Abstract
Survivin is a protein that is highly expressed in many embryonic tissues, as well as most human tumors. Prior studies have reported both positive and negative correlations between survivin expression and cancer prognosis, but these associations remain controversial. In the present study, we assessed the expression of nuclear and cytoplasmic survivin in gastrointestinal carcinomas. Using these data, we determined the correlation between nuclear and cytoplasmic survivin and, further, investigated correlations between survivin expression and clinicopathological parameters. Seventy-two advanced gastric adenocarcinomas and 78 colorectal adenocarcinomas were analyzed for survivin expression by immunohistochemistry. Expression of both nuclear and cytoplasmic survivin was significantly higher in colorectal carcinomas than in gastric carcinomas (P < 0.01). There was a positive correlation between nuclear and cytoplasmic expression of survivin (r = 0.42, P < 0.001). In gastric carcinomas, the level of survivin protein expression was associated with tumor differentiation, patient age, and lymphatic invasion (P < 0.05, 0.01, and 0.01, respectively). In colorectal carcinomas, the level of nuclear survivin expression was significantly higher in females than in males (P < 0.05). There were no significant associations between survivin expression and most of the clinicopathological parameters. Nevertheless, there was a trend towards an inverse correlation between nuclear survivin expression and tumor aggressiveness in gastric carcinoma; there was a similar trend for cytoplasmic survivin expression. In summary, our results suggest that levels of nuclear and cytoplasmic survivin expression differ between gastric carcinoma and colorectal carcinoma.
Keywords: Survivin, immunohistochemistry, gastrointestinal carcinoma, apoptosis
Introduction
Survivin, a member of the inhibitor of apoptosis protein (IAP) family, has a dual cellular function as an inhibitor of apoptosis and as a regulator of mitosis. Its anti-apoptotic function is related to its inhibition of caspase activity by directly or indirectly interfering with the function of the caspase-3, caspase-7, and caspase-9 [1-4]. In addition to its anti-apoptotic function, survivin functions as a chromosome passenger protein, regulating the G2 and M phases of the cell cycle. The chromosomal passenger complex consists of Aurora B kinase, INCENP, survivin, and Borealin [5-8].
Survivin is highly expressed in many embryonic tissues, as well as most human tumors of the lung, colon, breast, stomach, liver, ovary, and prostate. In contrast, it is either undetectable or expressed at a very low level in differentiated adult tissues [9-19]. Therefore, it has been suggested that survivin could be a marker for tumor progression and prognosis [20-32]. Furthermore, given its functional properties, survivin has been proposed as a molecular target for anti-cancer therapies [12,33-37]. However, the molecular mechanisms regulating survivin remain poorly understood.
Survivin localizes to the nucleus and cytoplasm of cancer cells. Several studies have proposed that the subcellular distribution of survivin is regulated by active import into the nucleus and CRM1-mediated export to the cytoplasm [7,37,38]. It has also been suggested that survivin could be a nuclear shuttling protein. Recent studies have suggested that the nuclear pool of survivin is involved in promoting cell proliferation, whereas the cytoplasmic pool of survivin controls cell survival [7,28,37].
Previous studies have reported both positive and negative correlations between survivin expression and cancer prognosis, but these results remain controversial (Tables 1 and 2). Several reports have suggested that nuclear expression of survivin is a prognostic marker. Some reports have demonstrated an association between nuclear expression of survivin and unfavorable outcomes in patients with gastric and colorectal carcinomas [23]. Conversely, other reports have demonstrated that nuclear expression of survivin is positively correlated with favorable prognoses in gastric and colorectal carcinomas [22,28]. Finally, other reports have demonstrated that cytoplasmic survivin expression is correlated with better, worse, or unchanged prognoses in patients with gastric and colorectal carcinomas [20,27-29,33].
Table 1.
Summary of previous reports on survivin expression in human gastric carcinomas
| Author/Year (ref. No), sample type/method |
|---|
| Lu CD./1998 [32], Surgical resection/Immunohistochemistry (IHC) |
| Cytoplasmic survivin was prominently expressed in tumors of the intestinal histological type without invasion. None of the prognostic parameters analyzed (tumor stage, tumor depth, and presence of lymph node metastasis) in cytoplasmic survivin-positive samples reached statistical significance. |
| Okada E./2001 [22], Surgical resection/IHC |
| Survivin nuclear staining was associated with a favorable prognosis. Survivin cytoplasmic positivity did not correlate with factors related to progression or prognosis. |
| Wakana Y./2002 [25], mRNA/real-time PCR |
| Survivin mRNA was significantly higher in the diffuse type than in the intestinal type. No significant relationship was found between survivin mRNA expression and depth of wall invasion, lymph-node metastasis, lymph invasion, or vein invasion. |
| Miyachi K./2003 [30], mRNA/real-time PCR |
| No significant differences were found in survivin mRNA expression based on histological classification or depth of tumor invasion. However, survivin mRNA expression was significantly higher (P < 0.01) in patients with lymph-node metastasis than in those without lymph-node metastasis. These results indicate that survivin mRNA expression increases in the early stage of carcinogenesis. Moreover, the level of survivin mRNA expression may indicate the potential for lymph-node metastasis. |
| Vallböhmer D./2009 [33], Endoscopic biopsy/IHC |
| A high level of cytoplasmic survivin expression was associated with a significant survival benefit. |
| Song KY./2009 [23], Tissue microarray/IHC |
| Nuclear survivin expression was frequently observed in large-sized tumors and was an important prognostic indicator of poor outcomes in patients with stage III gastric carcinoma. |
Table 2.
Summary of previous reports on survivin expression in human colorectal carcinomas
| Author/Year (ref. No), sample type/method |
|---|
| Ponnelle T./2005 [27], Surgical resection/Immunohistochemistry (IHC) |
| Cytoplasmic survivin expression was a significant prognostic factor. A high level of survivin expression was associated with a higher rate of survival. |
| Qi G./2009 [28], Surgical resection/IHC |
| Cytoplasmic survivin overexpression was associated with a poor prognosis, but nuclear survivin overexpression was associated with a better prognosis. |
| Fang YJ./2009 [21], Tissue microarray/IHC |
| Elevated expression of survivin was associated with lower survival rates. |
| Lee YY./2009 [29], Tissue microarray/IHC |
| A higher cytoplasmic survivin immunostaining score was associated with higher mortality in patients with colorectal cancer. |
| Xiayuan C./2010 [20], Surgical resection/IHC |
| Staining for survivin protein was strongly positive in the cytoplasm of colorectal carcinoma cells. Its expression was significantly correlated with tumor differentiation, Duke’s stage, and lymph-node metastasis. |
To date, no study has investigated the relationship between expression of nuclear survivin and cytoplasmic survivin. Therefore, the present study evaluated expression of nuclear and cytoplasmic survivin in gastrointestinal carcinomas in order to determine their correlation and, furthermore, to identify the relationship between survivin expression and clinicopathological parameters. Our results indicate that gastric carcinomas and colorectal carcinomas differ in levels of expression of nuclear and cytoplasmic survivin.
Materials and methods
Tissue samples
Seventy-two advanced gastric adenocarcinomas (36 well-to-moderately differentiated and 36 poorly differentiated) and 78 colorectal adenocarcinomas (68 well-to-moderately differentiated and 10 poorly differentiated) were analyzed for survivin expression by immunohistochemistry. Surgically resected tumor tissues were collected from the archives of the Department of Diagnostic Pathology of the Osaka Red Cross Hospital and the Kobe Central Hospital of Social Insurance. The study was approved by the local ethics committee. The tumors were classified according to the TNM classification of malignant tumors (TNM 2009) [39]. All specimens were preserved in 10% formalin and embedded in paraffin. Three-micrometer-thick sections were cut consecutively and mounted on aminopropyltriethoxysilane-coated slides.
Immunohistochemical staining
The sections were deparaffinized with xylene and rehydrated with a graduated series of ethanol solutions. Endogenous peroxidase was blocked by incubating the sections in 0.03% hydrogen peroxide in methanol for 30 min. Antigen retrieval was performed by immersing slides in 0.1 M citrate buffer (pH 7.0) and heating for 10 minutes in a pressure cooker (T-FAL; Rumily, France). After heating, the sections were cooled at room temperature in the soaking solution for 30 min. The sections were washed under running tap water followed by 0.01 M phosphate buffered saline (PBS; pH 7.2), and then incubated with a rabbit polyclonal antibody against survivin (1:1,000 dilution, R&D Systems Minneapolis, MN, USA) overnight at room temperature. The sections were then rinsed with PBS. To detect survivin, the sections were incubated with the Histofine Simple Stain MAX-PO (Nichirei, Tokyo, Japan) for 1 h at room temperature. The reaction products were developed with diaminobenzidine and counterstained with Mayer’s hematoxylin. As a negative control, a section was treated as described above, but with the primary antibody replaced by buffer.
Immunostaining evaluation
Sections were considered positive for survivin in the presence of nuclear or cytoplasmic staining. The mean percentage of positive tumor cells was determined using the average of at least five areas at 400× magnification. Samples with ≥ 30% of tumor cells positive for nuclear staining and ≥ 15% of tumor cells positive for cytoplasmic staining were considered to have positive survivin protein expression. These cutoff values of 30% and 15% for nuclear and cytoplasmic staining, respectively, were based on the median observed values. The survivin-stained sections were examined by two independent researchers blinded to other pathological information.
Statistical analysis
The chi-square test and Fisher’s exact test were used to determine statistical differences in marker expression between gastric adenocarcinomas and colorectal adenocarcinomas, well-to-moderately differentiated and poorly-differentiated adenocarcinomas, patient age and gender, and lymphatic invasion and vascular invasion. Correlations between marker expression and tumor location, depth of invasion, lymph node metastasis, or pathological stage were analyzed using the Kruskal-Wallis test. Spearman rank correlation was calculated to assess the correlation between nuclear survivin expression and cytoplasmic survivin expression. P-values of < 0.05 were considered statistically significant.
Results
The clinicopathological parameters of 72 gastric carcinomas and 78 colorectal carcinomas are reported in Table 3.
Table 3.
Clinicopathological parameters of 72 gastric carcinomas and 78 colorectal carcinomas
| Stomach | Colorectum | |
|---|---|---|
| Age (years) | ||
| < 60 | 10 | 22 |
| ≥ 60 | 62 | 56 |
| Gender | ||
| Male | 51 | 44 |
| Female | 21 | 34 |
| Histological differentiation | ||
| well to moderately differentiated | 36 | 68 |
| poorly differentiated | 36 | 10 |
| Location | ||
| Cardia / Right colon | 17 | 24 |
| Fundus / Left colon | 31 | 24 |
| Antrum / Rectum | 24 | 30 |
| Depth of invasion | ||
| pT2 | 17 | 7 |
| pT3 | 20 | 46 |
| pT4 | 35 | 25 |
| Lymph node metastasis | ||
| pN0 | 23 | 35 |
| pN1 | 15 | 31 |
| pN2 | 11 | 12 |
| pN3 | 23 | |
| Lymphatic invasion | ||
| Ly (-) | 16 | 24 |
| Ly (+) | 56 | 54 |
| Vascular invasion | ||
| V (-) | 38 | 40 |
| V (+) | 34 | 38 |
| UICC p-Stage | ||
| IB | 11 | 6 |
| IIA and IIB | 22 | 28 |
| IIIA, IIIB and IIIC | 39 | 44 |
Expression of survivin in gastric carcinoma
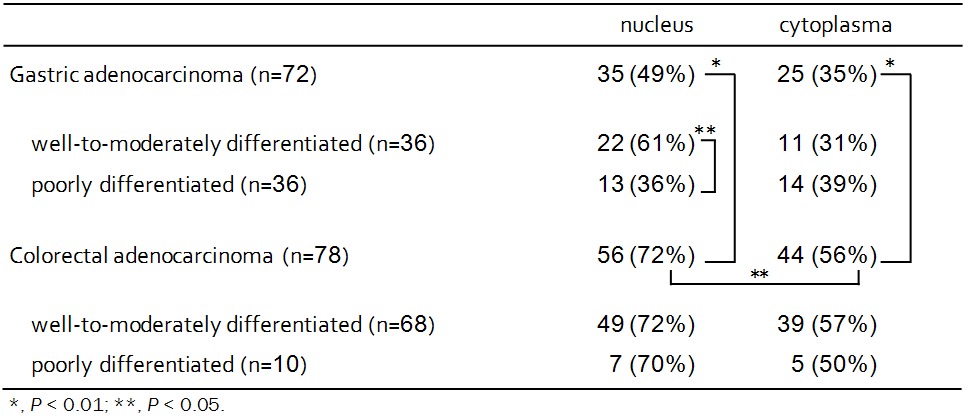
Survivin expression was observed in the nucleus and/or cytoplasm (Figure 1). Survivin-positive nuclear staining was observed in 49% (35/72) of gastric carcinomas, and cytoplasmic survivin expression was detected in 35% (25/72) of gastric carcinomas. Nuclear survivin expression in well-to-moderately differentiated samples (61%) was significantly higher than in poorly differentiated samples (36%; P < 0.05) (Table 4). Cytoplasmic survivin was detected in 31% (11/36) of well-to-moderately differentiated samples. There was a positive correlation between nuclear and cytoplasmic expression of survivin (r = 0.42, P < 0.001).
Figure 1.
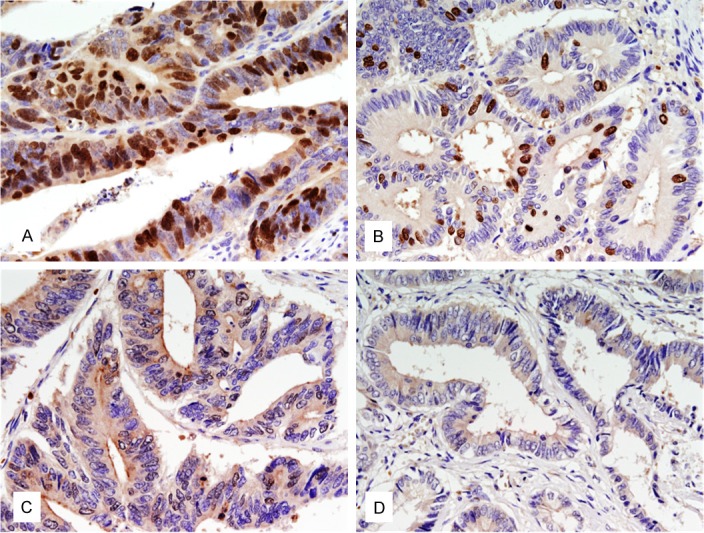
Immunohistochemical staining of survivin in paraffin-embedded tissues. A: Nuclear and cytoplasmic immunostaining of survivin in colonic carcinoma. B: Nuclear immunostaining of survivin in gastric carcinoma. C: Cytoplasmic immunostaining of survivin in colonic carcinoma. D: Nuclear and cytoplasmic negative immunostaining of survivin in rectal carcinoma.
Table 4.
Expression of survivin in gastrointestinal carcinomas
|
Expression of survivin in colorectal carcinoma
Survivin-positive nuclear staining was observed in 72% (56/78) of colorectal carcinomas, and cytoplasmic survivin expression was detected in 56% (44/78) of colorectal carcinomas. Expression of nuclear and cytoplasmic survivin was significantly higher in colorectal carcinomas than in gastric carcinomas (P < 0.01). Nuclear survivin expression was significantly higher than cytoplasmic survivin expression (P < 0.05). In contrast to gastric carcinomas, there was no relationship between nuclear survivin expression and cytoplasmic survivin expression in colorectal carcinomas.
Correlation between survivin expression and clinicopathological parameters
A clinicopathological analysis of the survivin-positive samples is shown in Figure 2. In gastric carcinomas, the level of survivin protein expression was associated with patient age, and lymphatic invasion (P < 0.01, and 0.01, respectively). None of the other parameters (patient gender, tumor location, depth of invasion, lymph-node metastasis, vascular invasion, or pathological stage) was associated with positive survivin expression.
Figure 2.
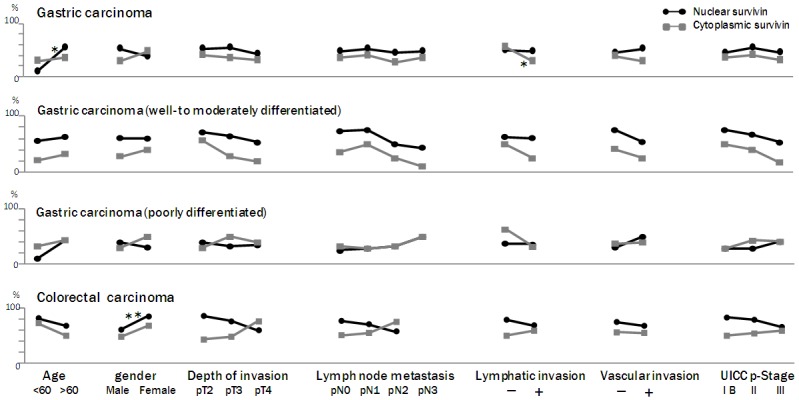
Correlation between survivin expression and clinicopathological parameters in gastric and colorectal carcinomas. In gastric carcinomas, the level of survivin protein expression was associated with patient age, and lymphatic invasion (P < 0.01, and 0.01, respectively). None of the other parameters (patient gender, tumor location, depth of invasion, lymph-node metastasis, vascular invasion, or pathological stage) was associated with positive survivin expression. In colorectal carcinomas, the level of nuclear survivin expression was significantly higher in females than in males (P < 0.05). None of the other parameters (patient age, tumor location, depth of invasion, lymph-node metastasis, lymphatic invasion, vascular invasion, or pathological stage) was associated with positive survivin expression. *, P < 0.01; **, P < 0.05.
In colorectal carcinomas, the level of nuclear survivin expression was significantly higher in females than in males (P < 0.05). None of the other parameters (patient age, tumor location, depth of invasion, lymph-node metastasis, lymphatic invasion, vascular invasion, or pathological stage) was associated with positive survivin expression. Although there were no significant differences between most of the clinicopathological parameters and survivin expression, there was a trend toward an association between decreased nuclear survivin expression and tumor aggressiveness in gastric carcinoma, with cytoplasmic survivin expression exhibiting a similar trend. In contrast, in colorectal carcinomas, cytoplasmic survivin expression increased - equaling or surpassing nuclear survivin expression - with increasing tumor aggressiveness. These data indicate that gastric carcinomas and colorectal carcinomas differ in their patterns of nuclear and cytoplasmic survivin expression.
Discussion
In this study, we used immunohistochemistry to investigate subcellular localization of survivin protein in gastric and colorectal carcinomas. Our data indicate that expression of both nuclear and cytoplasmic survivin was significantly higher in colorectal carcinomas (nuclear survivin, 72%; cytoplasmic survivin, 56%) than in gastric carcinomas (nuclear survivin, 49%; cytoplasmic survivin, 35%) (P < 0.01). Kawasaki et al. reported a higher incidence of cytoplasmic survivin expression in colorectal carcinomas than in gastric carcinomas (53.2% versus 34.5%) [26]. To our knowledge, ours is the first study comparing both nuclear and cytoplasmic survivin expression between gastric carcinomas and colorectal carcinomas. Furthermore, our results indicate that nuclear survivin expression is significantly higher than cytoplasmic survivin expression (P < 0.05) in colorectal carcinomas. This finding is consistent with the report from Qi et al., which suggested that survivin is more highly localized in the nucleus (78%; 109 of 142) than the cytoplasm (20%; 29 of 142) of colorectal carcinomas [28]. Together, these results suggest that the survivin protein is mainly localized in the nucleus rather than in the cytoplasm of colorectal carcinomas.
We next focused on the correlation between survivin expression and tumor differentiation. Among gastric carcinomas, nuclear survivin expression was significantly higher in well-to-moderately differentiated samples (61%) than in poorly differentiated samples (36%) (P < 0.05). However, there was no significant difference in cytoplasmic survivin expression between the well-to-moderately differentiated samples (31%) and the poorly differentiated samples (39%). Wakana et al. reported that cytoplasmic survivin expression was significantly higher in diffuse-type cases than in intestinal-type cases of gastric carcinomas [25]. However, Lu et al. used immunohistochemistry to demonstrate that cytoplasmic survivin is more highly localized in the intestinal-type gastric carcinomas than in the diffuse-type gastric carcinomas (P < 0.05) [32]. Miyachi et al. reported that survivin mRNA expression was independent of the histological type of gastric cancer [30]. Wakana et al. demonstrated that survivin mRNA expression was significantly higher in diffuse-type gastric carcinomas than in intestinal-type gastric carcinoma [25]. Our results demonstrate that nuclear survivin protein expression is higher in well-to-moderately differentiated samples than in poorly differentiated samples, and cytoplasmic survivin expression differed from nuclear survivin expression in gastric carcinomas. The discrepancies between the studies are likely due to analyzing survivin mRNA versus survivin protein. Wakana et al. suggested that these discrepancies might be due to differences in tumor cell volume in the tissue specimens or differences in the detection method [25].
We next investigated the relationship between nuclear survivin and cytoplasmic survivin expression. A positive correlation was observed between nuclear and cytoplasmic survivin expression (r = 0.42, P < 0.001) in gastric carcinomas; in contrast, no such relationship was observed in colorectal carcinomas. These results indicate that regulation of survivin expression is likely different between gastric carcinomas and colorectal carcinomas.
Finally, we investigated whether there was a correlation between nuclear or cytoplasmic survivin expression and clinicopathological parameters. In gastric carcinomas, survivin expression was correlated with tumor differentiation, patient age, and lymphatic invasion (P < 0.05, 0.01, and 0.01, respectively). Similarly, Lu et al. reported that cytoplasmic survivin was prominent in gastric carcinomas without lymphatic invasion [32]. In addition, we showed that nuclear survivin expression in colorectal carcinomas was significantly higher in females than in males (P < 0.05). None of the other parameters was associated with the expression of survivin. Nuclear and cytoplasmic survivin expression tended to decrease with increasing tumor aggressiveness in gastric carcinomas, but this effect was not statistically significant. Similarly, there was an inverse relationship between nuclear survivin expression and cytoplasmic survivin expression in colorectal carcinomas, which did not reach statistical significance.
There are several previous reports on the relationship between prognosis and expression of survivin mRNA or cytoplasmic survivin. Vallböhmer et al. reported that a high level of cytoplasmic survivin expression was associated with significantly higher rates of survival in patients with gastric carcinoma [33]. Similarly, Okada et al. reported that survivin nuclear staining was associated with a favorable prognosis [22]. Ponnelle et al. reported that high levels of survivin expression were associated with increased survival in patients with colorectal carcinomas [27]. On the other hand, Lee et al. reported that a higher cytoplasmic survivin immunostaining score was associated with higher mortality in patients with colorectal carcinoma [29]. Fang et al. reported that elevated expression of survivin was associated with lower rates of survival [21]. Qi et al. reported that higher cytoplasmic survivin expression was associated with a poor prognosis, while higher nuclear survivin expression was associated with a better prognosis [28]. Taken together, these reports indicate that nuclear and cytoplasmic survivin expression is associated with a better prognosis in gastric carcinomas. Conversely, upregulation of cytoplasmic survivin is associated with poor prognosis in colorectal carcinomas.
Survivin localizes to both the nucleus and cytoplasm. Nuclear survivin is thought to promote cell proliferation, and cytoplasmic survivin is thought to have cytoprotective activity [7,28,37]. Tu et al. reported that suppression of survivin expression or function in gastric carcinoma led to abnormal morphology, with decreased cell growth and increased rates of spontaneous apoptosis and mitotic catastrophe [24].
In the present study, we found that survivin expression was higher in both the nucleus and cytoplasm of colorectal carcinomas than in gastric carcinomas. Further, we identified a positive correlation between nuclear survivin and cytoplasmic survivin expression in gastric carcinomas but not in colorectal carcinomas. Our previous report suggested that different cell-death pathways are activated in gastric and colorectal carcinomas [40]. The extrinsic and intrinsic apoptotic pathways could be mutually regulated in gastric adenocarcinomas. In contrast, in colorectal carcinomas, autophagy might function as a cellular guardian to avoid caspase 9-dependent apoptosis. The present study suggests that survivin’s role in inhibiting apoptosis may be more prominent in colorectal carcinomas than in gastric carcinomas.
Based on our results, we conclude that gastric carcinomas and colorectal carcinomas differ in their levels of nuclear and cytoplasmic survivin expression. Although the role of survivin in tumor progression remains unknown, our results suggest that an inhibitor of survivin might be appropriate for individualized treatment of patients with gastric and colorectal cancer.
Acknowledgements
The authors thank Dr. Masayuki Shintaku and Dr. Toshihiko Miyake for their kind support. This study was supported by a Grant-in-Aid for Scientific Research (No. 23590396) from the Japan Society for the Promotion of Science.
Disclosure of conflict of interest
None.
References
- 1.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 2.Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene. 2003;22:8568–80. doi: 10.1038/sj.onc.1207101. [DOI] [PubMed] [Google Scholar]
- 3.Li F, Ackermann EJ, Bennett CF, Rothermel AL, Plescia J, Tognin S, Villa A, Marchisio PC, Altieri DC. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat Cell Biol. 1999;1:461–6. doi: 10.1038/70242. [DOI] [PubMed] [Google Scholar]
- 4.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 5.Li F. Survivin study: what is the next wave? J Cell Physiol. 2003;197:8–29. doi: 10.1002/jcp.10327. [DOI] [PubMed] [Google Scholar]
- 6.Li F, Ling X. Survivin study: an update of “what is the next wave”? J Cell Physiol. 2006;208:476–86. doi: 10.1002/jcp.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knauer SK, Mann W, Stauber RH. Survivin’s dual role: an export’s view. Cell Cycle. 2007;6:518–21. doi: 10.4161/cc.6.5.3902. [DOI] [PubMed] [Google Scholar]
- 8.Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006;244:164–71. doi: 10.1016/j.canlet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Andersen MH, Svane IM, Becker JC, Straten PT. The universal character of the tumor-associated antigen survivin. Clin Cancer Res. 2007;13:5991–4. doi: 10.1158/1078-0432.CCR-07-0686. [DOI] [PubMed] [Google Scholar]
- 10.Chen P, Li J, Ge LP, Dai CH, Li XQ. Prognostic value of survivin, X-linked inhibitor of apoptosis protein and second mitochondria-derived activator of caspases expression in advanced non-small-cell lung cancer patients. Respirology. 2010;15:501–9. doi: 10.1111/j.1440-1843.2010.01710.x. [DOI] [PubMed] [Google Scholar]
- 11.Choi J, Chang H. The expression of MAGE and SSX, and correlation of COX2, VEGF, and survivin in colorectal cancer. Anticancer Res. 2012;32:559–64. [PubMed] [Google Scholar]
- 12.Miura K, Fujibuchi W, Ishida K, Naitoh T, Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, Shibata C, Sasaki I. Inhibitor of apoptosis protein family as diagnostic markers and therapeutic targets of colorectal cancer. Surg Today. 2011;41:175–82. doi: 10.1007/s00595-010-4390-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang TT, Qian XP, Liu BR. Survivin: potential role in diagnosis, prognosis and targeted therapy of gastric cancer. World J Gastroenterol. 2007;13:2784–90. doi: 10.3748/wjg.v13.i20.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youssef NS, Hewedi IH, Abd Raboh NM. Immunohistochemical expression of survivin in breast carcinoma: relationship with clinicopathological parameters, proliferation and molecular classification. J Egypt Natl Canc Inst. 2008;20:348–57. [PubMed] [Google Scholar]
- 15.Sohn DM, Kim SY, Baek MJ, Lim CW, Lee MH, Cho MS, Kim TY. Expression of survivin and clinical correlation in patients with breast cancer. Biomed Pharmacother. 2006;60:289–92. doi: 10.1016/j.biopha.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Nassar A, Sexton D, Cotsonis G, Cohen C. Survivin expression in breast carcinoma: correlation with apoptosis and prognosis. Appl Immunohistochem Mol Morphol. 2008;16:221–6. doi: 10.1097/PAI.0b013e3180c317bc. [DOI] [PubMed] [Google Scholar]
- 17.Kim JY, Chung JY, Lee SG, Kim YJ, Park JE, Yoo KS, Yoo YH, Park YC, Kim BG, Kim JM. Nuclear interaction of Smac/DIABLO with Survivin at G2/M arrest prompts docetaxel-induced apoptosis in DU145 prostate cancer cells. Biochem Biophys Res Commun. 2006;350:949–54. doi: 10.1016/j.bbrc.2006.09.143. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M, Nakano T, Suzuki A. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080–5. doi: 10.1053/he.2000.6496. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura H, Torigoe T, Hirohashi Y, Asanuma H, Inoue R, Nishida S, Tanaka T, Masumori N, Sato N, Tsukamoto T. Nuclear, but not cytoplasmic, localization of survivin as a negative prognostic factor for survival in upper urinary tract urothelial carcinoma. Virchows Arch. 2013;462:101–7. doi: 10.1007/s00428-012-1343-7. [DOI] [PubMed] [Google Scholar]
- 20.Xiaoyuan C, Longbang C, Jinghua W, Xiaoxiang G, Huaicheng G, Qun Z, Haizhu S. Survivin: a potential prognostic marker and chemoradiotherapeutic target for colorectal cancer. Ir J Med Sci. 2010;179:327–35. doi: 10.1007/s11845-009-0448-8. [DOI] [PubMed] [Google Scholar]
- 21.Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW, Yun JP, Zhang MF, Wan DS. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis. 2009;24:875–84. doi: 10.1007/s00384-009-0725-z. [DOI] [PubMed] [Google Scholar]
- 22.Okada E, Murai Y, Matsui K, Isizawa S, Cheng C, Masuda M, Takano Y. Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer Lett. 2001;163:109–16. doi: 10.1016/s0304-3835(00)00677-7. [DOI] [PubMed] [Google Scholar]
- 23.Song KY, Jung CK, Park WS, Park CH. Expression of the antiapoptosis gene Survivin predicts poor prognosis of stage III gastric adenocarcinoma. Jpn J Clin Oncol. 2009;39:290–6. doi: 10.1093/jjco/hyp020. [DOI] [PubMed] [Google Scholar]
- 24.Tu SP, Jiang XH, Lin MC, Cui JT, Yang Y, Lum CT, Zou B, Zhu YB, Jiang SH, Wong WM, Chan AO, Yuen MF, Lam SK, Kung HF, Wong BC. Suppression of survivin expression inhibits in vivo tumorigenicity and angiogenesis in gastric cancer. Cancer Res. 2003;63:7724–32. [PubMed] [Google Scholar]
- 25.Wakana Y, Kasuya K, Katayanagi S, Tsuchida A, Aoki T, Koyanagi Y, Ishii H, Ebihara Y. Effect of survivin on cell proliferation and apoptosis in gastric cancer. Oncol Rep. 2002;9:1213–8. [PubMed] [Google Scholar]
- 26.Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071–4. [PubMed] [Google Scholar]
- 27.Ponnelle T, Chapusot C, Martin L, Bouvier AM, Plenchette S, Faivre J, Solary E, Piard F. Cellular localisation of survivin: impact on the prognosis in colorectal cancer. J Cancer Res Clin Oncol. 2005;131:504–10. doi: 10.1007/s00432-005-0682-z. [DOI] [PubMed] [Google Scholar]
- 28.Qi G, Tuncel H, Aoki E, Tanaka S, Oka S, Kaneko I, Okamoto M, Tatsuka M, Nakai S, Shimamoto F. Intracellular localization of survivin determines biological behavior in colorectal cancer. Oncol Rep. 2009;22:557–62. doi: 10.3892/or_00000471. [DOI] [PubMed] [Google Scholar]
- 29.Lee YY, Yu CP, Lin CK, Nieh S, Hsu KF, Chiang H, Jin JS. Expression of survivin and cortactin in colorectal adenocarcinoma: association with clinicopathological parameters. Dis Markers. 2009;26:9–18. doi: 10.3233/DMA-2009-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyachi K, Sasaki K, Onodera S, Taguchi T, Nagamachi M, Kaneko H, Sunagawa M. Correlation between survivin mRNA expression and lymph node metastasis in gastric cancer. Gastric Cancer. 2003;6:217–24. doi: 10.1007/s10120-003-0255-2. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Yang J, Ramnath N, Javle MM, Tan D. Nuclear or cytoplasmic expression of survivin: what is the significance? Int J Cancer. 2005;114:509–12. doi: 10.1002/ijc.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998;58:1808–12. [PubMed] [Google Scholar]
- 33.Vallböhmer D, Drebber U, Schneider PM, Baldus S, Bollschweiler E, Brabender J, Warnecke-Eberz U, Mönig S, Hölscher AH, Metzger R. Survivin expression in gastric cancer: Association with histomorphological response to neoadjuvant therapy and prognosis. J Surg Oncol. 2009;99:409–13. doi: 10.1002/jso.21271. [DOI] [PubMed] [Google Scholar]
- 34.Iwasa T, Okamoto I, Suzuki M, Nakahara T, Yamanaka K, Hatashita E, Yamada Y, Fukuoka M, Ono K, Nakagawa K. Radiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell lines. Clin Cancer Res. 2008;14:6496–504. doi: 10.1158/1078-0432.CCR-08-0468. [DOI] [PubMed] [Google Scholar]
- 35.Yao C, Liu J, Shao L. Rapamycin inhibits the proliferation and apoptosis of gastric cancer cells by down regulating the expression of survivin. Hepatogastroenterology. 2011;58:1075–80. [PubMed] [Google Scholar]
- 36.Shen X, Zheng JY, Shi H, Zhang Z, Wang WZ. Survivin knockdown enhances gastric cancer cell sensitivity to radiation and chemotherapy in vitro and in nude mice. Am J Med Sci. 2012;344:52–8. doi: 10.1097/MAJ.0b013e318239c4ee. [DOI] [PubMed] [Google Scholar]
- 37.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67:5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 38.Knauer SK, Krämer OH, Knösel T, Engels K, Rödel F, Kovács AF, Dietmaier W, Klein-Hitpass L, Habtemichael N, Schweitzer A, Brieger J, Rödel C, Mann W, Petersen I, Heinzel T, Stauber RH. Nuclear export is essential for the tumor-promoting activity of survivin. FASEB J. 2007;21:207–16. doi: 10.1096/fj.06-5741com. [DOI] [PubMed] [Google Scholar]
- 39.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th edition. New Jersey: Wiley-Blackwell; 2009. [Google Scholar]
- 40.Shintani M, Sangawa A, Yamao N, Miyake T, Kamoshida S. Immunohistochemical analysis of cell death pathways in gastrointestinal adenocarcinoma. Biomed Res. 2011;32:379–86. doi: 10.2220/biomedres.32.379. [DOI] [PubMed] [Google Scholar]


