Abstract
High-density cell culture is widely used for the analysis of cartilage development of human mesenchymal stem cells (HMSCs) in vitro. Several cell culture systems, as micromass, pellet culture and alginate culture, are applied by groups in the field to induce chondrogenic differentiation of HMSCs. A draw back of all model systems is the high amount of cells necessary for the experiments. Further, handling of large experimental approaches is difficult due to culturing e.g. in 15 ml tubes. Therefore, we aimed to develop a new model system based on “hanging drop” cultures using 10 to 100 fold less cells. Here, we demonstrate that differentiation of chondrogenic cells was induced as previously shown in other model systems. Real time RT-PCR analysis demonstrated that Collagen type II and MIA/CD-RAP were upregulated during culturing whereas for induction of hypertrophic markers like Collagen type X and AP-2 epsilon treatment with TGF beta was needed. To further test the system, siRNA against Sox9 was used and effects on chondrogenic gene expression were evaluated. In summary, the hanging drop culture system was determined to be a promising tool for in vitro chondrogenic studies.
Keywords: Chondrogenic differentiation, hanging drop, human mesenchymal stem cells, collagen
Introduction
Model systems to analyze cartilage development in vitro are widely used. With these, detailed analysis of molecular processes during differentiation of mesenchymal stem cells (MSC) is feasible. Here, cells can be manipulated e.g. using expression constructs or siRNA. These aspects made these models indispensable tools for studying chondrocyte differentiation in great detail in addition to in vivo studies. Several model systems are used to investigate chondrogenesis in vitro following the literature, e.g. micromass culture [1,2], alginate culture [3,4] or pellet culture [5,6]. All models are based on high-density cell culture preventing the MSCs from adhesion and therefore creating a suitable environment for the cells for differentiation and for maintaining the chondrocyte phenotype. In all these model systems the MSCs produce certain differentiation markers like for example Collagen type II, which is a marker for early stage chondrogenesis, or Collagen type X which is produced by hypertrophic chondrocytes. In alginate culture the alginate is used to provide a suitable environment for chondrocytes to e.g. migrate into defects and repair cartilage (Hunziker 1996). Alginate is an unbranched linear copolymer of 1-4 linked β-D-mannuronic acid and α-L-guluronic acid. It forms gels in the presence of Ca2+ and is used to entrap cells for in vitro cultivation. In this model the cells are able to completely differentiate into hypertrophic chondrocytes and produce extracellular matrix like in vivo
Another high density culture method is the pellet culture in which cells form a tightly aggregated cell mass which mimics embryonic cartilage development [6,7]. A third system which is widely used is the micromass culture, which was first described by Ahrens et al in 1977 [1]. Zhang et al. were able to show that in this culture method the expression of Col I and Col X was down-regulated compared to pellet culture [7]. This indicates that micromass culture is a good tool to mimic hyaline cartilage.
All these model systems nicely exemplify the cellular differentiation in the developing cartilage; however, the biggest disadvantage is the number of cells necessary and the culturing systems. In most cases, cell numbers of around 200.000 to 600.000 cells per treatment are needed. Further, several systems, like the pellet culture, are performed in 15 ml tubes making the handling and the space needed rather problematic and expensive. Therefore, we aimed to transfer a model known from analysis of tumor cells, the hanging drop model, to analysis of cartilage tissue.
Material and methods
Primary human mesenchymal stem cells
Human mesenchymal stem cells (HMSC; Provitro, Berlin, Germany) were cultivated in HMSC proliferation medium (Provitro) under a humidified atmosphere of 8% CO2 at 37°C. Cells of four different donors were used in passages 4 to 10. Transfection of the HSMCs with siRNA against Sox9 (CTCCACCTTCACCTACATGAA, Qiagen, Hilden, Germany) or the respective control was performed using Lipofectamine 2000 (Invitrogen, Darmstadt, Germany). The cells were cultured in T75 culture flasks to 30-50% confluency. For transfection 750 pmol siRNA were used in the transfection solutions. The transfection was performed following the manufacturer’s instructions. The cells were harvested 48 h later, and used for the hanging drops.
Hanging drop assay
To generate spheroids the HMSC were detached from the culture flask by adding 1 ml Trypsin-EDTA (Provitro). After incubation for 5 minutes 1 ml neutralizing solution (Provitro) and 8 ml HMSC proliferation medium were added. After centrifugation for 4 min at 1200 rpm and resuspension in incomplete chondrogenesis induction medium (DMEM with glucose (4.5 g/L), penicillin (400 units/ml) streptomycine (50 μg/ml), L-glutamine (300 μg/ml), sodium pyruvate (1 mM), L-ascorbic acid 2-phosphate (0.17 mM), L-proline (0.35 nM), dexamethasone (1 μM) and ITS premix (BD Biosciences, Heidelberg, Germany)), the cells were counted and adjusted to 50,000 cells/ml. 20% methocel (6 g methyl cellulose (Sigma-Aldrich, Munich, Germany), 250 ml basal medium)) was added and 25 μl of the cells suspension were dropped onto the cover of a 9 mm petri dish. For each experimental condition 10 drops were used. The petri dish was filled with PBS and the cover dish was inverted (Figure 1) and incubated for 72 h under a humidified atmosphere of 8% CO2 at 37°C for 1, 2, 4 and 7 d followed by RNA isolation or alcian blue staining. For treatment, TGF-beta3 (10 ng/ml, Biomol, Hamburg, Germany) was added. The experiment was repeated 4 times.
Figure 1.

Schematic drawing of the hanging drop model. A cell suspension containing 50,000 cells/ml, supplements (e.g. TGF-β) and 20% Methocel was dropped onto a petri dish cover. The cover was then carefully inverted and set on the respective petri dish filled with PBS. The hanging drops were incubated for 1, 2, 4 or 7 d in the CO2-incubator.
Pellet culture assays
HMSC differentiation was also performed as 3D pellets. For this, 2.5 × 105 HMSCs were seeded into 15 ml polypropylene tubes and pelleted. HMSCs were cultured for 0, 3, 7, 9, 11, 13, 17, 19, 23, 27, or 33 days as 3D pellets in serum-free induction medium (DMEM-high glucose, 0.1 μM dexamethasone, 1 mM sodium pyruvate, 0.17 mM ascorbic acid-2-phosphate, 0.35 mM proline and 1:500 insulin–transferrin– selenite (Becton Dickinson, Heidelberg, Germany)). The experiment was repeated three times.
Isolation of RNA and reverse transcription
For RNA isolation 10 spheroids were pooled. Total cellular RNA was isolated from cultured cells using the Micro Elute Total RNA Kit (VWR, Darmstadt, Germany) and cDNAs were generated by reverse transcriptase reaction performed in 20 μl reaction volume containing 4 μl of total cellular RNA, 4 μl of 5x first strand buffer (Invitrogen, Darmstadt, Germany), 2 μl of 0.1 M DTT, 1 μl of dN6-Primer (10 mM), 1 μl of dNTPs (10 mM) and DEPC-water as described previously [8]. The reaction mixture was incubated for 10 min at 70°C, 200 units of Superscript II reverse transcriptase (Invitrogen) were added and RNA were transcribed for 1 h 37°C. Reverse transcriptase was inactivated at 70°C for 10 min and RNA was degraded by digestion with 1 μl RNase A (10 mg/ml) at 37°C for 30 min.
Analysis of mRNA expression by quantitative real time PCR
Quantitative real time PCR was performed on a Lightcycler (Roche, Mannheim, Germany), cDNA template (2 μl), 0.5 μl (20 mM) of forward and reverse primers (Table 1) and 10 μl SybrPremix ExTaq (Takara (Lonza), Cologne, Germany) in a total of 20 μl were applied to the following PCR program: 30 s 95°C (initial denaturation); 20°C/s temperature transition rate up to 95°C for 15 s, 3 s – 60°C, 5 s – 72°C, 80°C acquisition mode single, repeated for 45 times (amplification). The PCR reaction was evaluated by melting curve analysis and checking the PCR products on 1.8% agarose gels. The values were calculated relative to ß-actin and relative to the expression of the gene of interest in HMSC monolayer.
Table 1.
Primer sequences
| Gene | Primer |
|---|---|
| ß-actin forward | 5’-CTA CGT CGC CCT GGA CTT CGA GC |
| ß-actin reverse | 5’-GAT GGA GCC GCC GAT CCA CAC GG |
| Coll X forward | 5’-AGCCAGGGTTGCCAGGACCA |
| Coll X reverse | 5’-TTTTCCCACTCCAGGAGGGC |
| Mia forward | 5’-CATGCATGCGGTCCTATGCCCAAGCTG |
| Mia reverse | 5’-GATAAGCTTTCACTGGCAGTAGAAATC |
| Sox9 forward | 5’-CGAACGCACATCAAGACGA |
| Sox9 reverse | 5’-AGGTGAAGGTGGAGTAGAGGC |
| Aggrecan forward | 5’-CCTACCAAGTGGCATAGC |
| Aggrecan reverse | 5’-TGTTGGAGCCTGGGTTAC |
| Coll II forward | 5’-AGGGCAATAGCAGGTTCACG |
| Coll II reverse | 5’-GGTCAGGTCAGCCATTCAGT |
| P54nrb forward | 5’-TTCCTCCCGACATCACTGAG |
| P54nrb reverse | 5’-ATCCACAATGACTACAGCCCT |
| AP2ε forward | 5’-GGAGTAAGGGAGGGTGGCCTCTC |
| AP2ε reverse | 5’-GCACCAACTCCTCAGTAGCACCTC |
Western blot and histological staining
Western blot analysis was performed as described before (Schubert et al., 2009). Sox9 antibody (Merck Millipore, Billerica, MA, USA) was used in a 1:1000 dilution in 5% dry milk/TBS-Tween (0.1%).
Enzyme-linked immunosorbent assay (ELISA)
MIA protein was quantified in cell culture supernatants by an ELISA system as described in 2003 by Bosserhoff et al. [9].
Statistical analysis
In the bar graphs, results are expressed as mean±S.D. (range) or percent. All calculations were made using the GraphPad Prism Software (GraphPad Software, Inc., San Diego, USA).
Results
The hanging drop model, established in the early 1900s, has been adapted to the application in the tumor field [10,11] in the last years, and was now adapted to HMSCs to approach the problems occurring using other 3D-model systems mentioned above.
As described in detail in the Material and Method section the assay was performed in petri dishes using hanging drops of medium each containing about 1000 HMSCs (see Figure 1 for schematic illustration). Usually for each experimental approach 10 parallels are used. The cells quickly formed spheroids and started to differentiate (data not shown). After incubation for 1, 2, 4 and 7 days RNA was isolated and used for further experiments. Of course, also analysis for protein content or GAGs in the supernatant is feasible.
To investigate the differentiation of the MSCs several marker genes, like Collagen II, MIA/CD-RAP, Collagen X and AP-2 epsilon were analyzed on mRNA level [8,12-15]. As shown in Figure 2A, Collagen II and MIA/CD-RAP expression increased strongly over time in MSCs. Sox9 protein expression could be detected at similar levels at all tested time-points (Figure 2B). MIA protein levels increased over time as shown in Figure 2C. As induction of both, Sox9 and MIA was already observed at day 2 in the hanging drop model, we also determined the expression in pellet culture and induction of these marker genes was not observed until day 11 (Figure 2D).
Figure 2.

Expression of marker genes for early chondrogenic differentiation. A: The HMSCs were cultivated in the hanging drop setting for 7 d and mRNA expression of Sox9, Collagen type II, and MIA/CD-RAP was measured on day 1, 2, 4 and 7 by quantitative RT-PCR (n=4). MIA/CD-RAP (p=0.0006) and Col II (p=0.0002) were significantly up-regulated over time, indicating that the HMSC undergo differentiation, although Sox9 expression did not change. B: Western blot analysis of Sox9. Protein levels of Sox9 were similar at all time-points. C: MIA ELISA showed that expression of MIA protein increased over time, therefore indicating higher differentiation states of the chondrocytes at day 7 compared to day 1 (n=3, p=0.02). D: RNAs from classical pellet culture [13,14] were analyzed for Col II and MIA/CD-RAP mRNA expression at day 0, 3, 7, 9, 11, 13, 17, 19, 23, 27, and 33 by quantitative RT-PCR. Both genes were up-regulated over time. The levels at days 23-30 were comparable to the mRNA levels of day 7 in the hanging drop setting. (*: p<0.05; ***: p<0.001; ns: p>0.05).
In contrast to early induction of cartilage differentiation marker like Collagen II or MIA/CD-RAP the mRNA levels of Collagen X and AP-2 epsilon, both expressed in later phases of cartilage differentiation, stayed only weakly induced until day 7. Here, TGF-beta treatment of the MSCs strongly enhances expression of late differentiation markers starting at day 2 (Figure 3).
Figure 3.

Expression of marker genes for late chondrogenic differentiation. HMSCs were cultivated in the hanging drop assay and treated with TGF-β to accelerate differentiation and to induce late stage chondrogenic markers. Therefore, Col X and AP-2 epsilon mRNA expression levels were determined by quantitative RT-PCR (n=4). Both expression levels increased over time. Col X expression was significantly increased by TGF-β treatment (p=0.0004) while AP-2 epsilon expression only showed a trend towards enhanced expression after TGF-β treatment.
In an experimental approach using siRNA against Sox9 the validity of the new assay system was evaluated. The siRNA used for the transfection was already published to decrease Sox9 expression efficiently by Wenke et al. in 2009 [8]. HMSCs were transfected with siRNA and used in the assay 2 days after transfection. The knock-down was stable for 7 d in the spheroids and in monolayer on mRNA and protein levels (Figure 4A, 4B). MessengerRNA expression of several chondrocyte genes was analyzed by quantitative RT-PCR (Figure 4C). At all time points strong reduction of Sox9 mRNA expression was found compared to siRNA control (set as 1). Significant reduction of gene expression was observed for Aggrecan (p=0.0057) in the siSox9 treated cells. MIA/CD-RAP, Col II showed a tendency towards reduced gene expression (Figure 4C). As we assumed this might be due to redundancy mechanisms of p54nrb, which was recently shown to play a supportive role in Sox9-mediated transcription [16,17], we also determined p54nrb mRNA expression levels (Figure 4C). Interestingly, we saw an induction of p54 expression (not significant) at day 4 potentially mediating induced expression of Aggrecan and Collagen II in spite of low Sox9 levels. However, expression of MIA/CD-RAP was not rescued.
Figure 4.
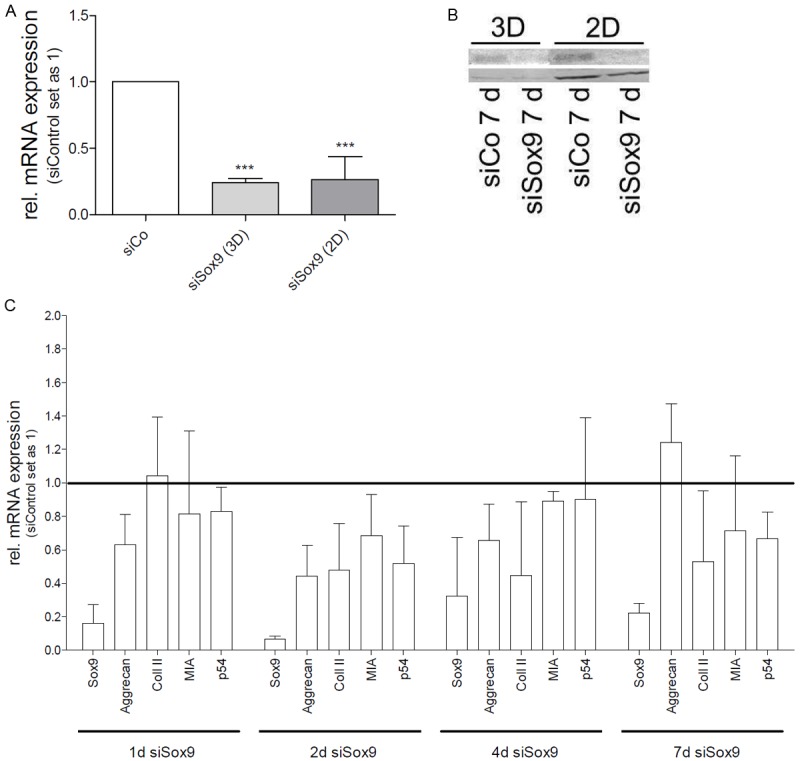
Analysis of Sox9 function in the hanging drop model. A: Sox9 knock-down from day 1 to day 7 in HMSCs in the hanging drop setting (3D) and monolayer (2D). In HMSCs, the knock-down is stable over 7 d (n=4). B: Western blot analysis of HMSCs 7 d after siCo or siSox9 transfection in 3D and 2D. A down-regulation of Sox9 protein is achieved in both, hanging drop and monolayer. C: Sox9 expression in HMSCs was down-regulated by siRNA and cells were seeded into the hanging drop model. Sox9 (p=0.0003), Aggrecan (p=0.0057), Collagen II (not significant), MIA/CD-RAP (not significant), and p54nrb (not significant) mRNA expression levels were determined by quantitative RT-PCR (n=3). SiControl treated HMSC cells were set as 1 for each individual day. (***: p<0.001).
Discussion
In this study we aimed to establish a model system for high-density cell culture models of chondrogenesis due to several disadvantages of the established systems. The main disadvantage of these is the high number of cells necessary. According to the literature all model systems require 5 × 105 to 4 × 106 cells per batch [18,19]. Either for experimental settings with many conditions, with murine or rat primary cells or using transfection these numbers are hard to reach. Further, handling of the established assay systems is time- and space-consuming and in some instances prone to contamination due to long periods of culturing and cell culture devices used.
The new format is based on “hanging drop” culturing. The hanging drop method has first been used to cultivate nerve fibers and fibroblasts isolated from chick embryos [20,21]. Since then, the method has been modified and applied to several kinds of cell types, e.g. human embryonic stem cells [22] or tumor cells [10]. Here, we applied the hanging drop method to human chondrocytes. The amount of cells is 10 to 100 fold less than in other 3D cell models although the methods can be equivalently used for studies of gene regulation of early chondrogenesis. Comparison of two assays, the new hanging drop and the classical pellet culture, showed that the results were comparable with regards to induction of differentiation and marker gene expression. These findings let us state that the hanging drop system provides a 3D environment similar to cartilage formation during embryonic development and allows HMSCs to undergo chondrogenesis. Interestingly, the cells in the classical pellet culture experiment differentiated much slower whereas differentiation was strongly induced in the hanging drop model already after 4 days. Potentially, this depicts another advantage for experiments where cells are transfected prior to differentiation or expensive, sensitive treatments are performed. Regarding this aspect it is also of importance that only small amounts of substance for treatment are needed because of the culturing volume of 25 μl. As bone marrow-derived MSCs are heterogeneous due to donor differences the quality of the new assay is supported by the low standard deviation (see Figure 2A) although cells from 4 different donors were used in independent experiments. The experimental setting applied in this study using siRNA against Sox9 demonstrated that application of siRNA is feasible and effective in this model. It, further, proves that effects on gene expression can be determined in detail. In summary, we suggest that the hanging drop model is a good alternative for analyzing chondrogenic differentiation which requires less space, time and costs but still provides results comparable to other, established 3D models for chondrocyte differentiation.
Acknowledgements
We thank Sibylla Lodermeyer for excellent technical assistance. This study was supported by a DFG grant to AKB.
Disclosure of conflict of interest
None.
References
- 1.Ahrens PB, Solursh M, Reiter RS. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol. 1977;60:69–82. doi: 10.1016/0012-1606(77)90110-5. [DOI] [PubMed] [Google Scholar]
- 2.DeLise AM, Stringa E, Woodward WA, Mello MA, Tuan RS. Embryonic limb mesenchyme micromass culture as an in vitro model for chondrogenesis and cartilage maturation. Methods Mol Biol. 2000;137:359–375. doi: 10.1385/1-59259-066-7:359. [DOI] [PubMed] [Google Scholar]
- 3.Grandolfo M, D’Andrea P, Paoletti S, Martina M, Silvestrini G, Bonucci E, Vittur F. Culture and differentiation of chondrocytes entrapped in alginate gels. Calcif Tissue Int. 1993;52:42–48. doi: 10.1007/BF00675625. [DOI] [PubMed] [Google Scholar]
- 4.Hunziker EB, Rosenberg LC. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am. 1996;78:721–33. doi: 10.2106/00004623-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 6.Chang CH, Lin HY, Fang HW, Loo ST, Hung SC, Ho YC, Chen CC, Lin FH, Liu HC. Chondrogenesis from immortalized human mesenchymal stem cells: comparison between collagen gel and pellet culture methods. Artif Organs. 2008;32:561–566. doi: 10.1111/j.1525-1594.2008.00575.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Su P, Xu C, Yang J, Yu W, Huang D. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett. 2010;32:1339–1346. doi: 10.1007/s10529-010-0293-x. [DOI] [PubMed] [Google Scholar]
- 8.Wenke AK, Grassel S, Moser M, Bosserhoff AK. The cartilage-specific transcription factor Sox9 regulates AP-2epsilon expression in chondrocytes. FEBS J. 2009;276:2494–2504. doi: 10.1111/j.1742-4658.2009.06973.x. [DOI] [PubMed] [Google Scholar]
- 9.Bosserhoff AK, Buettner R. Establishing the protein MIA (melanoma inhibitory activity) as a marker for chondrocyte differentiation. Biomaterials. 2003;24:3229–34. doi: 10.1016/s0142-9612(03)00184-4. [DOI] [PubMed] [Google Scholar]
- 10.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003;83:173–180. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- 11.Meierjohann S, Hufnagel A, Wende E, Kleinschmidt MA, Wolf K, Friedl P, Gaubatz S, Schartl M. MMP13 mediates cell cycle progression in melanocytes and melanoma cells: in vitro studies of migration and proliferation. Mol Cancer. 2010;9:201. doi: 10.1186/1476-4598-9-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schubert T, Schlegel J, Schmid R, Opolka A, Grassel S, Humphries M, Bosserhoff AK. Modulation of cartilage differentiation by melanoma inhibiting activity/cartilage-derived retinoic acid-sensitive protein (MIA/CD-RAP) Exp Mol Med. 2010;42:166–174. doi: 10.3858/emm.2010.42.3.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tscheudschilsuren G, Bosserhoff AK, Schlegel J, Vollmer D, Anton A, Alt V, Schnettler R, Brandt J, Proetzel G. Regulation of mesenchymal stem cell and chondrocyte differentiation by MIA. Exp Cell Res. 2006;312:63–72. doi: 10.1016/j.yexcr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Wenke AK, Bosserhoff AK. Roles of AP-2 transcription factors in the regulation of cartilage and skeletal development. FEBS J. 2010;277:894–902. doi: 10.1111/j.1742-4658.2009.07509.x. [DOI] [PubMed] [Google Scholar]
- 16.Hata K, Nishimura R, Muramatsu S, Matsuda A, Matsubara T, Amano K, Ikeda F, Harley VR, Yoneda T. Paraspeckle protein p54nrb links Sox9-mediated transcription with RNA processing during chondrogenesis in mice. J Clin Invest. 2008;118:3098–3108. doi: 10.1172/JCI31373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmid R, Schiffner S, Opolka A, Grassel S, Schubert T, Moser M, Bosserhoff AK. Enhanced cartilage regeneration in MIA/CD-RAP deficient mice. Cell Death Dis. 2010;1:e97. doi: 10.1038/cddis.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JY, Hall R, Pelinkovic D, Cassinelli E, Usas A, Gilbertson L, Huard J, Kang J. New use of a three-dimensional pellet culture system for human intervertebral disc cells: initial characterization and potential use for tissue engineering. Spine (Phila Pa 1976) 2001;26:2316–22. doi: 10.1097/00007632-200111010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch AD, Grady LM, Ablett MP, Katopodi T, Meadows RS, Hardingham TE. Chondrogenic differentiation of human bone marrow stem cells in transwel cultures: generation of scaffold-free cartilage. Stem Cells. 2007;25:2786–96. doi: 10.1634/stemcells.2007-0374. [DOI] [PubMed] [Google Scholar]
- 20.Harrison RG. Observations on the living developing nerve fibres. Proc Soc Exp Bio Med. 1907;4:140–143. [Google Scholar]
- 21.Carrel A. On the permanent life of tissues outside of the organism. J Exp Med. 1912;15:516–28. doi: 10.1084/jem.15.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz JC, Stumpf PS, Katsen-Globa A, Sachinidis A, Hescheler J, Zimmermann H. First steps towards the successful surface-based cultivation of human embryonic stem cells in hanging drop systems. Eng Life Sci. 2012;12:584–587. doi: 10.1002/elsc.201100213. [DOI] [PMC free article] [PubMed] [Google Scholar]


