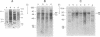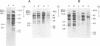Abstract
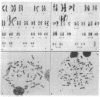
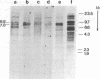
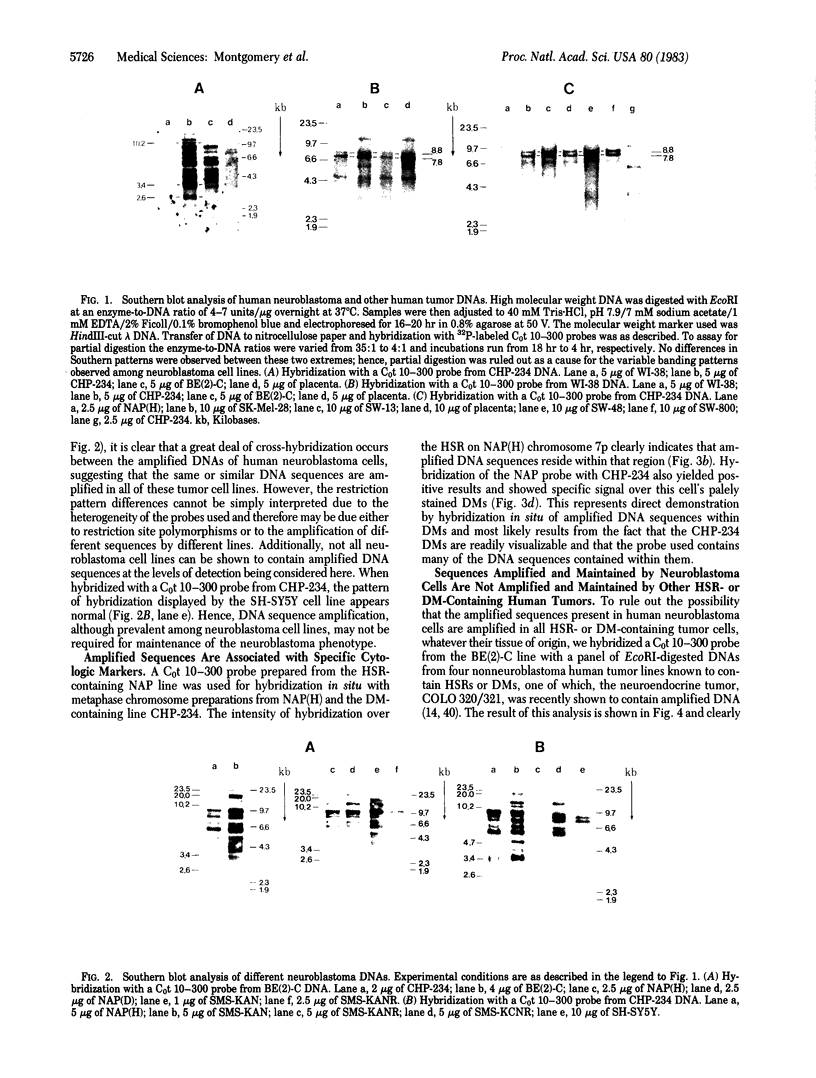
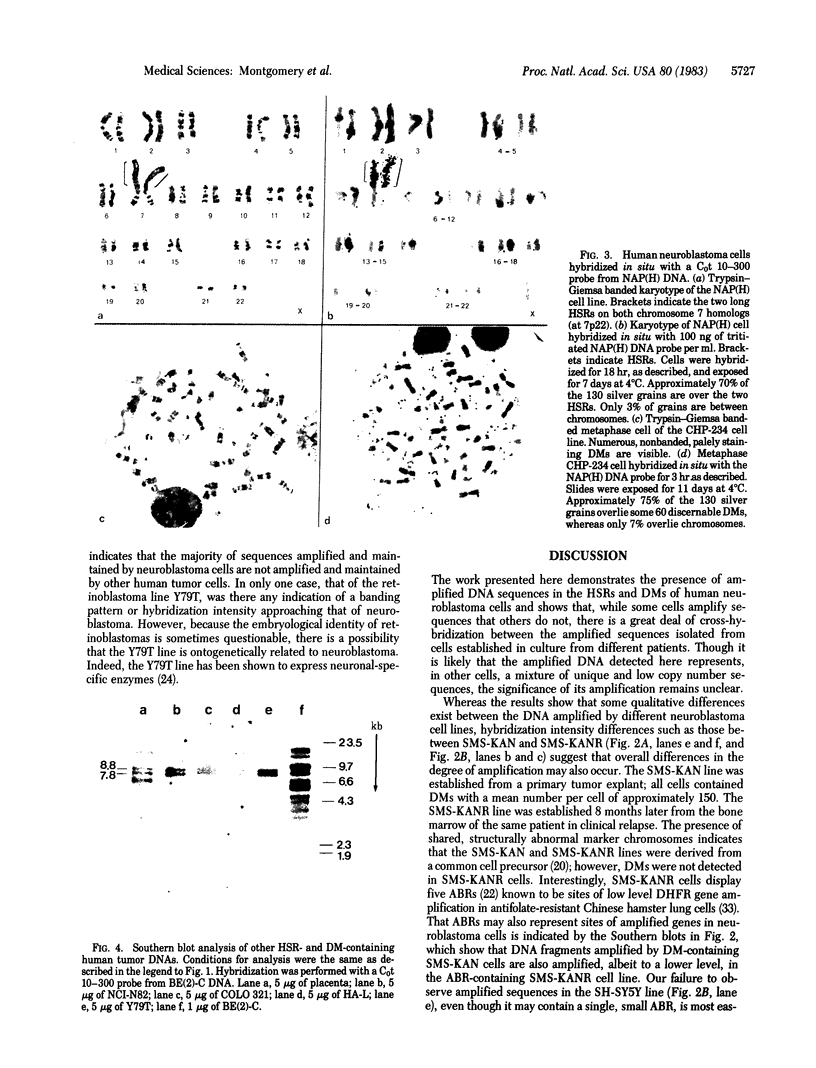
Southern blot analysis of a number of EcoRI-digested human neuroblastoma DNAs has revealed the presence of a family of discrete restriction fragments, the majority of which are amplified in most, but not all, of the neuroblastoma cell lines tested. None of these sequences is abundantly present in DNA from other human tumors of different tissue origins, including several either known or presumed to contain amplified DNA. Hence, these sequences appear to be specifically amplified by neuroblastoma cells. Hybridization with metaphase chromosomes in situ has localized these sequences to either the homogeneously staining regions or double-minute chromosomes of different neuroblastoma cell lines, indicating that these chromosomal structures, although present in cell lines established from different patients, share many sequences and may have a common, but as yet unknown, function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Balaban-Malenbaum G., Gilbert F. Double minute chromosomes and the homogeneously staining regions in chromosomes of a human neuroblastoma cell line. Science. 1977 Nov 18;198(4318):739–741. doi: 10.1126/science.71759. [DOI] [PubMed] [Google Scholar]
- Beach L. R., Palmiter R. D. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2110–2114. doi: 10.1073/pnas.78.4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A. Metaphase chromosome anomaly: association with drug resistance and cell-specific products. Science. 1976 Jan 16;191(4223):185–187. doi: 10.1126/science.942798. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock C. J., Clark E. M., Harding N. G., Mounts P. M., Tyler-Smith C., van Heyningen V., Walker P. M. The development of resistance to methotrexate in a mouse melanoma cell line. I. Characterisation of the dihydrofolate reductases and chromosomes in sensitive and resistant cells. Chromosoma. 1979;74(2):153–177. doi: 10.1007/BF00292270. [DOI] [PubMed] [Google Scholar]
- Bostock C. J., Tyler-Smith C. Gene amplification in methotrexate-resistant mouse cells. II. Rearrangement and amplification of non-dihydrofolate reductase gene sequences accompany chromosomal changes. J Mol Biol. 1981 Dec 5;153(2):219–236. doi: 10.1016/0022-2836(81)90275-8. [DOI] [PubMed] [Google Scholar]
- Brison O., Ardeshir F., Stark G. R. General method for cloning amplified DNA by differential screening with genomic probes. Mol Cell Biol. 1982 May;2(5):578–587. doi: 10.1128/mcb.2.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Brown P. C., Beverley S. M., Schimke R. T. Relationship of amplified dihydrofolate reductase genes to double minute chromosomes in unstably resistant mouse fibroblast cell lines. Mol Cell Biol. 1981 Dec;1(12):1077–1083. doi: 10.1128/mcb.1.12.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey T. E., Takahashi T., Resnick L. A., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3278–3282. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M. E., Yunis J. J. A high resolution in situ hybridization technique for the direct visualization of labeled G-banded early metaphase and prophase chromosomes. Cytogenet Cell Genet. 1978;22(1-6):352–356. doi: 10.1159/000130970. [DOI] [PubMed] [Google Scholar]
- Chen T. R., Hay R. J., Macy M. L. Karyotype consistency in human colorectal carcinoma cell lines established in vitro. Cancer Genet Cytogenet. 1982 Jun;6(2):93–117. doi: 10.1016/0165-4608(82)90076-0. [DOI] [PubMed] [Google Scholar]
- Dolnick B. J., Berenson R. J., Bertino J. R., Kaufman R. J., Nunberg J. H., Schimke R. T. Correlation of dihydrofolate reductase elevation with gene amplification in a homogeneously staining chromosomal region in L5178Y cells. J Cell Biol. 1979 Nov;83(2 Pt 1):394–402. doi: 10.1083/jcb.83.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J., Wright W. C., Loveless J. D. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst. 1977 Feb;58(2):209–214. doi: 10.1093/jnci/58.2.209. [DOI] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. L., Powers V. E. Amplified DNA sequences in Y1 mouse adrenal tumor cells: association with double minutes and localization to a homogeneously staining chromosomal region. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1597–1601. doi: 10.1073/pnas.79.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. L., Powers V. E. Cloning of DNA from double minutes of Y1 mouse adrenocortical tumor cells: evidence for gene amplification. Cell. 1981 Apr;24(1):117–123. doi: 10.1016/0092-8674(81)90507-9. [DOI] [PubMed] [Google Scholar]
- Gilbert F., Balaban G., Breg W. R., Gallie B., Reid T., Nichols W. Homogeneously staining region in a retinoblastoma cell line: relevance to tumor initiation and progression. J Natl Cancer Inst. 1981 Aug;67(2):301–306. [PubMed] [Google Scholar]
- Glover D. M., Zaha A., Stocker A. J., Santelli R. V., Pueyo M. T., De Toledo S. M., Lara F. J. Gene amplification in Rhynchosciara salivary gland chromosomes. Proc Natl Acad Sci U S A. 1982 May;79(9):2947–2951. doi: 10.1073/pnas.79.9.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Brown P. C., Schimke R. T. Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5669–5673. doi: 10.1073/pnas.76.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Biedler J. L., Melera P. W. Gene amplification accompanies low level increases in the activity of dihydrofolate reductase in antifolate-resistant Chinese hamster lung cells containing abnormally banding chromosomes. J Cell Biol. 1982 Aug;94(2):418–424. doi: 10.1083/jcb.94.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Davide J. P., Melera P. W. Selective amplification of polymorphic dihydrofolate reductase gene loci in Chinese hamster lung cells. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6961–6965. doi: 10.1073/pnas.79.22.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melera P. W., Lewis J. A., Biedler J. L., Hession C. Antifolate-resistant Chinese hamster cells. Evidence for dihydrofolate reductase gene amplification among independently derived sublines overproducing different dihydrofolate reductases. J Biol Chem. 1980 Jul 25;255(14):7024–7028. [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schlesinger H. R., Rorke L., Jamieson R., Hummeler K. Neuronal properties of neuroectodermal tumors in vitro. Cancer Res. 1981 Jul;41(7):2573–2575. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1096–1100. doi: 10.1073/pnas.77.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Whang-Peng J., Bunn P. A., Jr, Kao-Shan C. S., Lee E. C., Carney D. N., Gazdar A., Minna J. D. A nonrandom chromosomal abnormality, del 3p(14-23), in human small cell lung cancer (SCLC). Cancer Genet Cytogenet. 1982 Jun;6(2):119–134. doi: 10.1016/0165-4608(82)90077-2. [DOI] [PubMed] [Google Scholar]