Abstract
Background: Mycophenolate mofetil (MMF), the prodrug of mycophenolic acid (MPA) which has been widely used for the prevention of acute graft rejection, is a potent inhibitor of inosine monophosphate dehydrogenase (IMPDH) that is up-regulated in many tumors and potentially a target for cancer therapy. MPA is known to inhibit cancer cell proliferation and induces apoptosis; however, the underlying molecular mechanisms remain elusive. Methods: We first demonstrated MPA’s antiproliferative and proapoptotic activities using in vitro studies of 13 cancer cell lines and a xenograft model. Key proteins involved in cell cycle, proliferation and apoptosis were analyzed by Western blotting. Results: In vitro treatment of thirteen cancer cell lines indicated that five cell lines (AGS, NCI-N87, HCT-8, A2780 and BxPC-3) are highly sensitive to MPA (IC50 < 0.5 μg/ml), four cell lines (Hs746T, PANC-1, HepG2 and MCF-7) are very resistant to MPA (IC50 > 20 μg/ml) and the four other cell lines (KATO III, SNU-1, K562 and HeLa) have intermediate sensitivity. The anticancer activity of MPA was confirmed in vivo using xenograft model with gastric AGS cell line. Further in vitro analyses using AGS cells indicated that MPA can potently induce cell cycle arrest and apoptosis as well as inhibition of cell proliferation. Targeted proteomic analyses indicate that many critical changes responsible for MPA’s activities occur at the protein expression and phosphorylation levels. MPA-induced cell cycle arrest is achieved through reduction of many cell cycle regulators such as CDK4, BUB1, BOP1, Aurora A and FOXM1. We also demonstrate that MPA can inhibit the PI3K/AKT/mTOR pathway and can induce caspase-dependent apoptosis. Conclusions: These results suggest that MPA has beneficial activities for anticancer therapy through diverse molecular pathways and biological processes.
Keywords: MPA, drug repurposing, cancer, apoptosis, xenograft
Introduction
Mycophenolate mofetil (MMF) is approved for the prevention of acute graft rejection in transplantation [1,2]. MMF is the morpholinoethyl ester prodrug of mycophenolic acid (MPA), which is a potent uncompetitive inhibitor of inosine monophosphate dehydrogenase (IMPDH), the rate-limiting enzyme for the de novo synthesis of guanosine nucleotides [3,4], which play crucial roles in cell proliferation and other cellular functions [5]. Consequently, MPA blocks T and B lymphocyte proliferation and clonal expansion, and prevents the generation of cytotoxic T cells and other effector T cells. Other mechanisms may also contribute to the efficacy of MPA in preventing allograft rejection. Through depletion of guanosine nucleotides, MPA can suppress glycosylation and the expression of several adhesion molecules, thereby decreasing the recruitment of lymphocytes and monocytes into sites of inflammation and graft rejection [4]. It has recently been shown that IMPDH can function as a sequence-specific DNA-binding transcription factor [6]. Although IMPDH is largely cytoplasmic, IMPDH can accumulate in the nucleus, where it binds and represses histone genes and E2F, the master driver of the G1/S transition.
The expression of IMPDH, particularly IMPDH2, is significantly up-regulated in many tumor cells [7,8]. Therefore, IMPDH is potentially a target for cancer therapy in addition to immunosuppressive chemotherapy. MPA/MMF has been reported to inhibit cancer cell proliferation and induces apoptosis in vitro and in vivo, such as multiple myeloma cells [9], HL-60 cells [10], lymphoma [11], Walker’s carcinosarcoma [12], U87 glioblastoma cells [13], pancreatic, lung and colon cancer [14]. However, the precise signaling pathways underlying MPA’s activities remain elusive. The reported mechanisms include its ability to induce and activate p53 [15], a key tumor suppressor molecule involved in cell cycle control and apoptosis and its ability to inhibit the surface expression of some integrins [16].
To gain further insights into the biological and molecular mechanisms of MPA, we investigated the anticancer activity of MPA in multiple cancer cell lines and the molecular changes after MPA treatment using targeted proteomic analyses.
Materials and methods
Cell viability and proliferation assays
Cell viability was assayed using a cell counting kit-8 (CCK-8) from Dijindo Molecular Technolog-ies (Rockville, Maryland). Approximately 3500 cells were seeded into 96 well plate 24 h before MPA treatment. An equal volume (1 μl) of different concentration of MPA (0.4 μg/ml - 2 mg/ml) was added to each well. After incubation for 24, 48 or 72 hours, culture medium was removed and replaced by 100 μl of fresh medium containing 5 μl CCK-8. After 4 h incubation, OD values were measured at 450 nm using the multifunctional microplate reader.
In vivo experiments
This study was approved by the Georgia Regents University IACUC committee. About 2 × 106 AGS cells (per mouse) in 100 μl PBS with matrigel (Sigma) (1 : 1) were inoculated subcutaneously in the right leg flank of male Balb/c nude mice (4-6 weeks of age). Two weeks post-inoculation, when the tumor sizes reached approximately 100 mm3, mice were randomly divided into two groups. For the MPA treated group, mice were injected intraperitoneally with MPA (2 mg/mouse) once daily. For the control group, mice were treated with vehicle. The weights of mice were similar within each treatment group. Tumor volumes and body weight were measured once every three days.
Western blotting
Cells were harvested and resuspended in PBS. After centrifugation at 2000 rpm for 5 min, the pellet was lysed in ice cold M-PER Mammalian Protein Extraction Reagent containing 1% Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific) for 30 min. The supernatant was collected after 10 min of centrifugation at 12000 rpm, equaled by spectrophotometry, denatured with sample loading buffer for 10 min at 95°C and stored at 4°C for future use. Proteins were separated by 10% SDS-polya-crylamide gels and transferred to PVDF membrane, and incubated with primary antibodies of interest at 4°C overnight. The appropriate horseradish-conjugated secondary antibody at a dilution of 1 : 10000 in blocking buffer (3% BSA-TBST) was added and incubated for 1 h at room temperature. All antibodies were diluted in 3% BSA-TBST. Antibodies were purchased from Abcam (BOP1; Santa Cruz for GAPDH, p53, PI3-Kinase beta85), Cell Signaling (caspase 3, cleaved caspase 3, caspase 9, cleaved caspase 9, caspase 7, cleaved caspase 7, caspase 8, cleaved caspase 8, PARP, Bcl2, Bcl-xl, BUB1b, Aurora A, PI3K p110a, Akt, p-Akt (Ser473), p-p70S6 Kinase (Thr389)) and BD biosciences (AIF).
Results
MPA has potent in vitro anticancer activity
Thirteen cancer cell lines were treated with serial dilutions of MPA (0.04 - 20 μg/ml) for 72 hours to evaluate MPA’s activity on cell proliferation (Figure 1A, 1B). Five cell lines (AGS, NCI-N87, HCT-8, A2780 and B x PC-3) are highly sensitive to MPA (IC50 < 0.5 μg/ml), four cell lines (Hs746T, PANC-1, HepG2 and MCF-7) are very resistant to MPA (IC50 > 20 μg/ml) and the four other cell lines (KATO III, SNU-1, K562 and HeLa) have intermediate sensitivity. As the gastric cancer cell line, AGS, is one of the most sensitive cell lines which can establish xenograft tumors in mice in our lab, it was selected for the subsequent studies.
Figure 1.
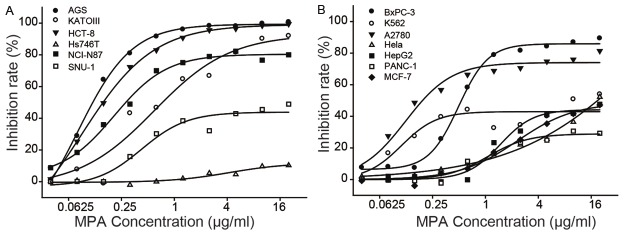
Anti-cancer activities of MPA. A and B: Inhibition rate of MPA on different cancer cell lines.
MPA inhibits cancer growth in vivo
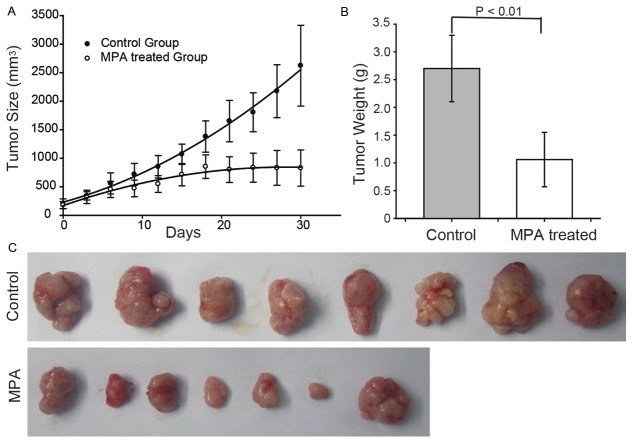
Based on the in vitro results, we evaluated the in vivo anti-tumor activity of MPA using the xenograft model with male BALB/c nude mice. As shown in Figure 2A, MPA significantly inhibited tumor growth in the first three weeks and completely halted tumor growth afterwards by MPA treatment (p = 5.5 × 10-5). Tumors were weighted after 30 days of MPA treatment and the average tumor weight was significantly lower in the treated group compared to vehicle control group (p = 6.2 × 10-4, Figure 2B, 2C).
Figure 2.
MPA inhibits tumor growth in Balb/c mice bearing AGS xenografts. A: Tumor size was measured once every three days for the MPA and vehicle treated groups. B: After 30 days of treatment, tumors were taken out and weighted. C: Images of tumors at the end of the experiment.
MPA induces cell cycle arrest
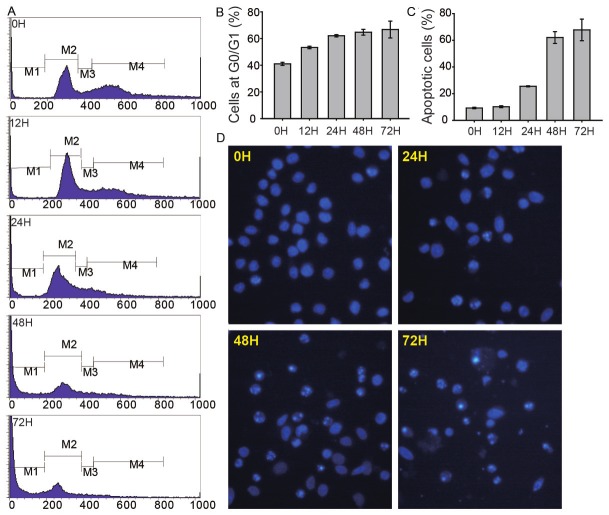
AGS cells at different time points of MPA treatment were stained with Propidium Iodide (PI) and analyzed by flow cytometry. As shown in Figure 3A, 3B, MPA treatment increased the percentages of cells in the G0/G1 phase starting at 12 h and in a time-dependent manner, suggesting that MPA treatment induces cell cycle arrest. The FACS data also indicated that MPA treatment significantly increased the percentage of apoptotic cells starting at the 24 h time point and in a time-dependent manner (Figure 3C). Treatment of AGS cells with MPA caused obvious morphological changes such as chromatin condensation and nuclear fragmentations as revealed by Hoechst staining (Figure 3D).
Figure 3.
MPA induces cell cycle arrest and apoptosis. AGS cells were treated with 2 μg/ml of MPA and stained with propidium iodide at different time point and analyzed by FACS. A: Representative FACS plots. B: Percentages of cells at the G0 phase (M2 gate). C: Percentage of apoptotic cells (M1 gate). D: Images of AGS cells stained with Hoechst dye after treatment with MPA for 0, 24, 48 and 72 hours. Apoptotic cells with chromatin condensation and nuclear fragmentations are indicated the bright staining.
The tumor suppressor protein p53 mediates cell cycle G1 phase arrest in response to a variety of stress stimuli. We confirmed by Western blot analysis that p53 protein expression is significantly increased after MPA treatment (Figure 4A). Several kinases implicated in cell cycle control are also significantly altered by MPA treatment. Western blot analysis revealed a dramatic reduction of the CDK4 and BUB1 protein after MPA treatment (Figure 4A). BUB1 and BUB1B are two related mitotic checkpoint serine/threonine-protein kinases, which are involved in spindle checkpoint function. Many cancers have impaired spindle checkpoint function. The Aurora A and Aurora B kinases play important roles in mitosis. Aurora A kinase regulates spindle assembly and stability, while Aurora B kinase regulates chromosome segregation and cytokinesis. The Aurora A protein (Figure 4A) was significantly down-regulated by MPA treatment.
Figure 4.
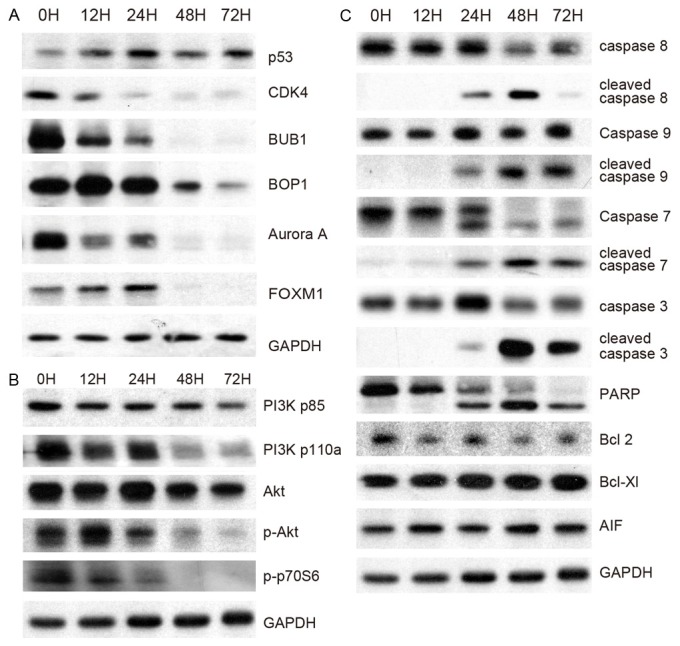
Western blotting analyses of key signaling proteins. A: Proteins involved in cell cycle regulation. B: Proteins involved in cell proliferation. C: Proteins involved in apoptosis.
FOXM1 is known to play a key role in cell cycle progression. Endogenous FOXM1 expression peaks at S and G2/M phases [17]. FOXM1 knockout mice are neonatal lethal as a result of the development of polyploidy cardiomyocytes and hepatocytes, highlighting the role of FOXM1 in mitotic division. FOXM1 regulates expression of a large array of G2/M-specific genes. It plays an important role in maintaining chromosomal segregation and genomic stability [18]. The FOXM1 protein is severely down-regulated (Figure 4A) by MPA treatment.
BOP1 is a ribosome biogenesis protein involved in rRNA processing, thereby controlling the cell cycle [19]. It is required for the maturation of the 25 S and 5.8 S ribosomal RNAs and it may serve as an essential factor in ribosome formation. The BOP1 complex is involved in ribosome biogenesis and altered chromosome segregation. The expression of BOP1 protein (Figure 4A) is significantly reduced by MPA treatment.
MPA inhibits cell proliferation via the PI3K-AKT-mTOR pathway
The PI3K-AKT-mTOR pathway, which is activated by receptor tyrosine kinases (RTK), plays a critical role in cell proliferation. Western blot analyses revealed that MPA treatment slightly decreased PI3K p85beta protein expression but drastically decreased phosphorylated PI3K p110a in a time-dependent manner (Figure 4B). The total AKT protein only showed slight change at later time points; however, phospho-Akt (Ser473) was severely decreased by MPA treatment (Figure 4B). Furthermore, the phosphop70S6 kinase (Thr 389), an mTOR component, was also dramatically down-regulated by MPA treatment. These results suggest that inhibition of the PI3K/AKT/mTOR pathway is a major mechanism through which MPA inhibits AGS cell proliferation. In many cancers, the PI3K/AKT/mTOR pathway is overactive, allowing proliferation of cancer cells [20-22]. A number of experimental anti-cancer drugs aim to inhibit this signaling pathway. Our results suggest that MPA treatment results in, most likely through an indirect mechanism, effective inhibition of the PI3K/AKT/mTOR pathway.
MPA induces caspase-dependent apoptosis
Next, we attempted to elucidate the apoptotic pathways induced by MPA treatment using Western blotting technique. Caspases are proteolytic enzymes that play critical roles in apoptosis. Caspase 8 can self-activate through proteolytic cleavage upon biding with FADD. Active caspase 8 is then released into the cytosol, where it cleaves other effector caspases, eventually leading to DNA degradation, membrane blebbing, and other hallmarks of apoptosis. Caspase 8 is highly activated in AGS cells after MPA treatment (Figure 4C), suggesting that the Fas-mediated apoptosis pathway is activated by MPA treatment. The mitochondrial apoptosis pathway is also activated by MPA treatment as indicated by the activation of another initiator caspase, caspase 9 (Figure 4C). As expected, two effector caspases, caspase 3 and caspase 7, are also highly activated in MPA-treated AGS cells. Poly (ADP-ribose) polymerase (PARP) is activated by caspase cleavage (Figure 4C). Activation of PARP expedites cellular disassembly by rapid catalysis of NAD+ and subsequent ATP depletion. It is also a robust indicator of cells undergoing apoptosis. These proteins are significantly altered after 24 h treatment time point, which is consistent with the apoptotic phenotype observed starting at the 24 h time point (Figure 3B). However, MPA treatment did not alter the protein expression of two anti-apoptotic members of the Bcl family (namely Bcl-2 and Bcl-xL) (Figure 4C).
There also exists a caspase-independent apoptotic pathway that is mediated by apoptosis-inducing factor (AIF) [23]. In our experiments, the expression of AIF was not altered by MPA treatment (Figure 4C), suggesting that the caspase-independent apoptosis pathway is not activated by MPA treatment.
Discussion
Our extensive functional studies indicate that MPA can exert potent antitumor activities including cell cycle arrest, inhibition of proliferation, and induction of apoptosis. MPA can inhibit cell proliferation in 9 of the 13 cell lines. Consistent with MPA’s in vitro functions, our study also demonstrated the in vivo anticancer activity of MPA in mice bearing AGS xenografts.
Although the anticancer activities of MPA are now well established, it is still unclear how this drug exerts its functions. In this study, we used proteomic and functional technologies to elucidate the molecular and biological pathways activated or inhibited by MPA. Our targeted proteomic studies allowed us to identify several key signaling pathways involved in the MPA regulatory network. For example, the phosphorylated PI3K, AKT and mTOR were dramatically reduced by MPA treatment.
MPA-induced cell cycle arrest already occurred at the 12 h time point, while alterations in cell proliferation and apoptosis only became apparent at the 24 h time point (Figure 3B). At the proteomic level, many key proteins involved in cell cycle arrest are also significantly altered at the 12 h time points (Figure 4A), while the proteins involved in proliferation (Figure 4B) and especially those involved in apoptosis are only altered at the 24 h and later time points. Therefore, both functional and molecular data suggest that arresting cell cycle may be the first and most important mechanism underlying MPA’s anticancer activity.
One of the main consequences of MPA treatment is the reduced expression or phosphorylation of a number of kinases such as PI3K, AKT and PKC that are key signaling nodes known to be implicated in multiple cellular functions including cell cycle, proliferation and apoptosis. Consistent with a previous report [15], p53, a key tumor suppressor molecule involved in cell cycle control and apoptosis, is up-regulated by MPA. Furthermore, a number of kinases involved in cell cycle regulation such as BUB1, BUB1B, AURKA, AURKB and BOP are drastically reduced by MPA. The changes in these key node proteins can account for the expression changes of a large number of down-stream effector molecules implicated in the observed biological functions. Our functional and molecular data suggest that the FDA-approved immunosuppressive drug MMF may be a good candidate that may be repurposed for anticancer therapy in certain human cancer patients.
Acknowledgements
JXS is partially supported by the Georgia Res-earch Alliance as an eminent scholar.
Disclosure of conflict of interest
The authors have declared that no competing interests exist.
References
- 1.Millan O, Oppenheimer F, Brunet M, Vilardell J, Rojo I, Vives J, Martorell J. Assessment of mycophenolic acid-induced immunosuppression: a new approach. Clin Chem. 2000;46:1376–1383. [PubMed] [Google Scholar]
- 2.Allison AC, Eugui EM. Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation. 2005;80:S181–190. doi: 10.1097/01.tp.0000186390.10150.66. [DOI] [PubMed] [Google Scholar]
- 3.Morath C, Schwenger V, Beimler J, Mehrabi A, Schmidt J, Zeier M, Muranyi W. Antifibrotic actions of mycophenolic acid. Clinical Transplantation. 2006;20(Suppl 17):25–29. doi: 10.1111/j.1399-0012.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 4.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 5.Jackson RC, Weber G, Morris HP. IMP dehydrogenase, an enzyme linked with proliferation and malignancy. Nature. 1975;256:331–333. doi: 10.1038/256331a0. [DOI] [PubMed] [Google Scholar]
- 6.Kozhevnikova EN, van der Knaap JA, Pindyurin AV, Ozgur Z, van Ijcken WF, Moshkin YM, Verrijzer CP. Metabolic enzyme IMPDH is also a transcription factor regulated by cellular state. Mol Cell. 2012;47:133–139. doi: 10.1016/j.molcel.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Fellenberg J, Bernd L, Delling G, Witte D, Zahlten-Hinguranage A. Prognostic significance of drug-regulated genes in high-grade osteosarcoma. Mod Pathol. 2007;20:1085–1094. doi: 10.1038/modpathol.3800937. [DOI] [PubMed] [Google Scholar]
- 8.Fellenberg J, Kunz P, Sahr H, Depeweg D. Overexpression of inosine 5’-monophosphate dehydrogenase type II mediates chemoresistance to human osteosarcoma cells. PLoS One. 2010;5:e12179. doi: 10.1371/journal.pone.0012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takebe N, Cheng X, Fandy TE, Srivastava RK, Wu S, Shankar S, Bauer K, Shaughnessy J, Tricot G. IMP dehydrogenase inhibitor mycophenolate mofetil induces caspase-dependent apoptosis and cell cycle inhibition in multiple myeloma cells. Mol Cancer Ther. 2006;5:457–466. doi: 10.1158/1535-7163.MCT-05-0340. [DOI] [PubMed] [Google Scholar]
- 10.Gortz A, Franklin TJ, Dive C, Hickman JA. Cell cycle specific induction of HL-60 cell differentiation and apoptosis by mycophenolic acid. Cell Death Differ. 1997;4:787–795. doi: 10.1038/sj.cdd.4400300. [DOI] [PubMed] [Google Scholar]
- 11.Vegso G, Sebestyen A, Paku S, Barna G, Hajdu M, Toth M, Jaray J, Kopper L. Antiproliferative and apoptotic effects of mycophenolic acid in human B-cell non-Hodgkin lymphomas. Leuk Res. 2007;31:1003–1008. doi: 10.1016/j.leukres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Silva SL, Silva SF, Cavalcante RO, Mota RS, Carvalho RA, Moraes MO, Campos HH, Moraes ME. Mycophenolate mofetil attenuates Walker’s tumor growth when used alone, but the effect is lost when associated with cyclosporine. Transplant Proc. 2004;36:1004–1006. doi: 10.1016/j.transproceed.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 13.Domhan S, Muschal S, Schwager C, Morath C, Wirkner U, Ansorge W, Maercker C, Zeier M, Huber PE, Abdollahi A. Molecular mechanisms of the antiangiogenic and antitumor effects of mycophenolic acid. Mol Cancer Ther. 2008;7:1656–1668. doi: 10.1158/1535-7163.MCT-08-0193. [DOI] [PubMed] [Google Scholar]
- 14.Tressler RJ, Garvin LJ, Slate DL. Anti-tumor activity of mycophenolate mofetil against human and mouse tumors in vivo. Int J Cancer. 1994;57:568–573. doi: 10.1002/ijc.2910570421. [DOI] [PubMed] [Google Scholar]
- 15.Sun XX, Dai MS, Lu H. Mycophenolic acid activation of p53 requires ribosomal proteins L5 and L11. J Biol Chem. 2008;283:12387–12392. doi: 10.1074/jbc.M801387200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engl T, Makarevic J, Relja B, Natsheh I, Muller I, Beecken WD, Jonas D, Blaheta RA. Mycophenolate mofetil modulates adhesion receptors of the beta1 integrin family on tumor cells: impact on tumor recurrence and malignancy. BMC Cancer. 2005;5:4. doi: 10.1186/1471-2407-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388:1257–1274. doi: 10.1515/BC.2007.159. [DOI] [PubMed] [Google Scholar]
- 18.Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–136. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Goo SY, Chung HJ, Yang HW, Yong TS, Lee KH, Park SJ. Interaction of beta-giardin with the Bop1 protein in Giardia lamblia. Parasitol Res. 2006;98:138–144. doi: 10.1007/s00436-005-0040-8. [DOI] [PubMed] [Google Scholar]
- 20.Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16:797–803. doi: 10.1097/01.cad.0000173476.67239.3b. [DOI] [PubMed] [Google Scholar]
- 21.Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Cortot A, Armand JP, Soria JC. [PI3K-AKT-mTOR pathway inhibitors] . Bull Cancer. 2006;93:19–26. [PubMed] [Google Scholar]
- 23.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]