Abstract
Context:
Rhinosporidiosis is a chronic granulomatous disease endemic in certain regions of India. Computed tomography (CT) imaging appearances of rhinosporidiosis have not been previously described in the literature.
Aims:
To study imaging features in rhinosporidiosis with contrast-enhanced CT and elucidate its role in the evaluation of this disease.
Materials and Methods:
Sixteen patients with pathologically proven rhinosporidiosis were included in the study. Contrast-enhanced CT images were analyzed retrospectively and imaging findings were correlated with surgical and histopathologic findings.
Results:
A total of 29 lesions were found and evaluated. On contrast-enhanced CT, rhinosporidiosis was seen as moderately enhancing lobulated or irregular soft tissue mass lesions in the nasal cavity (n = 13), lesions arising in nasal cavity and extending through choana into nasopharynx (n = 5), pedunculated polypoidal lesions arising from the nasopharyngeal wall (n = 5), oropharyngeal wall (n = 2), larynx (n = 1), bronchus (n = 1), skin and subcutaneous tissue (n = 2). The inferior nasal cavity comprising nasal floor, inferior turbinate, and inferior meatus was the most common site of involvement (n = 13). Surrounding bone involvement was seen in the form of rarefaction (n = 6), partial (n = 3) or complete erosion (n = 3) of inferior turbinate, thinning of medial maxillary wall (n = 2), and septal erosion (n = 2). Nasolacrimal duct involvement was seen in four cases.
Conclusions:
Contrast-enhanced CT has an important role in delineating the site and extent of the disease, as well as the involvement of surrounding bone, nasolacrimal duct and tracheobronchial tree. This provides a useful roadmap prior to surgery.
Keywords: Bronchus, CT, disseminated, nasolacrimal duct, rhinosporidiosis
Introduction
Rhinosporidiosis is a chronic granulomatous condition caused by Rhinosporodium seeberi.[1] Earlier, R. seeberi was classified as a protozoan. This organism is now believed to be a protistal microbe belonging to the class Mesomycetozoea at the animal–fungus boundary.[2] Recently, infection with the cyanobacterium, Microcystis aeruginosa, has also been suggested as the possible alternative etiology of the disease.[3] Source of infection is usually contact with infected soil and water.[1] Frequent bathing in pond water is known to predispose to this infection. The organism gains access to the tissues through an abrasion in the skin or mucosa. It is known to be prevalent mainly in India, Sri Lanka, and Pakistan.[1] In India, it is endemic in southern India (Tamil Nadu and Kerala), Orissa, West Bengal, and Chhattisgarh, and known to occur sporadically in many parts of the country.[1,4,5] This condition is known to have a high rate of recurrence after surgical excision of lesions.[4]
Although the diagnosis of rhinosporidiosis affecting the nose and throat is largely clinical and confirmation is possible by histopathology, radiological evaluation is often required to delineate the extent of the lesion and the status of underlying structures. Many of these patients have undergone multiple previous surgeries, and hence, radiological assessment of deep-seated lesion in the nose and nasopharynx is essential to plan further surgery. The imaging appearances of rhinosporidiosis affecting nose and throat have not been previously described in the literature. A few anecdotal case reports have briefly alluded to the computed tomography (CT) findings in lesions affecting the trachea and bronchus.[4,6] The purpose of our study was to systematically analyze the imaging findings in patients with biopsy-proven rhinosporidiosis and suggest the role of imaging in evaluation of the disease.
Materials and Methods
The study was performed after obtaining approval from the institutional review board. We reviewed case records of patients with rhinosporidiosis who were operated at our institution from 2003 to 2012. Patients with rhinosporidiosis confirmed by histopathology and who had a preoperative contrast-enhanced CT imaging were included in the study. Patient demographics, clinical presentation, diagnostic nasal endoscopy (DNE), nasopharyngolaryngoscopy (NPL) findings, imaging characteristics of lesion on CT, operative and histopathologic findings were evaluated. CT scan was performed with a 6-slice CT scanner (Brilliance, Philips Medical Systems, Netherlands) using a routine CT Neck protocol with 130 kVp and 150-220 mA tube current and slice thickness of 3 mm. Intravenous contrast medium (low osmolar, non-ionic, 300 mg/mL iodine content) was used routinely at a dose of 1 ml/kg administered by a single head pressure injector. The intensity of contrast enhancement (HU values) was measured with region of interest (ROI) varying from 25 to 60 mm2. ROI was placed manually in central portion of the lesion, avoiding any surrounding bone. The imaging findings of the lesion at imaging were correlated with operative and histopathologic findings.
Results
A total of 16 patients with rhinosporidiosis having preoperative contrast-enhanced CT imaging and histopathology confirmation were identified.
Demographics
The age of the patients in our study ranged from 14 to 73 years. All patients in the study were males. Of the 16 patients, 5 were from West Bengal, 3 each from Tamil Nadu and Jharkhand, 2 from Tripura, and 1 patient each from Orissa, Kerala, and Bangladesh.
Clinical findings
The clinical symptoms reported in our series are summarized in Table 1. A history of bathing in ponds was present in four cases.
The DNE and NPL findings in our study were presence of a reddish mass with whitish dots on surface in the nasal cavity (15 cases) and pedunculated polypoidal lesions arising from the nasopharyngeal/oropharyngeal wall (6 cases). A clinical diagnosis of rhinosporidiosis prior to imaging was made in all 16 cases based on clinical features and NPL findings.
Table 1.
Clinical symptoms

Imaging findings
Number of lesions [Table 2]: In the 16 patients included in our study, a total of 29 lesions were found and evaluated. A single lesion was noted in 10 patients, two lesions in 3 patients, and three lesions were found in 1 patient. Two patients presented with disseminated disease with multiple skin and subcutaneous lesions and multiple polypoidal lesions in the airway. One of the patients with disseminated disease was found to be HIV-positive while the other patient was immunocompetent.
-
Site and shape:
- Ear, Nose, and Throat (ENT) sites: On evaluating the site of involvement of disease [Table 2] [Figures 1-6], lesions were seen arising from the nasal cavity proper (n = 13), arising in nasal cavity and extending through choana into nasopharynx (n = 5), nasopharyngeal wall (n = 5), oropharyngeal wall (n = 2), and larynx (n = 1). In the nasal cavity, the inferior nasal cavity (13 lesions) consisting of nasal floor, inferior meatus, and inferior turbinate was most commonly involved. On evaluation of shape of the lesions, the nasal cavity lesions were seen as well-defined, lobulated, or irregular soft tissue lesions extending into vestibule anteriorly and through the choana into the nasopharynx posteriorly. The nasopharyngeal and oropharyngeal wall lesions were seen as pedunculated, polypoidal lesions with a bulbous tip. Of the four cases with oropharyngeal involvement, one case had polypoid attachment to the posterior soft palate and one was seen arising from the lateral oropharyngeal wall, while in two cases, the lesion was seen to project from the nasopharynx into the oropharynx. In one case with laryngeal involvement, a polypoid lesion was noted in the subglottis connected by a stalk to the right arytenoid cartilage.
- Tracheal and bronchial involvement: In one patient with bronchial involvement, the lesion was seen arising from the right main bronchus with extension into the right upper lobe bronchus. There was associated consolidation and volume loss in the right lower lobe and right upper lobe.
- Skin and subcutaneous tissue: In two patients with disseminated disease, the visualized imaging field revealed multiple, disseminated, irregular, and smooth nodular skin and subcutaneous lesions. Some of the subcutaneous lesions showed areas of necrosis.
Enhancement [Table 2]: All lesions showed moderate-to-intense enhancement compared to surrounding pharyngeal tissue/masticator muscles with HU ranging from 70 to 160. Lesions arising from oropharynx (HU 60), larynx (HU 60), and bronchus (HU 40) showed relatively less enhancement. In case of pedunculated, polypoidal lesions in the nasopharynx/oropharynx, the bulbous end of the polyp was relatively hypodense (HU 50) in enhancement compared to the stalk (HU 70). This was probably related to chronic dependency related mucosal edema of the tip. A prominent leash of blood vessels in the stalk of polyp was seen in three lesions originating from the nasopharyngeal wall. Due to irregular variegated surface of the polypoidal lesions, multiple small trapped air foci were seen along the surface of the lesions in nine cases. Small foci of calcification in the bulbous end of the polyp were noted in one case.
Bone involvement [Table 2]: Surrounding bone involvement was seen in the form of rarefaction (n = 6), partial (n = 3) or complete erosion (n = 3) of inferior turbinate, thinning of medial maxillary wall (n = 2), and septal erosion (n = 2). Histopathologic correlation in two cases revealed chronic osteomyelitis-related changes in the bone.
Nasolacrimal duct (NLD) involvement was seen in four cases. NLD extension was defined as extension of enhancing soft tissue within the NLD, having similar attenuation as the mass in the nasal cavity. NLD extension and resultant obstruction leading to lacrimal sac pyocele (1 case) and distal nasolacrimal duct mucocele (1 case) were noted.
A previous history of surgical excision of lesion with recurrence was found in nine cases. Both the patients with disseminated disease had a previous history of multiple recurrences. Previous imaging and operative details were not available for comparison. All 16 patients with rhinosporidiosis underwent surgical excision of their lesions. One patient with a bronchial lesion underwent bronchoscopy-guided excision of the lesion. On comparison with surgical findings, CT could correctly define the site and extent of involvement, number of lesions, and surrounding bone involvement in all cases. Associated imaging findings noted were bilateral ethmoidal polyps (1 case), maxillary (3 cases) and ethmoidal sinusitis (2 cases) due to obstruction by mass lesion.
Table 2.
Summary of cases
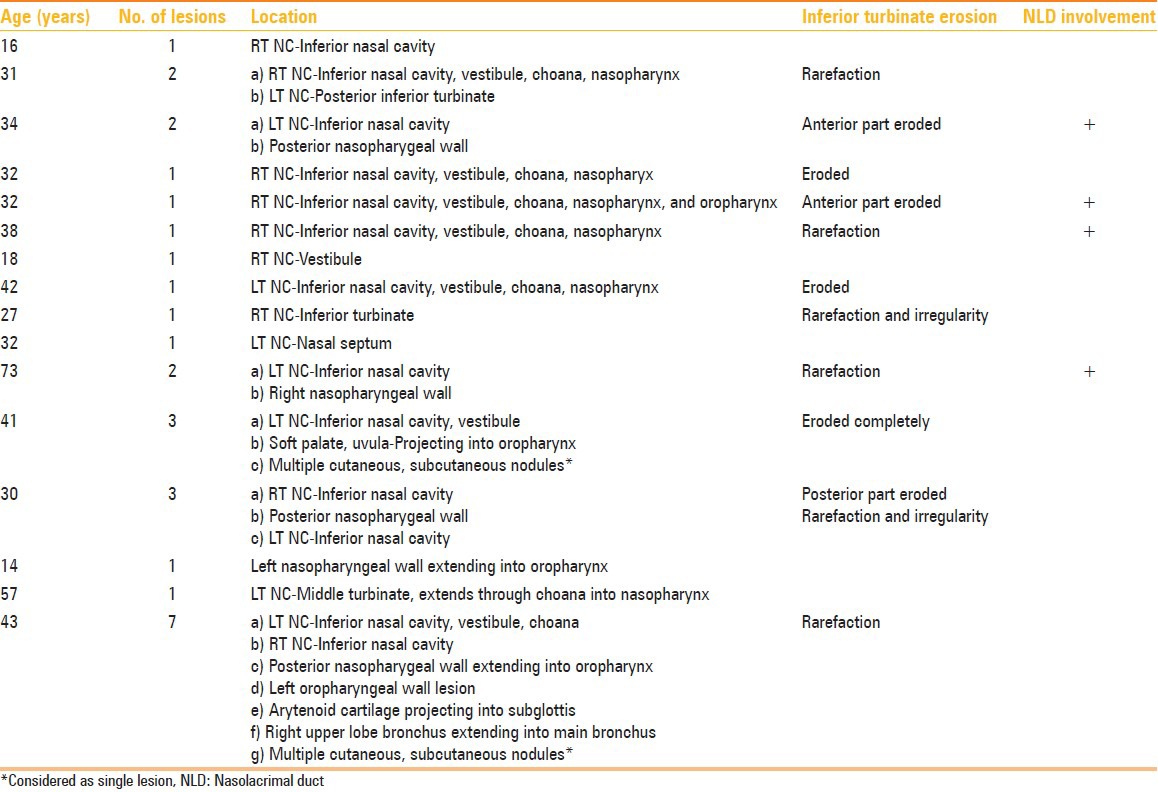
Figure 1(A-C):
A 31-year-old male with epistaxis and nasal mass. Contrast-enhanced CT PNS (A) Axial and (B) Coronal sections show an enhancing soft tissue mass lesion in the right inferior nasal cavity (thin black arrow) extending anteriorly into the vestibule and posteriorly into the nasopharynx. Another similar mass lesion is seen arising from the posterior aspect of the left inferior turbinate (thick black arrow). (C) Axial CT PNS image shows associated rarefaction of the right inferior turbinate (white arrow)
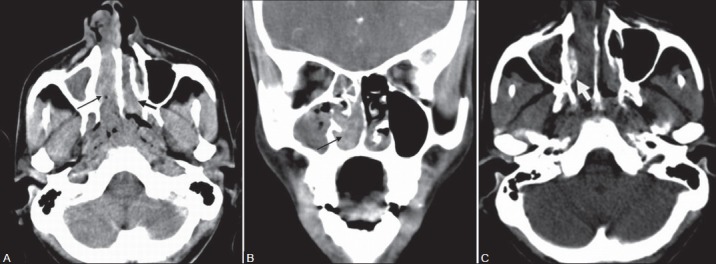
Figure 6(A-D):
A 41-year-old male with disseminated rhinosporidiosis and multiple cutaneous and subcutaneous nodules. Contrast-enhanced CT (A) Axial sections of PNS show an irregular enhancing soft tissue lesion (black arrow) in the left nasal cavity extending anteriorly into vestibule and posteriorly into the nasopharynx. (B) Coronal and (C) Axial PNS sections at the level of oropharynx show a pedunculated polypoidal enhancing soft tissue lesion (thin white arrows) arising from the posterior soft palate and projecting into oropharynx. Note the distal bulbous tip is relatively hypoattenuating compared to the stalk. Subcutaneous nodules are noted in the imaging field (thick white arrows). (D) Axial CT section of chest shows multiple subcutaneous nodules (thick white arrow)
Figure 3(A-C):
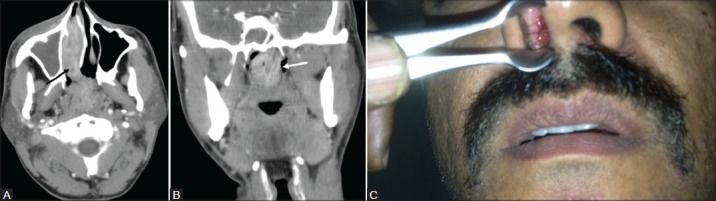
A 38-year-old male with epistaxis and nasal mass. Contrast-enhanced CT PNS (A) Axial image shows an enhancing soft tissue lesion (black arrow) in the right inferior nasal cavity extending through the choana into the nasopharynx. (B) Coronal section shows lobulated nasopharyngeal extension of the lesion (white arrow) with prominent leash of blood vessels. (C) Anterior rhinoscopy shows a red fleshy mass with whitish spots in the right nasal cavity. Diagnosis after surgery confirmed as rhinosporidiosis
Discussion
Rhinosporidiosis is a non-contagious chronic granulomatous infection seen mainly in adult males aged between 20 and 40 years.[3] In our study, all the cases were male patients aged between 14 and 73 years. The 14-year-old child had a history of previous surgery 3 years back with recurrence of lesion indicating that infection can be seen in younger children.
Usual presenting symptoms of disease include nasal mass, nasal obstruction, and recurrent epistaxis.[7] A history of pond bathing can be associated indicating the possible source of infection. On clinical examination, reddish vascular polyps studded with whitish dots are seen in the nasal cavity and extending into the vestibule. Polyploid lesions may be seen to project into the oropharynx from its nasopharyngeal wall attachment.[7] Similar results were noted in our study.
Rhinosporidiosis is commonly diagnosed clinically based on a history of origin from an endemic area, clinical symptoms, and classic nasal and endoscopic findings. The common sites of occurrence are the nasal cavity and nasopharynx. However, it is known to involve the lacrimal sac, larynx, conjunctiva, parotid duct, tracheobronchial tree, skin, urethra, vagina, and bones.[1,5,7,8] In our study, the sites of occurrence of disease were nasal cavity, nasopharynx, oropharynx, larynx, nasolacrimal duct, bronchus, skin, and subcutaneous tissue [Figures 1-6]. In the nasal cavity, the most common site involved was the inferior nasal cavity, comprising nasal floor, inferior turbinate, and inferior meatus. According to Banjara et al., the most common sites of involvement in rhinosporidiosis in order of frequency are nasal cavity, nasopharynx, lacrimal sac, and conjunctiva.[4]
Despite being an endemic disease in certain parts of the world, the imaging features of the disease have not been previously described in the literature. The most common imaging feature in our study was presence of a well-defined, lobulated, or irregular moderately enhancing soft tissue mass with HU ranging from 70 to 160, primarily centered in the inferior nasal cavity. Polypoidal lesions arising from oropharynx, larynx, and bronchus were minimally enhancing compared to nasal lesions indicating that the site of attachment of lesion plays an important role in determining the degree of blood supply and resultant enhancement. NLD involvement [Figure 2] was noted in four cases and is considered to be the route of spread for lacrimal sac involvement.[9] NLD involvement is likely a result of close proximity of its opening in the inferior meatus which is commonly involved in rhinosporidiosis and should be looked for in all cases of rhinosporidiosis. NLD involvement was defined when soft tissue extension of similar attenuation to the nasal mass was seen. Mere distension of the NLD with fluid without soft tissue extension is probably due to the presence of the obstructing nasal mass and should not be mistaken for NLD involvement.
Figure 2(A-C):
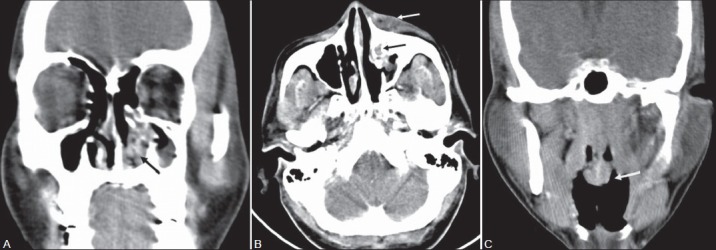
A 34-year-old male with left nasal mass and periorbital swelling. Contrast-enhanced CT PNS (A) Coronal image shows an irregular enhancing soft tissue lesion in the left inferior nasal cavity (black arrow) with partial erosion of the left inferior turbinate. (B) Axial CT image shows extension of enhancing soft tissue into the left nasolacrimal duct (black arrow) with left lacrimal sac pyocele (white arrow). (C) Coronal section shows another pedunculated polyploidal lesion (white arrow) with bulbous tip arising from the posterior nasopharyngeal wall. Diagnosis after surgery confirmed as rhinosporidiosis
Involvement of surrounding bones is common in rhinosporidiosis, as seen in our study. Bone involvement was seen as irregularity, rarefaction, partial or complete erosion of inferior turbinate, thinning of medial maxillary wall, and septal erosion [Figures 1, 2, and 5]. Although majority of the patients had a history of previous surgery, the bone involvement was likely a result of the disease process rather than surgical excision, as evidenced by the histopathology evidence of chronic osteomyelitis related changes in two cases and the rarefaction and irregular pattern of bone erosion on imaging. However, it is possible that in some cases with complete erosion of inferior turbinate, findings were likely due to a previous operative procedure. Maxillary sinus extension was not seen in any of the cases in our study and this feature may help in differentiating it from other nasal masses such as antrochoanal polyps and inverted papillomas.
Figure 5(A, B):
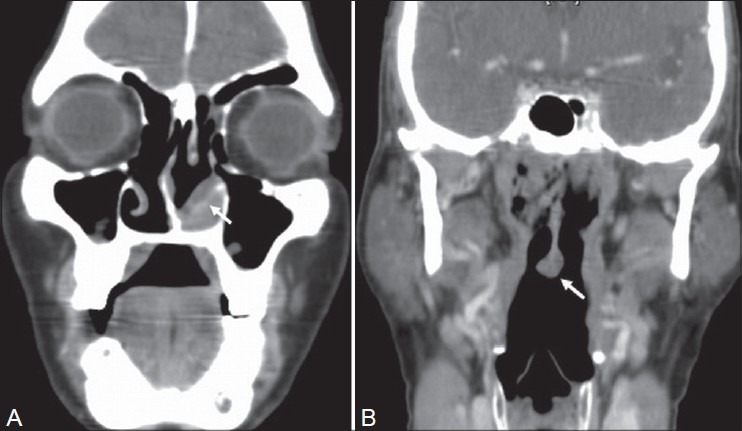
A 73-year-old male with nasal mass and epistaxis. Contrast-enhanced CT PNS (A) Coronal sections show an enhancing irregular soft tissue lesion (white arrow) in the left inferior nasal cavity with rarefaction of the left inferior turbinate and (B) A pedunculated polyploidal lesion (white arrow) with bulbous tip arising from the posterior nasopharyngeal wall. Histopathology confirmed it as rhinosporidiosis
Disseminated disease with multiple cutaneous and subcutaneous lesions was seen in two cases [Figures 4 and 6]. This form of the disease is known to occur in both immunodeficient (HIV-positive) and immunocompetent individuals, as was noted in our study.[10,11,12] Laryngeal and bronchial involvement [Figure 4] was seen in one case with disseminated disease. This patient had a history of previous excision of nasal/nasopharyngeal mass, and the new lesions probably were a result of drop lesions into the airway during surgery. Similar observations have been made by Banjara et al., wherein they reported a case of bronchial rhinosporidiosis with a previous history of surgery for nasal lesions.[4] Laryngeal and tracheal lesions may also be due to repeated intubation.
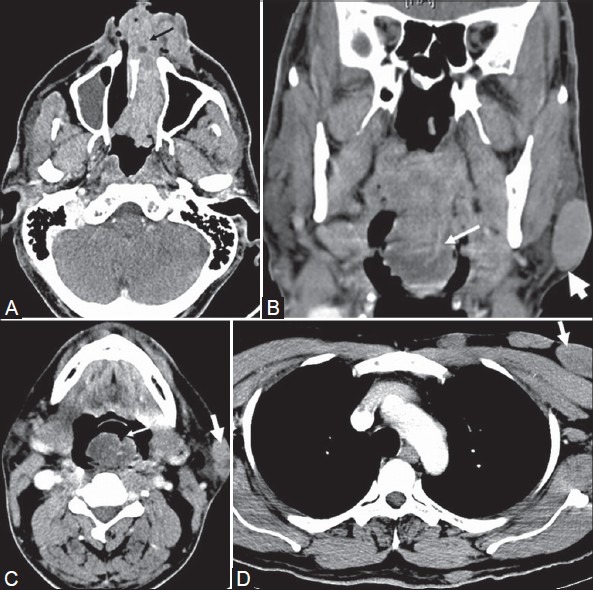
Figure 4(A-C):
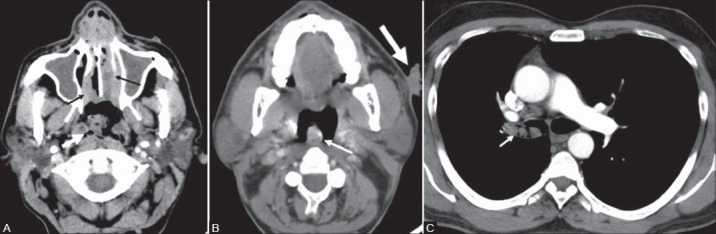
A 43-year-old male with disseminated rhinosporidiosis and multiple cutaneous and subcutaneous nodules. Contrast-enhanced CT (A) Axial section of PNS shows enhancing soft tissue lesions in the bilateral inferior nasal cavity (black arrows) and a lobulated soft tissue lesion (white arrow) arising from the posterior nasopharyngeal wall showing multiple small trapped air foci along its surface. (B) Axial section of oropharynx shows a lobulated enhancing soft tissue lesion (thin white arrow) arising from the oropharyngeal wall. An irregular verrucous cutaneous nodule (thick white arrow) is noted in the imaging field. (C) Axial section of chest shows irregular enhancing soft tissue lesion (white arrow) in the right main bronchus. Histopathology revealed multiple disseminated rhinosporidiosis
The major imaging differentials for rhinosporidiosis include juvenile angiofibroma, inverted papilloma, hemangioma, angiomatous polyp, lobular capillary hemangioma, and sinonasal malignancy. Juvenile angiofibroma is seen in young males and can be a potential diagnostic problem in patients presenting with epistaxis. On imaging, they are seen to arise close to the sphenopalatine foramen, causing erosion of the pterygoid process and anterior displacement of the posterior superior antral wall.[13] Inverted papilloma usually arises from both surfaces of the lateral nasal wall with early spread into maxillary sinus. On CT, it presents as a lobulated enhancing mass with high densities within, representing residual bone fragments. On magnetic resonance imaging (MRI), it shows a characteristic cerebriform appearance on T2-weighted (T2W) and T1 post-contrast imaging.[14] Sinonasal malignancies generally originate from the maxillary sinus and secondarily invade the nasal cavity.[15] On imaging, they are heterogeneous in appearance, showing necrosis and extensive bone destruction. Lobular capillary hemangioma is an idiopathic benign capillary proliferation commonly seen to arise from the inferior turbinate. On CT, it appears as lobular, intensely enhancing lobular mass with an iso- or hypodense cap around the enhancing region of the mass. Associated bone changes like erosions can be seen.[16] Angiomatous polyps are rare variants of sinonasal polyps which are fibrosed and vascularized and show large vascular spaces. On CT, the intranasal part of the polyps shows intense enhancement due to vascular stasis. Surrounding bone thickening rather than erosions may be associated. All the nasal cavity lesions in our study showed homogenous enhancement with no characteristic enhancing pattern. In addition, extension into the sphenopalatine foramen or maxillary sinus was not seen in any of the cases, thus helping in differentiating it from other enhancing nasal cavity lesions.
The role of imaging in rhinosporidiosis is to evaluate the number of lesions, the location and extent of disease, surrounding bone involvement, nasolacrimal duct involvement, and any associated complications. In cases of laryngeal and tracheobronchial involvement, CT helps in detecting the location of disease, extent of airway obstruction, and associated complications like airway obstruction, consolidation, and volume loss. On CT, rhinosporidiosis is commonly seen as a homogenously enhancing lobulated lesion in the inferior nasal cavity, extending into the vestibule anteriorly and through the choana into the nasopharynx posteriorly with erosion/rarefaction of the inferior turbinate. Multiple sites of involvement may be seen in cases with disseminated disease.
Our study had potential limitations. Due to the retrospective nature of the study, correlation of certain imaging findings could not be made based on available surgical findings. For example, certain areas of hyperenhancement in the nasopharyngeal wall which could represent small focus of disease could not be confirmed based on the available surgical/endoscopy notes and, hence, were not included in the study.
Conclusion
Rhinosporidiosis is a chronic granulomatous disease endemic in certain regions of India and shows a high rate of recurrence after surgery. On contrast-enhanced CT, rhinosporidiosis is commonly seen as enhancing lobulated mass in the inferior nasal cavity or as a polypoidal mass in the oro or nasopharynx with the bulbous end of the polypoidal lesion being relatively hypoattenuating. Adjacent bone erosion is also a common finding. Contrast-enhanced CT, however, has an important role in delineating the site and extent of the disease, as well as bone, nasolacrimal duct, and tracheobronchial tree involvement and detection of associated complications, thus providing a useful roadmap prior to surgery.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mallick AA, Majhi TK, Pal DK. Rhinosporidiosis affecting multiple parts of the body. Trop Doct. 2012;42:174–5. doi: 10.1258/td.2012.120035. [DOI] [PubMed] [Google Scholar]
- 2.Fredricks DN, Jolley JA, Lepp PW, Kosek JC, Relman DA. Rhinosporidium seeberi: A human pathogen from a novel group of aquatic protistan parasites. Emerg Infect Dis. 2000;6:273–82. doi: 10.3201/eid0603.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumari R, Nath AK, Rajalakshmi R, Adityan B, Thappa DM. Disseminated cutaneous rhinosporidiosis: Varied morphological appearances on the skin. Indian J Dermatol Venereol Leprol. 2009;75:68–71. doi: 10.4103/0378-6323.45225. [DOI] [PubMed] [Google Scholar]
- 4.Banjara H, Panda RK, Daharwal AV, Sudarshan V, Singh D, Gupta A. Bronchial rhinosporidiosis: An unusual presentation. Lung India. 2012;29:173–5. doi: 10.4103/0970-2113.95336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudarshan V, Gahine R, Daharwal A, Kujur P, Hussain N, Krishnani C, et al. Rhinosporidiosis of the parotid duct presenting as a parotid duct cyst-A report of three cases. Indian J Med Microbiol. 2012;30:108–11. doi: 10.4103/0255-0857.93079. [DOI] [PubMed] [Google Scholar]
- 6.Rekha P, Thomas B, Pappachan JM, Venugopal KP, Jayakumar TK, Sukumaran P. Tracheal rhinosporidiosis. J Thorac Cardiovasc Surg. 2006;132:718–9. doi: 10.1016/j.jtcvs.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Pal DK, Mallick AA, Majhi TK, Biswas BK, Chowdhury MK. Rhinosporidiosis in southwest Bengal. Trop Doct. 2012;42:150–3. doi: 10.1258/td.2012.120177. [DOI] [PubMed] [Google Scholar]
- 8.Arora R, Gupta R, Dinda AK. Rhinosporidiosis of trachea: A clinical cause for concern. J Laryngol Otol. 2008;122:e13. doi: 10.1017/S0022215108001849. [DOI] [PubMed] [Google Scholar]
- 9.Saha J, Basu AJ, Sen I, Sinha R, Bhandari AK, Mondal S. Atypical presentations of rhinosporidiosis: A clinical dilemma? Indian J Otolaryngol Head Neck Surg. 2011;63:243–6. doi: 10.1007/s12070-011-0275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma R, Vasudevan B, Pragasam V, Deb P, Langer V, Rajagopalan S. A case of disseminated cutaneous rhinosporidiosis presenting with multiple subcutaneous nodules and a warty growth. Indian J Dermatol Venereol Leprol. 2012;78:520. doi: 10.4103/0378-6323.98101. [DOI] [PubMed] [Google Scholar]
- 11.Padmavathy L, Rao IL, Selvam SS, Sahoo CG. Disseminated cutaneous rhinosporidiosis in a HIV sero--positive patient. Indian J Dermatol Venereol Leprol. 2001;67:332–3. [PubMed] [Google Scholar]
- 12.Nayak S, Rout TK, Acharjya B, Patra MK. Subcutaneous Rhinosporidiosis. Indian J Dermatol. 2008;53:41–3. doi: 10.4103/0019-5154.39746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd G, Howard D, Phelps P, Cheesman A. Juvenile angiofibroma: The lessons of 20 years of modern imaging. J Laryngol Otol. 1999;113:127–34. doi: 10.1017/s0022215100143373. [DOI] [PubMed] [Google Scholar]
- 14.Ojiri H, Ujita M, Tada S, Fukuda K. Potentially distinctive features of sinonasal inverted papilloma on MR imaging. AJR Am J Roentgenol. 2000;175:465–8. doi: 10.2214/ajr.175.2.1750465. [DOI] [PubMed] [Google Scholar]
- 15.Tiwari R, Hardillo JA, Mehta D, Slotman B, Tobi H, Croonenburg E, et al. Squamous cell carcinoma of maxillary sinus. Head Neck. 2000;22:164–9. doi: 10.1002/(sici)1097-0347(200003)22:2<164::aid-hed8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Lee DG, Lee SK, Chang HW, Kim JY, Lee HJ, Lee SM, et al. CT features of lobular capillary hemangioma of the nasal cavity. AJNR Am J Neuroradiol. 2010;31:749–54. doi: 10.3174/ajnr.A1908. [DOI] [PMC free article] [PubMed] [Google Scholar]


