the mRNA and protein levels of CD82 in the primary normal ESCs from endometrium without endometriosis are significantly higher than that of the primary ESCs from eutopic endometrium and ectopic tissue. CD82 inhibits the invasiveness of ESCs by down-regulating CCL2 secretion and CCR2 expression via mitogen-activated protein kinase (MAPK) and integrinβ1 signal pathway, and in turn up-regulating the expression of TIMP1 and TIMP2 in an autocrine manner. We have further found that miR-195 significantly enhance CD82 expression by targeting FKN, which suggests that the abnormal lower miR-195 in the eutopic and ectopic ESCs may induce abnormal increase and implantation of the shed endometrium to peritoneum.
Gebel et al. [25] found for the first time that the pattern of apoptosis in the eutopic endometrium of women with endometriosis is lower than that of the normal eutopic endometrium from healthy controls. Apoptosis of both endometrial stroma and glands in endometrium associated with endometriosis was lower compared with that in endometrium from women without endometriosis [26]. Taking into account our results, we hypothesize that in women with endometriosis a significantly lower miR-195 may decrease apoptosis in the eutopic and ectopic endometrial tissue, which favors ectopic growth, survival and invasion. Interestingly, we have further demonstrated in the present work that miR-195 regulates the expression of survival-relative molecules, survivin by targeting FKN. Survivin is an inhibitor of apoptosis expressed during fetal development and in cancer tissues. Several studies have demonstrated that survivin play an important role in physiological homeostasis during the normal menstrual cycle, and up-regulation of survivin may contribute to survival and or invasion of ESCs in the progression of endometriosis [27,28]. Therefore, our current results led us to propose that an decrease in survivin expression, may inhibits the survival of ESCs.
In conclusion, our findings suggest that downregulation of miR-195 expression in the eutopic and ectopic ESCs of women with endometriosis may promote the expression of survivin, MMP9, and enhance ESC survival via targeting FKN. These effects will promote survival of the retrograde endometrial cells in the peritoneal cavity, and further give rise to the formation of endometriotic lesions. Therefore, this information will be helpful in further investigation on the pathogenesis and therapeutics of endometriosis.
Acknowledgements
This work was supported by National Natural Foundation of China (NSFC) (31071275 to QF Lyu, 81270749 to YP Kuang) and the Natural Science Foundation of Shanghai, China (11411950105 to YP Kuang); Foundation for shanghai ninth hospital affiliated to JiaoTong University School of Medicine (No. JY2011A02 to Y.W.).
Disclosure of conflict of interest
None.
References
- 1.Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, Giudice LC. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod. 2009;15:625–31. doi: 10.1093/molehr/gap068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15:587–607. doi: 10.1093/molehr/gap064. [DOI] [PubMed] [Google Scholar]
- 3.Xu P, Vernooy SY, Guo M, Hay BA. The Drosophila microRNA mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol. 2003;13:790–5. doi: 10.1016/s0960-9822(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 4.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophia. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 5.Dostie J, Mourelatos Z, Yang M, Sharma A, Dreyfuss G. Numerous microRNPs in neuronal cells containing novel microRNAs. RNA. 2003;9:180–6. doi: 10.1261/rna.2141503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filigheddu N, Gregnanin I, Porporato PE, Surico D, Perego B, Galli L, Patrignani C, Graziani A, Surico N. Differential Expression of MicroRNAs between Eutopic and Ectopic Endometrium in Ovarian Endometriosis. J Biomed Biotechnol. 2010;2010:369549. doi: 10.1155/2010/369549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13:797–806. doi: 10.1093/molehr/gam063. [DOI] [PubMed] [Google Scholar]
- 8.Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP, Zhuang SM. MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology. 2009;50:113–21. doi: 10.1002/hep.22919. [DOI] [PubMed] [Google Scholar]
- 9.Guo J, Miao Y, Xiao B, Huan R, Jiang Z, Meng D, Wang Y. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol. 2009;24:652–7. doi: 10.1111/j.1440-1746.2008.05666.x. [DOI] [PubMed] [Google Scholar]
- 10.Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Newell J, Kerin MJ. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251:499–505. doi: 10.1097/SLA.0b013e3181cc939f. [DOI] [PubMed] [Google Scholar]
- 11.Hui W, Yuntao L, Lun L, WenSheng L, ChaoFeng L, HaiYong H, Yueyang B. MicroRNA-195 Inhibits the Proliferation of Human Glioma Cells by Directly Targeting Cyclin D1 and Cyclin E1. PLoS One. 2013;8:e54932. doi: 10.1371/journal.pone.0054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Yu J, Luo X, Wang X, Li M, Wang L, Li D. Abnormal regulation of chemokine TECK and its receptor CCR9 in the endometriotic milieu is involved in pathogenesis of endometriosis by way of enhancing invasiveness of endometrial stromal cells. Cell Mol Immunol. 2010;7:51–60. doi: 10.1038/cmi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egorina EM, Sovershaev MA, Østerud B. In-cell Western assay: a new approach to visualize tissue factor in human monocytes. J Thromb Haemost. 2006;4:614–20. doi: 10.1111/j.1538-7836.2005.01781.x. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, He JH, Xiao ZD, Zhang QQ, Chen YQ, Zhou H, Qu LH. Liver-enriched transcription factors regulate microRNA-122 that targets CUTL1 during liver development. Hepatology. 2010;52:1431–42. doi: 10.1002/hep.23818. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Wang J, Ma H, Zhang J, Zhou X. Downregulation of miR-195 correlates with lymph node metastasis and poor prognosis in colorectal cancer. Med Oncol. 2012;29:919–27. doi: 10.1007/s12032-011-9880-5. [DOI] [PubMed] [Google Scholar]
- 16.Aoyama T, Inokuchi S, Brenner DA, Seki E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology. 2010;52:1390–400. doi: 10.1002/hep.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 18.Haskell CA, Cleary MD, Charo IF. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J Biol Chem. 1999;274:10053–8. doi: 10.1074/jbc.274.15.10053. [DOI] [PubMed] [Google Scholar]
- 19.Lucas AD, Chadwick N, Warren BF, Jewell DP, Gordon S, Powrie F, Greaves DR. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am J Pathol. 2001;158:855–66. doi: 10.1016/S0002-9440(10)64034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol. 2008;28:1897–908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- 21.Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–8001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 22.Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, Kallen KJ, Rose-John S, Ludwig A. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–95. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 23.Shah R, Hinkle CC, Ferguson JF, Mehta NN, Li M, Qu L, Lu Y, Putt ME, Ahima RS, Reilly MP. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes. 2011;60:1512–8. doi: 10.2337/db10-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li MQ, Hou XF, Lv SJ, Meng YH, Wang XQ, Tang CL, Li DJ. CD82 gene suppression in endometrial stromal cells leads to increase of the cell invasiveness in the endometriotic milieu. J Mol Endocrinol. 2011;47:195–208. doi: 10.1530/JME-10-0165. [DOI] [PubMed] [Google Scholar]
- 25.Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil Steril. 1998;69:1042–7. doi: 10.1016/s0015-0282(98)00073-9. [DOI] [PubMed] [Google Scholar]
- 26.Szymanowski K. Apoptosis pattern in human endometrium in women with pelvic endometriosis. Eur J Obstet Gynecol Reprod Biol. 2007;132:107–10. doi: 10.1016/j.ejogrb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Konno R, Yamakawa H, Utsunomiya H, Ito K, Sato S, Yajima A. Expression of survivin and Bcl-2 in the normal human endometrium. Mol Hum Reprod. 2000;6:529–34. doi: 10.1093/molehr/6.6.529. [DOI] [PubMed] [Google Scholar]
- 28.Ueda M, Yamashita Y, Takehara M, Terai Y, Kumagai K, Ueki K, Kanda K, Yamaguchi H, Akise D, Hung YC, Ueki M. Survivin gene expression in endometriosis. J Clin Endocrinol Metab. 2002;87:3452–9. doi: 10.1210/jcem.87.7.8682. [DOI] [PubMed] [Google Scholar]


