Abstract
Objective: The aim of this study was to investigate the expression of Zonula Occludens-1 (ZO-1) and its potential value as prognostic indicator of survival in patients with primary non-small cell lung cancer (NSCLC). Methods: Quantitative real-time polymerase chain reaction (qRT-PCR) and immunohistochemistry with tissue microarrays were used to characterize the expression of the ZO-1 mRNA and protein in NSCLC. The correlation of ZO-1 expression with clinical characteristics and prognosis was determined by statistical analysis. Results: The ZO-1 mRNA and protein levels were significantly lower in NSCLC tissue compared with corresponding peritumoral tissue (P<0.05). ZO-1 protein expression in NSCLC was related to age (P=0.042) and 5-year survival (P<0.001). Kaplan-Meier survival and Cox regression analyses revealed that low ZO-1 expression (P<0.001) and later stage grouping by TNM (P=0.031) were independent factors predicting poor prognosis for patients with NSCLC. Conclusions: Our findings provide the first evidence that high expression of ZO-1 is associated with good prognosis in NSCLC.
Keywords: Non-small cell lung cancer, zonula occludens-1, immunohistochemistry, prognosis
Introduction
Lung cancer is one of the primary causes of cancer-related mortality world-wide [1], and more than 85% of lung cancer patients are diagnosed with non-small cell lung cancer (NSCLC) [2]. Patients with NSCLC have a poor prognosis with a 5-year survival rate of <10%, due to cancer metastasis and resistance to treatment [3,4]. Thus, improving patient cure rates is the main therapeutic goal in lung cancer management. During the past decade, there have been significant advances in the overall survival (OS) of patients with NSCLC. This may be attributed to the use of emerging new techniques and novel biologic agents (e.g, bevacizumab, erlotinib) in combination with conventional chemotherapy, as well as other agents targeting specific mutations [5,6]. Molecular medicine has changed the paradigm of many oncologic diseases, and the expression of specific genes has been associated with prognosis and/or predicted response to therapeutic agents. Analysis of the molecular phenotype of a patient’s tumor may facilitate the delivery of a “personalized” therapy, which may translate into increased survival advantage.
Tumorigenesis is a multi-step process involving several genes, including the activation of proto-oncogenes, mutation of tumor-suppressor genes and the inactivation of genes involved in apoptosis [7,8]. Recently, numerous studies have shown that the inactivation of tumor suppressor genes plays an important role in the onset and progression of lung cancer [9,10]. Alteration of tumor suppressor genes leads to the dysregulation of important cellular processes including cell cycle, apoptosis and signal transduction, leading to malignant transformation and tumorigenesis [11-13].
Previous studies have shown that tight junctions are a specialized type of intercellular contact and are directly engaged in paracellular sealing and membrane domain differentiation [14,15]. Metastasis is the major cause of lethality in cancer patients [16]. Tight junctions may prevent cell dissociation in epithelial cells through adhesive manner. However, these proteins give full scope to be a barrier by the passing of molecules and inflammatory in endothelial cells [17]. Thus, tight junctions represent the first barrier that cancer cells must overcome in order to metastasize [18]. The molecular organization of tight junctions implies an interaction between transmembrane proteins including occludin, members of the claudin family and tricellulin, and intracellular scaffold proteins such as Zonula Occludens-1 (ZO-1), ZO-2 and cingulin, which function as a bridge between tight junctions and the actin cytoskeleton [19]. The tight junction protein ZO-1, which is part of a multi-protein complex, binds actin and the integral tight junction proteins occludin and claudins [20]. Decreased ZO-1 expression has been shown to correlate with increased invasiveness in breast cancer [21], colorectal cancer [22] and cancers of the human digestive tract [23]. Furthermore, ZO-1 was reported to be involved in tumor invasion-associated epithelial-mesenchymal transition (EMT) processes [24]. Hence, ZO-1 may play an important role in processes underlying tumor growth and expression may be closely related to patient prognosis.
In this study, we explored the expression of ZO-1 in primary human NSCLC tumors compared with adjacent tissues and examined the correlation between ZO-1 levels and patient prognosis. We demonstrated the prognostic significance of ZO-1 protein expression in NSCLC and presented the potential value of this marker as a prognostic indicator of survival in patients with NSCLC.
Materials and methods
Patients and tissue microarray analysis (TMA)
Formalin-fixed, paraffin-embedded tumor tissues and corresponding tumor-adjacent specimens undergoing surgical therapy were obtained from 104 patients with NSCLC treated at the Affiliated Hospital of Nantong University between January 2005-December 2006. The average age of patients was 62.5 years (range, 35-81 years). Clinical data including sex, age, histological type, grade, stage, tumor size, differentiation, lymph node metastasis status and other information were obtained from individual patient medical records. The 5-year actuarial overall survival was calculated from the date of surgery until the date of death or last follow-up. Patients had not received neoadjuvant chemotherapy, radiation therapy or immunotherapy prior to surgery. Tumor staging was performed in accordance with the guidelines of the 7th edition of TNM staging in lung cancer [25]. An additional 20 freshly frozen NSCLC tissues and matching peritumoral tissues obtained from the Affiliated Hospital of Nantong University were also included in this study. This research was approved by the local Human Research Ethics Committee of the Affiliated Hospital of Nantong University, Nantong, China.
Tissue microarrays were manufactured by Shanghai Outdo Biotech (China). Representative cancer areas were labeled in paraffin blocks in accordance with hematoxylin and eosin staining (H&E) results. Tissue samples (2 mm-diameter) were obtained and sequentially aligned into prepared blank paraffin blocks. The quality of TMA sections was also confirmed using H&E staining.
Quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR was performed as previously described [26]. Total RNA was extracted from frozen samples using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed to cDNA using a Revert Aid™ First Strand cDNA synthesis kit (Fermentas, Glen Burnie, MD, USA) in accordance with the manufacturer’s instructions. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a normalization control. Real-time RT-PCR primers were as follows: ZO-1 forward, 5’-GTGTTGTGGATACCTTGT-3’ and reverse, 5’-GATGATGCCTCGTTCTAC-3’; GAPDH forward, 5’-TCGGAGTCAACGGATTTGGTCGT-3’ and reverse, 5’-TGCCATGGGTGGAATCATATTGGA-3’ (Sangon. Shanghai, China). Real-time PCR was performed using SyberGreen on an ABI 7500 thermal cycler (Applied Biosystems). The PCR conditions were as follows: UDG pre-treatment at 50°C for 2 min, 1 cycle; initial denaturation at 95°C for 10 min; denaturation at 95°C for 15 s, annealing and extension at 60°C for 60 s, 40 cycles. All experiments were performed in triplicate.
Immunohistochemical analysis
Immunohistochemistry (IHC) was performed as previously described [27]. In brief, sections (4 μm) were deparaffinized and rehydrated. Antigen retrieval was performed by boiling under pressure in citrate buffer (pH 6.0) for 3 min. Non-specific binding was blocked by incubation with 5% goat serum in PBS for 15 min. Tissues were then incubated with primary rabbit anti-ZO-1 antibody (1:300, ab59720; Abcam, Cambridge, MA) and subsequently with Envision goat anti-rabbit HRP secondary antibody (DAKO, Carpinteria, CA). All immunostaining was performed at the same time under the same conditions. Immunostained sections were evaluated by two trained pathologists under blinded experimental conditions.
The percentage of ZO-1-positive cells was scored as follows: 0 for 0-19%, 1 for 20-39%, 2 for 40-59% and 3 for 60-100%. ZO-1 staining intensity was also scored as follows: 0, 1, 2, or 3, from negative and weak to strong intensity. The cutoff point for a statistically significant ZO-1 expression score in terms of OS was set using the X-tile software program (The Rimm Lab at Yale University; http://www.tissuearray.org/rimmlab) [28]. The sum of the percentage and intensity scores was used as the final ZO-1 staining score and was defined as follows: 0-4, low expression, and 6-9, high expression.
Statistical methods
The Wilcoxon signed rank nonparametric test was used to compare the expression of ZO-1 mRNA in fresh-frozen NSCLC tissues compared with corresponding tumor-adjacent tissues. A chi-square statistics test was conducted to examine the association of clinicopathologic variables and ZO-1 protein expression. Kaplan-Meier and compared log-rank test were used to calculate the survival curves. Factors shown to be of prognostic significance in the univariate models were evaluated using a multivariate Cox regression model. P values less than 0.05 were considered statistically significant. All data were analyzed using STATA 9.0 software (Stata Corporation, College Station, TX).
Results
ZO-1 expression in NSCLC and corresponding tumor-adjacent tissues
The expression of ZO-1 mRNA was analyzed by qRT-PCR in tissue specimens obtained from 20 patients with NSCLC, including peritumoral tissues. ZO-1 transcript levels were significantly lower in cancer tissues compared with corresponding non-cancerous tissues (0.951±0.556 vs 1.752±1.094, 0.543-fold, p<0.05) (Figure 1).
Figure 1.
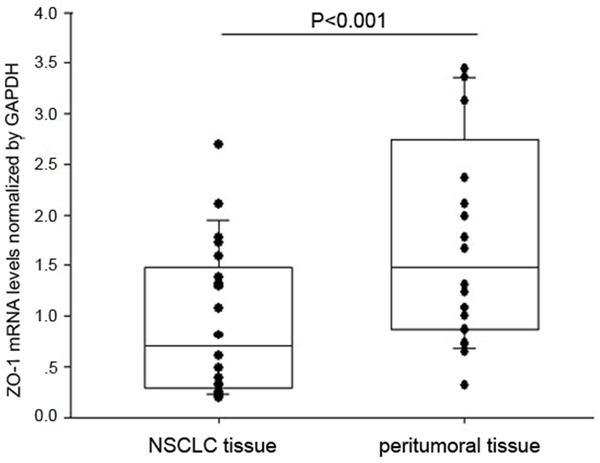
Expression of ZO-1 in NSCLC and adjacent noncancerous tissue. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed to detect the expression of ZO-1 mRNA in NSCLC compared with peritumoral tissue using GAPDH as a normalization control. ZO-1 mRNA levels were significantly lower in NSCLC compared with corresponding non-cancerous tissues (P<0.05). Bars indicate standard error (S.E.).
Expression of ZO-1 protein in NSCLC and peritumoral tissues
To further investigate the expression of ZO-1 protein in carcinoma and corresponding tumor-adjacent tissues, we performed immunohistochemical analysis on primary patient NSCLC specimens. As shown in Figure 2, ZO-1 was detected at various levels, primarily in the cytoplasm of cells. High ZO-1 expression was detected in 66.35% (69/104) of NSCLC samples compared with 83.51% (81/97) of adjacent matched tumor tissues. Thus, in accordance with ZO-1 mRNA analysis, we observed that ZO-1 protein expression was significantly lower in NSCLC tissue than in corresponding peritumoral tissue (χ2=7.8041, P=0.005).
Figure 2.
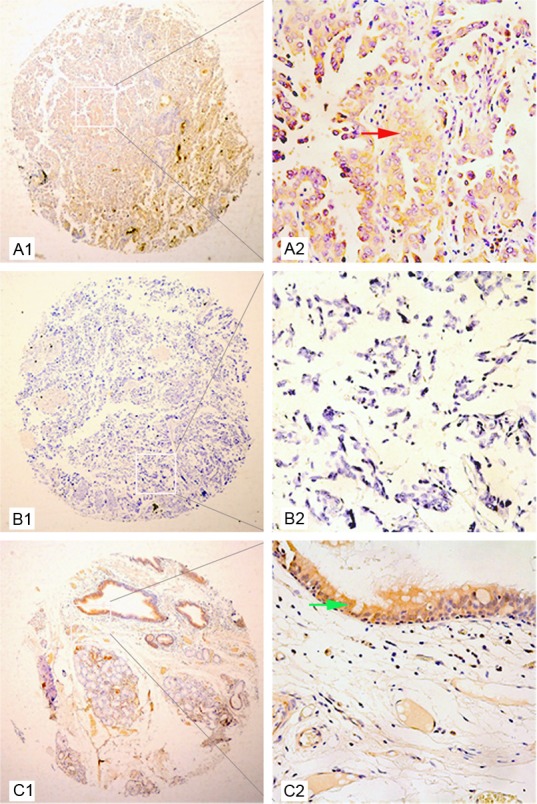
Representative patterns of ZO-1 protein expression in NSCLC and peritumoral tissue. A1 and A2: Well differentiated adenocarcinoma tissue and adjacent normal lung tissue. The expression of ZO-1 in tumor tissue was lower than that in adjacent normal lung tissue by IHC staining. The red arrow indicates positive ZO-1 protein expression in well differentiated adenocarcinoma. B1 and B2: Poorly differentiated carcinoma. The expression of ZO-1 protein was negative in poorly differentiated lung carcinoma tissues. C1 and C2: Peritumoral tissue. Strong staining of ZO-1 protein was observed in the adjacent normal lung tissue. The green arrow indicates strong ZO-1 staining in glandular epithelium of the bronchus.
Relationship between ZO-1 protein expression and clinical parameters
We next investigated the relationship between ZO-1 protein levels and patient clinicopathologic parameters (Table 1). High expression of ZO-1 in NSCLC tumors was significantly associated with age at diagnosis (χ2=4.151, P=0.042). However, we observed no significant association between ZO-1 levels and other clinical-pathological characteristics including gender, histology, TNM stage, tumor size and lymph node metastasis (Table 1).
Table 1.
Correlation of Zonula Occludens-1 (ZO-1) expression in tumor tissues with clinicopathologic characteristics in non-small cell lung cancer (NSCLC) patients
| NSCLC | n | Low or no expression | High expression | Pearson χ2 | P |
|---|---|---|---|---|---|
|
| |||||
| Total | 104 | 35 | 69 | ||
| Gender | 0.633 | 0.426 | |||
| male | 72 | 26 (36.11) | 46 (63.89) | ||
| female | 32 | 9 (28.13) | 23 (71.88) | ||
| Age at diagnosis (years) | 4.151 | 0.042* | |||
| ≤60 | 41 | 9 (21.95) | 32 (78.05) | ||
| >60 | 63 | 26 (41.27) | 37 (58.73) | ||
| Tumor diameter (cm) | 0.610 | 0.435 | |||
| ≤3 | 35 | 10 (28.57) | 25 (71.43) | ||
| >3 | 69 | 25 (36.23) | 44 (63.77) | ||
| Histological type | 0.003 | 0.957 | |||
| squamous cell carcinoma | 47 | 16 (34.04) | 31 (65.96) | ||
| adenocarcinoma | 55 | 19 (34.55) | 36 (65.45) | ||
| others | 2 | 0 | 2 | ||
| Differentiation | 0.926 | 0.629 | |||
| Well | 8 | 3 (37.50) | 5 (62.50) | ||
| Moderate | 66 | 24 (36.36) | 42 (63.64) | ||
| Poorly | 30 | 8 (26.67) | 22 (73.33) | ||
| Lymph node metastasis | 0.976 | 0.614 | |||
| No regional lymph node metastasis | 53 | 16 (30.19) | 37 (69.81) | ||
| Metastasis in ipsilateral peribronchial | 27 | 9 (33.33) | 18 (66.67) | ||
| Metastasis in mediastinal | 24 | 10 (41.67) | 14 (58.33) | ||
| Stage Grouping with TNM | 1.641 | 0.440 | |||
| Stage I | 50 | 14 (28.00) | 36 (72.00) | ||
| Stage II | 28 | 10 (35.71) | 18 (64.29) | ||
| Stage III | 26 | 11 (33.65) | 15 (66.35) | ||
P<0.05.
Low ZO-1 levels strongly correlate with poorly differentiated tumors and predict poor prognosis
Univariate cox regression analyses demonstrated that high ZO-1 expression was a significant prognostic factor for increased survival (P<0.001) (Table 2). The multivariate Cox proportional hazards regression model revealed that positive ZO-1 expression (P< 0.001), lymph node metastasis (P=0.025) and tumor stage (P=0.031) were the strongest predictors of survival (Table 2). Kaplan-Meier survival curves also demonstrated that patients with ZO-1 positive tumors exhibited prolonged overall and disease-free survival compared with those with ZO-1 negative tumors. This analysis also showed that early cancer stage was also correlated with improved survival (Figure 3).
Table 2.
Univariate and multivariate analysis of prognostic factors in NSCLC for 5-year overall survival
| Characteristic | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR | P | 95% CI | HR | P | 95% CI | |||
| ZO-1 expression | ||||||||
| High vs low | 0.188 | <0.001 | 0.109 | 0.326 | 0.281 | <0.001* | 0.169 | 0.466 |
| Gender | ||||||||
| Male vs Female | 1.302 | 0.329 | 0.766 | 2.212 | ||||
| Age (years) | ||||||||
| ≤60 vs >60 | 1.643 | 0.053 | 0.993 | 2.719 | ||||
| Diameter (cm) | ||||||||
| ≤3 vs >3 | 1.373 | 0.235 | 0.814 | 2.318 | ||||
| Histological type | ||||||||
| Sq vs Ad | .987 | 0.956 | 0.628 | 1.552 | ||||
| Differentiation | ||||||||
| Well vs Moderate vs Poorly | 1.364 | 0.154 | 0.890 | 2.092 | ||||
| Lymph node metastasis | ||||||||
| No metastasis vs metastasis | 1.201 | 0.200 | 0.906 | 1.604 | .070 | 0.025* | .007 | 0.712 |
| Stage | ||||||||
| I vs II vs III-IV | 1.312 | 0.060 | .989 | 1.740 | 13.065 | 0.031* | 1.265 | 134.6 |
P<0.05.
Figure 3.
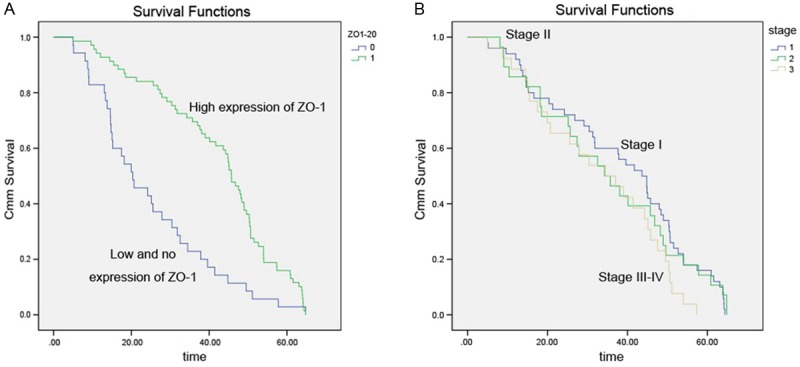
Kaplan-Meier survival curves following surgical therapy in NSCLC. A: Patients with low or no ZO-1 expression (blue line) exhibited significantly poorer survival compared with the high expression group (green line). B: Kaplan-Meier survival curves demonstrate that life spans of patients with stage III/IV tumors is significantly shorter than those of patients with stage II and stage I.
Discussion
NSCLC is the most common subtype of lung cancer and further studies are required to develop molecularly targeted therapies and improve the survival of patients. In present study, we investigated the expression of ZO-1 in 104 NSCLC specimens compared with tumor-adjacent tissue. We observed that both ZO-1 mRNA and protein levels were significantly lower in NSCLC tumors than in the corresponding tumor-adjacent tissues. Our study is the first to quantitatively analyze the expression of tight junction proteins in lung cancer, and demonstrates that ZO-1 expression may play an important role in the NSCLC tumorigenesis.
The expression pattern of ZO-1, which was predominantly localized in the cytoplasm of NSCLC cells is in accordance with previous reports [21,22]. Given the critical role of ZO-1 in tight junction assembly [29], it is possible that the entire tight junction may be lost with the loss of tumor differentiation. Indeed, our study indicates that loss of ZO-1 expression is associated with decreased tumor differentiation and with increased metastatic status. In highly differentiated adenocarcinoma specimens, ZO-1 expression is still observed in the cytoplasm of cancer cells, however in low differentiated adenocarcinoma, we observed loss of ZO-1 expression. These observations are supported by a murine study which demonstrated the absence of tight junctions in breast adenocarcinomas [21].
As expected, our clinical data demonstrated that low ZO-1 expression is associated with a poor prognosis in patients with NSCLC. Patients expressing high levels of ZO-1 exhibited a higher 5-year survival rate. These results are similar to previous studies in breast cancer [21,30] and colon cancer [22]. Several studies have described various relationships between ZO-1 expression and clinicopathologic parameters in different cancers. Studies by Martin et al [30] in a panel of 114 primary breast cancer tumors, confirmed that loss of tight junction plaque molecules in breast cancer tissues is associated with low survival rates. Kaihara et al [22] demonstrated that dedifferentiation and decreased expression of E-cadherin and ZO-1 are closely related to liver metastasis. Furthermore, in a study by Giri et al, down-regulation of ZO-1 expression in malignant endometrium was linked with disruption of cell-cell interactions leading to carcinogenesis and deep myometrial invasion [31]. Moreover, ZO-1 redistribution has been shown to be involved in the regulation of cell dissociation in pancreatic cancer cells [32,33]. Finally, phosphorylation leads to degradation of ZO-1 protein and a decrease in TER induced by patulin treatment of Caco-2 human colon cancer cells [34].
The integrity of intercellular contacts is crucial to epithelial homeostasis. During carcinogenesis, epithelial cells lose their characteristic epithelial features and become increasingly motile and invasive [35]. Previous investigations have shown that ZO-1 relocalizes from tight junctions to the nucleus during the EMT processes [24], and the domains of ZO-1 are sufficient to induce EMT-like changes in Madin-Darby canine kidney (MDCK) cells [36]. Studies by Hoover et al also demonstrate a significant correlation between reduced E-cadherin and decreased ZO-1 expression in breast cancer samples [21]. Given that several groups have shown that decreased E-cadherin is linked to lung cancer metastasis [37], we propose that the expression of ZO-1 in NSCLC may be relevant. This view is also supported in a review of tight junctions in lung cancer and lung metastasis [38].
Previous studies have shown that certain members of the protein kinase C (PKC) family play a role in regulating the disassembly of tight junctions and phosphorylating ZO proteins in vitro [39,40]. Loss of ZO-1 from intercellular adhesions led to an increase in α5β1 integrin, and disruption of ZO-1 prevented the polarized localization of ZO-1 and α5 at the leading edge by binding to an internal PDZ-binding motif in the α5 cytoplasmic tail. However, integrin α5 gene expression has previously been confirmed as a prognostic factor in node-negative NSCLC [41]. Additionally, activation of PKCε has also been shown to correlate with increased invasion and poor prognosis in lung cancer [40]. Previous studies have shown that ZO-1 up-regulates HER-2/neu expression in vitro by sequestering a repressor of the HER-2/neu gene promoter [42], which also mediated signaling and apical junction formation involving ZO-1 in human endometrial Ishikawa cells [31]. Thus, ZO-1 may be involved in multiple regulatory pathways, facilitating a better understanding of its role in lung cancer.
In this study, we focused on the relationship between ZO-1 protein expression and clinical characteristics in NSCLC using tumor tissue arrays and statistical analyses. However, this study is limited to clinical observations, and further in vitro and in vivo murine tumor studies are required to investigate the role of ZO-1 in NSCLC tumor biology.
Taken together, these findings demonstrate that ZO-1 may act as a tumor suppressor gene in human cancer. In accordance with this, we show that high ZO-1 protein expression was associated with increased survival of NSCLC patients. Interestingly, ZO-1 expression was also associated with patient age, a finding that has not been reported in previous studies. It might concern to the features of NSCLC and physiology characteristic of elderly patients. Moreover, this study also revealed that high ZO-1 expression in NSCLC tissue and early stages of cancer are independent prognostic biomarkers for patients with NSCLC. Tumor staging has previously been confirmed as a prognostic factor in lung cancer in clinical settings [25,43].
In summary, we conclude that ZO-1 may be used as a prognostic factor in NSCLC. ZO-1 may also represent a novel therapeutic target in patients with NSCLC. Further studies are required to investigate the biological functions of ZO-1 in NSCLC.
Acknowledgements
This research is supported in part by grants from the Social Development and Applied Research Projects (K2010048 and K2010054), Nantong, China.
Disclosure of conflict of interest
None.
References
- 1.Lin J, Beer DG. Molecular predictors of prognosis in lung cancer. Ann Surg Oncol. 2012;19:669–676. doi: 10.1245/s10434-011-1967-x. [DOI] [PubMed] [Google Scholar]
- 2.Reckamp KL. Antiangiogenic agents as second-line therapy for advanced non-small cell lung cancer. Cancer Lett. 2012;321:101–109. doi: 10.1016/j.canlet.2012.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Yang P. Epidemiology of lung cancer prognosis: quantity and quality of life. Methods Mol Biol. 2009;471:469–486. doi: 10.1007/978-1-59745-416-2_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih JY, Yang PC. The EMT regulator slug and lung carcinogenesis. Carcinogenesis. 2011;32:1299–1304. doi: 10.1093/carcin/bgr110. [DOI] [PubMed] [Google Scholar]
- 5.Berghmans T, Pasleau F, Paesmans M, Bonduelle Y, Cadranel J, Cs Toth I, Garcia C, Giner V, Holbrechts S, Lafitte JJ, Lecomte J, Louviaux I, Markiewicz E, Meert AP, Richez M, Roelandts M, Scherpereel A, Tulippe C, Van Houtte P, Van Schil P, Wachters C, Westeel V, Sculier JP. Surrogate markers predicting overall survival for lung cancer: ELCWP recommendations. Eur Respir J. 2012;39:9–28. doi: 10.1183/09031936.00190310. [DOI] [PubMed] [Google Scholar]
- 6.Sudhindra A, Ochoa R, Santos ES. Biomarkers, prediction, and prognosis in non-small-cell lung cancer: a platform for personalized treatment. Clin Lung Cancer. 2011;12:360–368. doi: 10.1016/j.cllc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Yoo NJ, Park SW, Lee SH. Mutational analysis of tumour suppressor gene NF2 in common solid cancers and acute leukaemias. Pathology. 2012;44:29–32. doi: 10.1097/PAT.0b013e32834c3599. [DOI] [PubMed] [Google Scholar]
- 8.Prager GW, Poettler M. Angiogenesis in cancer. Basic mechanisms and therapeutic advances. Hamostaseologie. 2012;32:105–114. doi: 10.5482/ha-1163. [DOI] [PubMed] [Google Scholar]
- 9.Ma C, Wei S, Song Y. T790M and acquired resistance of EGFR TKI: a literature review of clinical reports. J Thorac Dis. 2011;3:10–18. doi: 10.3978/j.issn.2072-1439.2010.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Li Y, Ling X, Liu L, Liu B, Xu K, Bin X, Ji W, Lu J. A common genetic variant (97906C>A) of DAB2IP/AIP1 is associated with an increased risk and early onset of lung cancer in Chinese males. PLoS One. 2011;6:e26944. doi: 10.1371/journal.pone.0026944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pustisek N, Situm M. UV-radiation, apoptosis and skin. Coll Antropol. 2011;35(Suppl 2):339–341. [PubMed] [Google Scholar]
- 12.Naguib A, Cooke JC, Happerfield L, Kerr L, Gay LJ, Luben RN, Ball RY, Mitrou PN, McTaggart A, Arends MJ. Alterations in PTEN and PIK3CA in colorectal cancers in the EPIC Norfolk study: associations with clinicopathological and dietary factors. BMC Cancer. 2011;11:123. doi: 10.1186/1471-2407-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcel V, Dichtel-Danjoy ML, Sagne C, Hafsi H, Ma D, Ortiz-Cuaran S, Olivier M, Hall J, Mollereau B, Hainaut P, Bourdon JC. Biological functions of p53 isoforms through evolution: lessons from animal and cellular models. Cell Death Differ. 2011;18:1815–1824. doi: 10.1038/cdd.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 15.Jiang WG, Martin TA, Matsumoto K, Nakamura T, Mansel RE. Hepatocyte growth factor/scatter factor decreases the expression of occludin and transendothelial resistance (TER) and increases paracellular permeability in human vascular endothelial cells. J Cell Physiol. 1999;181:319–329. doi: 10.1002/(SICI)1097-4652(199911)181:2<319::AID-JCP14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742–1757. doi: 10.1016/S0140-6736(07)60781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollande F, Blanc EM, Bali JP, Whitehead RH, Pelegrin A, Baldwin GS, Choquet A. HGF regulates tight junctions in new nontumorigenic gastric epithelial cell line. Am J Physiol Gastrointest Liver Physiol. 2001;280:G910–921. doi: 10.1152/ajpgi.2001.280.5.G910. [DOI] [PubMed] [Google Scholar]
- 18.Martin TA, Mansel RE, Jiang WG. Antagonistic effect of NK4 on HGF/SF induced changes in the transendothelial resistance (TER) and paracellular permeability of human vascular endothelial cells. J Cell Physiol. 2002;192:268–275. doi: 10.1002/jcp.10133. [DOI] [PubMed] [Google Scholar]
- 19.Balda MS, Matter K. Tight junctions at a glance. J Cell Sci. 2008;121:3677–3682. doi: 10.1242/jcs.023887. [DOI] [PubMed] [Google Scholar]
- 20.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoover KB, Liao SY, Bryant PJ. Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am J Pathol. 1998;153:1767–1773. doi: 10.1016/S0002-9440(10)65691-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaihara T, Kusaka T, Nishi M, Kawamata H, Imura J, Kitajima K, Itoh-Minami R, Aoyama N, Kasuga M, Oda Y, Hattori M, Fujimori T. Dedifferentiation and decreased expression of adhesion molecules, E-cadherin and ZO-1, in colorectal cancer are closely related to liver metastasis. J Exp Clin Cancer Res. 2003;22:117–123. [PubMed] [Google Scholar]
- 23.Kimura Y, Shiozaki H, Hirao M, Maeno Y, Doki Y, Inoue M, Monden T, Ando-Akatsuka Y, Furuse M, Tsukita S, Monden M. Expression of occludin, tight-junction-associated protein, in human digestive tract. Am J Pathol. 1997;151:45–54. [PMC free article] [PubMed] [Google Scholar]
- 24.Polette M, Mestdagt M, Bindels S, Nawrocki-Raby B, Hunziker W, Foidart JM, Birembaut P, Gilles C. Beta-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs. 2007;185:61–65. doi: 10.1159/000101304. [DOI] [PubMed] [Google Scholar]
- 25.Goldstraw P. The 7th Edition of TNM in Lung Cancer: what now? J Thorac Oncol. 2009;4:671–673. doi: 10.1097/JTO.0b013e31819e7814. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Zhu H, Wang X, Tang Q, Huang H, Wu K, Zhu J, Feng Z, Shi G. The patterns and expression of KDR in normal tissues of human internal organs. J Mol Histol. 2011;42:597–603. doi: 10.1007/s10735-011-9355-1. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Zhang X, Tang Q, Zhang F, Li Y, Feng Z, Zhu J. Prognostic significance and potential therapeutic target of VEGFR2 in hepatocellular carcinoma. J Clin Pathol. 2011;64:343–348. doi: 10.1136/jcp.2010.085142. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Zhang J, Li H, Lu Z, Shan W, Mercado-Uribe I, Liu J. VCAM1 expression correlated with tumorigenesis and poor prognosis in high grade serous ovarian cancer. Am J Transl Res. 2013;5:336–346. [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 30.Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur J Cancer. 2004;40:2717–2725. doi: 10.1016/j.ejca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Giri S, Poindexter KM, Sundar SN, Firestone GL. Arecoline induced disruption of expression and localization of the tight junctional protein ZO-1 is dependent on the HER 2 expression in human endometrial Ishikawa cells. BMC Cell Biol. 2010;11:53. doi: 10.1186/1471-2121-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan X, Egami H, Ishikawa S, Kurizaki T, Hirota M, Ogawa M. Zonula occludens-1 (ZO-1) redistribution is involved in the regulation of cell dissociation in pancreatic cancer cells. Dig Dis Sci. 2005;50:1402–1409. doi: 10.1007/s10620-005-2853-9. [DOI] [PubMed] [Google Scholar]
- 33.Kleeff J, Shi X, Bode HP, Hoover K, Shrikhande S, Bryant PJ, Korc M, Buchler MW, Friess H. Altered expression and localization of the tight junction protein ZO-1 in primary and metastatic pancreatic cancer. Pancreas. 2001;23:259–265. doi: 10.1097/00006676-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Kawauchiya T, Takumi R, Kudo Y, Takamori A, Sasagawa T, Takahashi K, Kikuchi H. Correlation between the destruction of tight junction by patulin treatment and increase of phosphorylation of ZO-1 in Caco-2 human colon cancer cells. Toxicol Lett. 2011;205:196–202. doi: 10.1016/j.toxlet.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Berx G, Raspe E, Christofori G, Thiery JP, Sleeman JP. Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clin Exp Metastasis. 2007;24:587–597. doi: 10.1007/s10585-007-9114-6. [DOI] [PubMed] [Google Scholar]
- 36.Reichert M, Muller T, Hunziker W. The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells. Evidence for a role of beta-catenin/Tcf/Lef signaling. J Biol Chem. 2000;275:9492–9500. doi: 10.1074/jbc.275.13.9492. [DOI] [PubMed] [Google Scholar]
- 37.Wang G, Dong W, Shen H, Mu X, Li Z, Lin X, Liu Y, Du J. A comparison of Twist and E-cadherin protein expression in primary non-small-cell lung carcinoma and corresponding metastases. Eur J Cardiothorac Surg. 2011;39:1028–1032. doi: 10.1016/j.ejcts.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Soini Y. Tight junctions in lung cancer and lung metastasis: a review. Int J Clin Exp Pathol. 2012;5:126–136. [PMC free article] [PubMed] [Google Scholar]
- 39.Basu A, Sivaprasad U. Protein kinase Cepsilon makes the life and death decision. Cell Signal. 2007;19:1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuomi S, Mai A, Nevo J, Laine JO, Vilkki V, Ohman TJ, Gahmberg CG, Parker PJ, Ivaska J. PKCepsilon regulation of an alpha5 integrin-ZO-1 complex controls lamellae formation in migrating cancer cells. Sci Signal. 2009;2:ra32. doi: 10.1126/scisignal.2000135. [DOI] [PubMed] [Google Scholar]
- 41.Adachi M, Taki T, Higashiyama M, Kohno N, Inufusa H, Miyake M. Significance of integrin alpha5 gene expression as a prognostic factor in node-negative non-small cell lung cancer. Clin Cancer Res. 2000;6:96–101. [PubMed] [Google Scholar]
- 42.Bell J, Walsh S, Nusrat A, Cohen C. Zonula occludens-1 and Her-2/neu expression in invasive breast carcinoma. Appl Immunohistochem Mol Morphol. 2003;11:125–129. doi: 10.1097/00129039-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Cagle PT, Allen TC, Dacic S, Beasley MB, Borczuk AC, Chirieac LR, Laucirica R, Ro JY, Kerr KM. Revolution in lung cancer: new challenges for the surgical pathologist. Arch Pathol Lab Med. 2011;135:110–116. doi: 10.5858/2010-0567-RA.1. [DOI] [PubMed] [Google Scholar]


