Abstract
The common causes of pseudoaneurysms of internal carotid artery (ICA) in the neck are penetrating trauma, head and neck surgeries, carotid endarterectomies, infiltrating metastatic lymph nodes and neoplasms. We report a young male patient who presented with a swelling in left upper neck diagnosed as carotid body tumor with ultrasonography and magnetic resonance imaging. Subadventitial excision of the tumor was done. The patient developed dense right hemiplegia in the immediate postoperative period. Doppler study of neck revealed left ICA dissection with partial thrombosis of the lumen. Computed tomography of the brain revealed nonhemorrhagic left capsuloganglionic infarct and he was managed conservatively with heparin. Follow-up Doppler study done 2 weeks later revealed pseudoaneurysm of the ICA. Attempts to obliterate the pseudoaneurysm by interventional procedures failed due to the narrow neck of the pseudoaneurysm. Heparin was stopped and patient was maintained only on oral aspirin. Doppler study repeated 1 week later showed spontaneous thrombosis of the pseudoaneurysm with good flow in the distal ICA. To the best of our knowledge, only one case of pseudo-pseudoaneurysm complicating surgical resection of carotid body tumor has been reported so far The etiology, imaging features, and treatment options of pseudoaneurysms are discussed.
Keywords: Capsuloganglionic infarct, carotid body tumor, color Doppler, dissection, excision, internal carotid artery, magnetic resonance imaging, pseudoaneurysm
Introduction
Pseudoaneurysms of internal carotid artery (ICA) in the neck are caused by penetrating trauma, post head and neck surgeries, carotid endarterectomies, infiltrating metastatic lymph nodes and neoplasms.[1] The management options for ICA pseudoaneurysm include ultrasound (USG) guided compression, percutaneous thrombin injection, coil embolization, endovascular stent graft insertion, and surgery.[2] We managed our patient conservatively due to failure to negotiate intravascular catheter across the narrow neck of the pseudoaneurysm, and after 1 week, there was complete spontaneous resolution of the pseudoaneurysm. We report this case to focus on this rare complication after carotid body tumor (CBT) excision and to highlight the success of conservative management.
Case Report
A 35-year-old male patient presented with swelling in the left side of the neck. USG and magnetic resonance imaging (MRI) revealed a well-marginated mass lesion measuring 3.8 cm × 2.8 cm at the left carotid bifurcation [Figure 1] splaying the proximal internal and external carotid arteries, with circumferential area of contact with ICA of 180°-270°, consistent with the imaging diagnosis of Shamblin stage 2 CBT. Subadventitial excision of CBT was done after retraction of the carotids. No special technique was used to visualize the tumor and the excision was simple. Proximal and distal control of the internal, external, and common carotid arteries was done over vascular loops and the vessel loops were used to retract the external and internal carotid artery bifurcation. There were no arterial or nerve injuries. No preoperative embolization was planned. The postoperative course was uneventful and the patient was given intraoperative heparin based on body weight. No neurological deficits were observed at recovery or in the immediate postoperative period. The patient developed right-sided dense hemiplegia on the morning of the first postoperative day, with a score of 29/48 according to the Scandinavian Stroke scale.[3] Plain computed tomography (CT) of the brain [Figure 2] revealed a recent infarct involving the left capsuloganglionic region and corona radiata (middle cerebral artery territory). Color Doppler of the carotid arteries [Figure 3] revealed partial thrombosis of the left ICA with approximately 50% reduction in vessel lumen and hypoechoic hematoma around the proximal ICA, consistent with a diagnosis of postoperative dissection. Patient was managed conservatively with low molecular weight (LMW) heparin. In view of ICA thrombus, oral warfarin was started and LMW heparin was stopped after 5 days. Two weeks later, a follow-up carotid Doppler study [Figure 4] showed a pseudoaneurysm measuring 2.3 cm × 1.5 cm in the medial aspect of proximal left ICA with a central patent lumen of size 10 mm × 8 mm and a mural thrombus around it. Neck of the pseudoaneurysm measured 2 mm. ICA thrombus had significantly resolved with good distal flow. Carotid angiography done on the 17th postoperative day [Figure 5A] showed a pseudoaneurysm measuring 2.3 cm × 1.8 cm, medial to the proximal left ICA filling through a small neck of 2 mm [Figure 5B]. Intimal irregularity was noted in the lateral wall of the left ICA, suggesting arterial injury/dissection. The neck of the pseudoaneurysm was very narrow, so a 0.018-inch guide wire could not be negotiated and attempts to close the pseudoaneurysm were not successful. Microcatheters and stent grafts were not available in our hospital, as it was a primitive hospital set-up. Non-invasive methods were avoided in this patient because he developed stroke on the first postoperative day, mandating anticoagulation. In view of the ICA pseudoaneurysm and no significant intraluminal thrombus in ICA on angiogram, he was started on oral antiplatelets (aspirin) on the 18th postoperative day and oral anticoagulant was stopped after 2 days. But the pseudoaneurysm had spontaneously thrombosed with no demonstrable flow into the aneurysm in follow-up Doppler done after a week of stopping anticoagulants. There was minimal hypoechoic intimal thickening and irregularity of the proximal ICA with no significant lumen narrowing, thrombus, or false lumen. The patient was continued on oral antiplatelets for 6 months, and is on regular follow-up for 5 years. His neurological weakness has improved significantly with regular physiotherapy.
Figure 1.
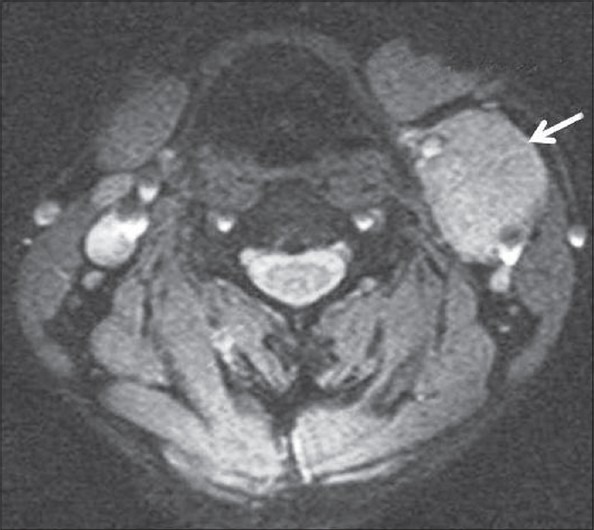
Axial gradient recalled echo MRI of neck showing mass at the carotid bifurcation (arrow) splaying the internal and external carotid arteries
Figure 2.
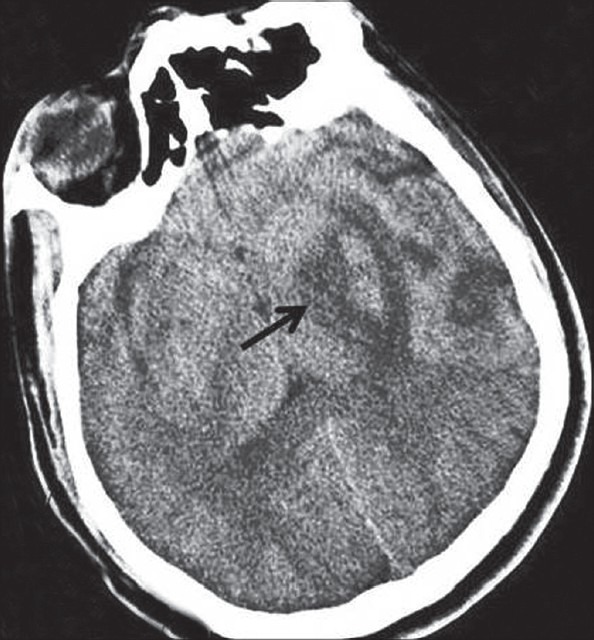
CT scan of brain showing infarct in left capsuloganglionic region (arrow)
Figure 3.
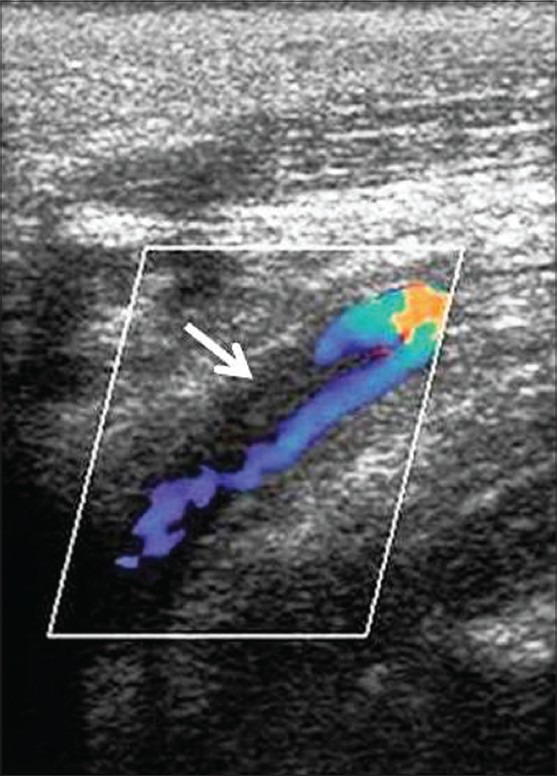
Carotid Doppler immediate postoperative period showing dissection with partial thrombosis of proximal left internal carotid artery (arrow)
Figure 4(A, B):
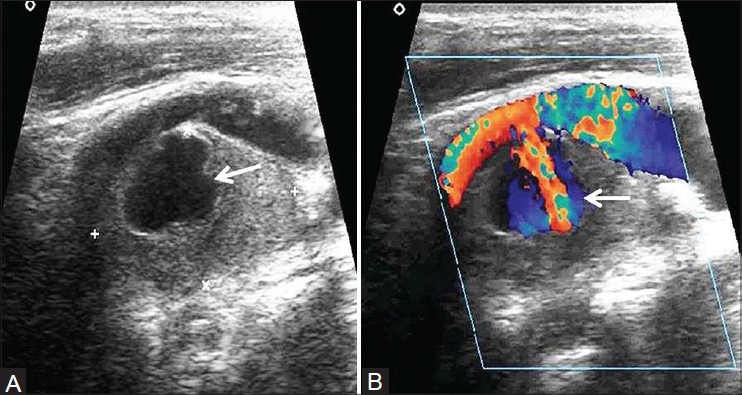
A) Gray scale and (B) Color Doppler ultrasound showing pseudoaneurysm of left internal carotid artery fed by a narrow lumen (arrow)
Figure 5(A, B):
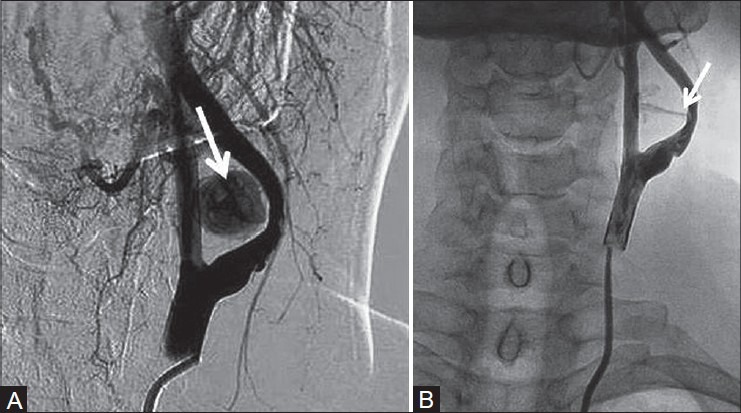
Digital subtraction angiography of left internal carotid artery showing the (A) pseudoaneurysm medial to ICA filling up with contrast with narrowed and irregular lumen of ICA (arrow) and (B) narrow neck of the pseudoaneurysm with contrast jet (arrow)
Discussion
A pseudoaneurysm is a pulsatile extravascular contained hematoma communicating with the vessel lumen lined by adventitious layer, perivascular connective tissue, or just a blood clot. Pseudoaneurysm of cervical portion of ICA is a rare and potentially lethal condition. It may result from retropharyngeal or parapharyngeal sepsis, penetrating trauma, invasion by malignancy, radiotherapy, radical neck dissection, and iatrogenic causes such as vascular surgeries, needle puncture, and tonsillectomy.[4] Clinically, ICA pseudoaneurysms present as a pulsatile lateral neck mass, medial bulge in pharyngeal wall, or as carotid thrill or bruit. CBTs are slow-growing, highly vascular tumors arising from paraganglionic cells of carotid body located in the posteromedial wall of the common carotid artery at the level of carotid bifurcation. Subadventitial excision is the treatment of choice. Preoperative embolization has been shown to reduce intraoperative bleeding in Shamblin type 3 lesions encasing the carotids. Operative complications of excision of CBT include cerebrovascular complications in the form of stroke (0-7%), ICA injury with or without need for vascular reconstruction (2-13%), and injury to cranial nerves, commonly hypoglossal and vagal nerves with resultant deficit (6.9-42%).[5]
Dissection and thrombosis of ICA/common carotid artery have been described to occur after CBT excision and dissection can potentially evolve into a pseudoaneurysm.[4] There is only one report of ICA pseudo-pseudoaneurysm complicating CBT excision, which was proved to be a postoperative fluid collection with no demonstrable arterial flow on CT scan.[6] In our case, Color Doppler done on the first postoperative day showed dissection with partial thrombosis of ICA. Dissection of the carotid artery results from arterial injury disrupting the intimo-medial integrity with resultant hemorrhage in vessel wall, favoring local thrombus formation or formation of a false lumen. The accepted standard medical treatment for dissection is systemic anticoagulation, and spontaneous healing occurs in a significant number of cases. In a subset of patients, restoration of native vessel anatomy does not occur, and false lumen, residual focal stenosis of varying degree, or pseudoaneurysm form.[7] In cases of arterial dissection site pseudoaneurysms, anticoagulation, though critical to prevent thromboembolic events in the initial stages of disease, may be contraindicated because of the risk of pseudoaneurysm rupture.[7] We faced this dilemma and we changed over to oral antiplatelets in our case.
Investigations aiding in the diagnosis of pseudoaneurysm include Color Doppler sonography, contrast-enhanced CT (CECT), or MRI, and cervical angiography. Color Doppler shows swirling of blood flow within the pseudoaneurysm with a communicating channel to the parent artery (Yin Yang phenomenon). Pulse Doppler shows to-and-fro waveforms. CECT or CT angiography shows an intensely enhancing mass projecting outside the confines of the arterial wall, with or without mural thrombus.[4] MRI shows flow void in a patent aneurysm and variable signal intensity in athrombosed aneurysm. The various treatment options for pseudoaneurysms include USG-guided compression, percutaneous thrombin injection, coil embolization, endovascular stent graft insertion, and surgery.[2] Spontaneous thrombosis can occur in small pseudoaneurysms, but it is unpredictable and less likely to occur in those greater than 1.8 cm in diameter and in the presence of continued anticoagulation.[4] In our case, smaller size of pseudoaneurysm with a short neck, early diagnosis, and withdrawal of anticoagulant were the factors that favored spontaneous thrombosis of the pseudoaneurysm.
USG-guided compression is routinely practiced for the treatment of iatrogenic femoral pseudoaneurysms following catheterization, and it is noninvasive, cost-effective, and well tolerated by patients.[8] Treatment failures can occur in pseudoaneurysms which are larger, multiloculated, and longstanding, with wider neck and high flow rates and in patients receiving high levels of anticoagulation.[4] However, there are no reports of USG-guided compression in ICA pseudoaneurysms, probably due to the higher risk of catastrophic rupture and thromboemboli leading to cerebrovascular events.
Endoluminal stent graft placement has emerged as the frontline treatment option for wide-necked pseudoaneurysms.[9] It is minimally invasive, reduces hospital stay, and has eliminated the need for surgical procedures under general anesthesia. Endovascular stent was not required in this case because the neck of the pseudoaneurysm was very short and there was no significant luminal narrowing or residual false lumen. Our patient is on regular follow-up, Doppler US showing minimal thickening and irregularity of intimal layer of the proximal left ICA, with no evidence of pseudoaneurysm, false lumen, or intramural thrombus. Proximal and distal control of the carotids was done over loops. This handling of the arteries could have led to the dissection in the ICA. This complication may possibly be avoided by gentle and minimal handling of the vessels.
Conclusion
Pseudoaneurysm as a complication of CBT surgery has not been reported in literature so far. This case is presented for its rarity and also to focus the success of conservative approach in our patient.
Acknowledgment
The authors sincerely acknowledge the efforts of (Late) Dr. J. R. Daniel, Professor, Department of Radiology, for performing carotid angiography in this patient.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kim HO, Ji YB, Lee SH, Jung C, Tae K. Cases of common carotid artery pseudoaneurysm treated by stent graft. Case Rep Otolaryngol 2012. 2012 doi: 10.1155/2012/674827. 674827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom AI, Sasson T, Verstandig A, Wolf YG, Anner H, Berlatzky Y, et al. Ultrasound-guided thrombin injection for the treatment of iatrogenic pseudoaneurysm of the femoral artery. Isr Med Assoc J. 2001;3:649–52. [PubMed] [Google Scholar]
- 3.Scandinavian Stroke Study Group. Multicenter trial of hemodilution in ischemic stroke: Background and study protocol. Stroke. 1985;16:885–90. doi: 10.1161/01.str.16.5.885. [DOI] [PubMed] [Google Scholar]
- 4.Pearson SE, Choi SS. Pseudoaneurysm of the internal carotid artery: A case report and review of the literature. Arch Otolaryngol Head Neck Surg. 2005;131:454–6. doi: 10.1001/archotol.131.5.454. [DOI] [PubMed] [Google Scholar]
- 5.Arya S, Rao V, Juvekar S, Dcruz AK. Carotid body tumors: Objective criteria to predict the Shamblin group on MR imaging. AJNR Am J Neuroradiol. 2008;29:1349–54. doi: 10.3174/ajnr.A1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotze TE, Smith TA, Clagett GP. Carotid artery pseudo-pseudoaneurysm after excision of carotid body tumor. J Vasc Surg. 2011;54:864. doi: 10.1016/j.jvs.2010.06.111. [DOI] [PubMed] [Google Scholar]
- 7.Malek AM, Higashida RT, Phatouros CC, Lempert TE, Meyers PM, Smith WS, et al. Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol. 2000;21:1280–92. [PMC free article] [PubMed] [Google Scholar]
- 8.Hajarizadeh H, LaRosa CR, Cardullo P, Rohrer MJ, Cutler BS. Ultrasound-guided compression of iatrogenic femoral pseudoaneurysm failure, recurrence, and long-term results. J Vasc Surg. 1995;22:425–30. doi: 10.1016/s0741-5214(95)70010-2. [DOI] [PubMed] [Google Scholar]
- 9.Matsuura JH, Rosenthal D, Jerius H, Clark MD, Owens DS. Traumatic carotid artery dissection and pseudoaneurysm treated with endovascular coils and stent. J Endovasc Surg. 1997;4:339–43. doi: 10.1177/152660289700400403. [DOI] [PubMed] [Google Scholar]


