Extraocular sebaceous carcinoma is a relatively rare skin appendage carcinoma [1]. Although ocular sebaceous carcinoma is thought to arise from the Meibomian glands and glands of Zeis, there is no evidence that extraocular sebaceous carcinoma arises from pre-existing sebaceous glands [2]. Most sebaceous carcinomas are invasive [1], however, only a few cases of sebaceous carcinoma associated with intraepidermal squamous neoplasia (actinic keratosis or Bowen’s disease) have been reported [3-8]. These cases suggest that extraocular sebaceous carcinoma can originate from pre-existing intraepidermal squamous neoplasia [8]. Herein, we report an additional case of sebaceous carcinoma associated with Bowen’s disease and discuss the pathogenesis of sebaceous carcinoma.
A 67-year-old Japanese female presented with a left buttock tumor, which had been first detected approximately 1 year earlier, and had recently gradually enlarged. Physical examination revealed a relatively well-circumscribed skin-colored tumor with hyperkeratosis, measuring 43 x 38 mm in diameter, in her left buttock. The biopsy specimen revealed Bowen’s disease (squamous cell carcinoma in situ), and subsequently, total resection of the tumor was performed.
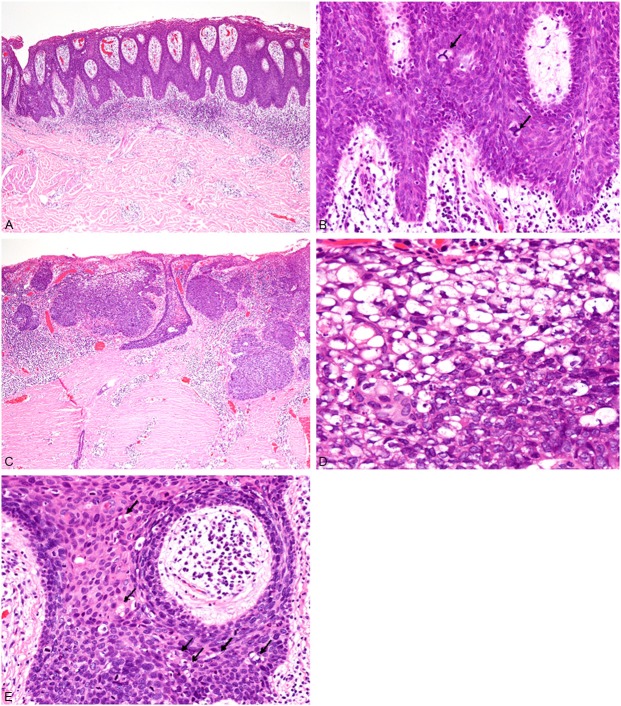
Histopathological study of the resected specimen revealed proliferation of atypical squamous cells in the entire layer of the acanthotic epidermis accompanied by hyperparakeratosis (Figure 1A). These atypical squamous cells had enlarged and hyperchromatic nuclei (Figure 1B), and multinucleated atypical squamous cells were also present. Mitotic figures were scattered and present in the upper portion of the epidermis. Atypical mitotic figures were also observed (Figure 1B). No invasive neoplastic growth was noted (Figure 1A). Approximately 95% of the lesion was composed of the above-mentioned Bowen’s disease (squamous cell carcinoma in situ), and superficial sebaceous carcinoma was present in the central area of the lesion. This latter component was composed of proliferation of nests of atypical cells with vacuolated clear cytoplasm and large nuclei containing conspicuous nucleoli (Figure 1C, 1D). Sebaceous carcinoma had invaded into the superficial reticular dermis (Figure 1C). Moreover, atypical cells containing vacuolated cytoplasm were present within the lesion of Bowen’s disease adjacent to the sebaceous carcinoma (Figure 1E).
Figure 1.
Histopathological features of the buttock tumor. A: Bowen’s disease component. Proliferation of atypical squamous cells in the entire layer of acanthotic epidermis accompanied by hyperparakeratosis. HE, x 40. B: These atypical squamous cells have large and hyperchromatic nuclei, and atypical mitotic figures are observed (arrows). HE, x 200. C: Sebaceous carcinoma component. Nests of atypical cells with clear cytoplasm have invaded into the superficial reticular dermis. HE, x 40. D: The neoplastic cells have vacuolated clear cytoplasm and large nuclei containing conspicuous nucleoli. HE, x 400. E: Some atypical cells containing vacuolated cytoplasm are present within Bowen’s disease. HE, x 200.
Immunohistochemical studies were performed using an autostainer (Ventana) by the same method as previously reported [9-12]. Epithelial membrane antigen (EMA), cytokeratin 7, and adipophilin were expressed in the sebaceous carcinoma component, but not in Bowen’s disease (Figure 2A). Androgen receptor was also expressed in some of the tumor cells of the sebaceous carcinoma, but not in Bowen’s disease. Moreover, atypical vacuolated cells within the lesion of Bowen’s disease were also positive for adipophilin (Figure 2B). Overexpression of p53 protein was observed in both the sebaceous carcinoma and Bowen’s disease.
Figure 2.
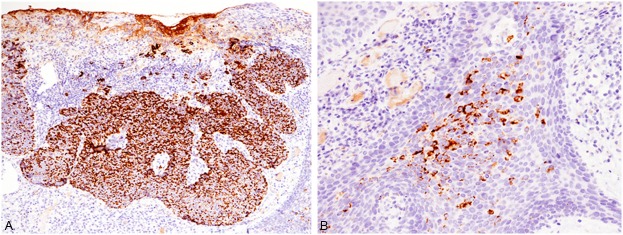
Immunohistochemical features of the buttock tumor. A: Adipophilin is diffusely expressed in the sebaceous carcinoma component. x 100. B: Adipophilin-positive atypical cells are present within the lesion of Bowen’s disease. x 200.
Accordingly, an ultimate diagnosis of sebaceous carcinoma associated with Bowen’s disease was made.
In this report, we describe an extremely rare case of sebaceous carcinoma associated with Bowen’s disease. The peculiar findings of the present case were the presence of Bowen’s disease, which comprised approximately 95% of the tumor, around the sebaceous carcinoma and atypical cells with vacuolated cytoplasm showing positive immunoreactivity for adipophilin within the lesion of Bowen’s disease. Only a limited number of sebaceous carcinoma cases associated with intraepidermal squamous neoplasia (actinic keratosis or Bowen’s disease) have been documented [3-8]. These cases included sebaceous carcinoma in situ in continuity with intraepidermal squamous neoplasia without association to pre-existing sebaceous glands [3,5,8] and invasive sebaceous carcinoma associated with intraepidermal squamous neoplasia, as seen in the present case [4-8]. Nakashima et al. reported an interesting case of invasive sebaceous carcinoma in continuity with actinic keratosis [6]. In their case, there were intraepidermal atypical cells expressing EMA and adipophilin, which were also observed in the present case. These adipophilin-positive intraepidermal atypical cells with vacuolated cytoplasm may represent the tumor cells with sebaceous differentiation. Therefore, they speculated that a close relationship between actinic keratosis and sebaceous carcinoma exists [6].
Moreover, only a limited number of sebaceous carcinoma in situ cases without any association to intraepidermal squamous neoplasia have been reported [2,13]. To the best of our knowledge, there has been no evidence that sebaceous carcinoma arises from pre-existing sebaceous glands [2]. Therefore, these results suggest that extraocular sebaceous carcinoma may originate de novo from intraepidermal pluripotential cells or pre-existing intraepidermal squamous neoplasia.
Disclosure of conflict of interest
None.
References
- 1.Rütten A, Wick MR, Sangüeza OP, Wallace C. Tumours with sebaceous differentiation. In: LeBoit PE, Burg G, Weedon D, Sarasain A, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Skin Tumours. Lyon: IARC Press; 2006. pp. 160–163. [Google Scholar]
- 2.Kazakov DV, Kutzner H, Spagnolo DV, Rütten A, Mukensnabl P, Michal M. What is extraocular cutaneous sebaceous carcinoma in situ? Am J Dermatopathol. 2010;32:857–8. doi: 10.1097/DAD.0b013e3181e1d1e8. [DOI] [PubMed] [Google Scholar]
- 3.Fulling KH, Strayer DS, Santa Cruz DJ. Adnexal metaplasia in carcinoma in situ of the skin. J Cutan Pathol. 1981;8:79–88. doi: 10.1111/j.1600-0560.1981.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 4.Escalonilla P, Grilli R, Canamero M, Soriano ML, Farina MC, Manzarbeitia F, Sainz R, Matuskura T, Requena L. Sebaceous carcinoma of the vulva. Am J Dermatopathol. 1999;21:468–472. doi: 10.1097/00000372-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Ansai S, Mihara I. Sebaceous carcinoma arising on actinic keratosis. Eur J Dermatol. 2000;10:385–388. [PubMed] [Google Scholar]
- 6.Nakashima K, Adachi K, Yamasaki A, Yamada N, Yoshida Y, Yamamoto O. Sebaceous carcinoma with actinic keratosis. Acta Derm Venereol. 2010;90:196–198. doi: 10.2340/00015555-0761. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs DM, Sandles LG, LeBoit PE. Sebaceous carcinoma arising from Bowen’s disease of the vulva. Arch Dermatol. 1986;122:1191–1193. [PubMed] [Google Scholar]
- 8.Ishida M, Okabe H. Intraepidermal sebacoues carcinoma occuring concurrently with actinic keratosis. J Cutan Pathol. 2012;39:731–2. doi: 10.1111/j.1600-0560.2012.01881.x. [DOI] [PubMed] [Google Scholar]
- 9.Ishida M, Fukami T, Nitta N, Iwai M, Yoshida K, Kagotani A, Nozaki K, Okabe H. Xanthomatous meningioma: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:2242–2246. [PMC free article] [PubMed] [Google Scholar]
- 10.Ishida M, Hodohara K, Yoshii M, Okuno H, Nakanishi R, Horinouchi A, Nakanishi R, Harada A, Iwai M, Yoshida K, Kagotani A, Yoshida T, Okabe H. Methotrexate-related Epstein-Barr virus-associated lymphoproliferative disorder occurring in the gingiva of a patient with rheumatoid arthritis. Int J Clin Exp Pathol. 2013;6:2237–2241. [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida M, Kodama N, Takemura Y, Iwai M, Yoshida K, Kagotani A, Matsusue Y, Okabe H. Primary bone carcinosarcoma of the fibula with chondrosarcoma and squamous cell carcinoma components. Int J Clin Exp Pathol. 2013;6:2216–2223. [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida M, Iwai M, Yoshida K, Kagotani A, Okabe H. Metastatic Crohn’s disease accompanying granulomatous vasculitis and lymphangitis in the vulva. Int J Clin Exp Pathol. 2013;6:2263–2266. [PMC free article] [PubMed] [Google Scholar]
- 13.Oka K, Katsumata M. Intraepidermal sebaceous carcinoma: case report. Dermatologica. 1990;180:181–5. doi: 10.1159/000248026. [DOI] [PubMed] [Google Scholar]