Abstract
Purpose:
To evaluate the spectrum of celiac axis, common hepatic artery (CHA), right, left, middle hepatic artery and gastroduodenal artery variations by using spiral computed tomography (CT).
Materials and Methods:
A retrospective review of Multidetector CT (MDCT) abdominal angiography scans was performed in patients sent for various liver and other abdominal pathologies between January 2012 and February 2013. A total of 600 patients were evaluated. Definitions of CHA, ambiguous celiac axis, course and division patterns of CHA, replaced hepatic artery, accessory hepatic artery and middle hepatic artery were used as proposed by Song et al., Covey et al., and Wang et al. The pattern of the aortic origin of branches of celiac axis, common hepatic artery and its branches was analyzed.
Results:
Six types of celiac axis anatomic variations were identified in our study. A total of 546 of the 600 patients had a normal celiac axis anatomy. Anatomic variations were seen in 5.5% of patients. Ambiguous anatomy was seen in 3.5% of the patients. CHA originated from celiac axis in 95.83% of the patients. Variations in anatomic origin of CHA were seen in 8 patients. Ambiguous dual pathway was seen in 4 patients. Normal Sp-preportal course of CHA was identified in 97.78% of cases, Sp-retroportal course in 7 patients, Tp-preportal course in 2, Tp-retroportal in 1, Ip-preportal in 1 and through Ligamentum venosum in 2 patients. Normal origin of RHA from HAP was seen in 79.6% patients. Replaced origin of RHA was seen in 15.16% cases and Accessory origin of RHA was seen in 5.16% cases. LHA originated from HAP in 81.5% patients. Replaced LHA origin was seen in 10.8% cases and Accessory LHA origin seen in 7.6% cases. MHA originated from RHA in 41.3% patients, LHA in 27.83% and from CHA in 4.5% cases. Origin of MHA could not be defined in 26.3% of patients. GDA originated from CHA in 97.6% of patients, from celiac axis in 1.6%, from RHA in 0.33% in patients. Trifurcation of CHA was seen in 7.16% and quadrifurcation of CHA in 2.16%.
Conclusion:
CT Angiography is a safe and highly sensitive and accurate modality for evaluation of arterial anatomy and its variants.
Keywords: Abdominal angiography, accessory, ambiguous celiac axis, celiac axis, common hepatic artery, replaced hepatic artery
Introduction
The importance of celiac axis, common hepatic artery variations, and origin of middle hepatic artery has been an area of great interest and research since 1756 when Haller first described celiac axis variations. In 1955, Michel described the classification scheme for describing anatomic variation in the hepatic arterial blood supply based on the results of dissecting 200 cadavers [Table 1].[1] In 1969, Vandamme et al., did extensive research on hepatic artery anomalies on 156 cadavers.[2] Then, in 1971, Suzuki et al., published their series on 200 patients based on angiography study and also highlighted the importance of hepatic artery variations.[3] In 2010, Song et al., published the largest series of their work in celiac axis and hepatic artery variations in 5002 patients.[4]
Table 1.
Hepatic arterial variants according to the Michel's classification[1]
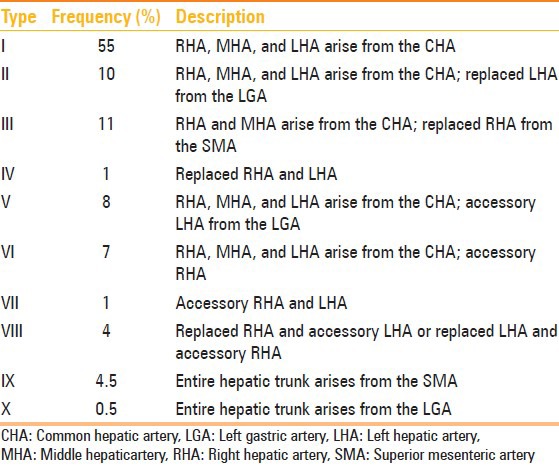
Recently, with the advent of newer interventional and surgical options for patients with primary and metastatic hepatic malignancies, partial hepatectomy for liver transplantation and laparoscopic cholecystectomy, surgeons and interventional radiologists are now relying on accurate imaging and assessment of the hepatic arterial supply. A road map of the arterial vascularity of the donor and recipient is a prerequisite for transplant surgery [Table 2].[5] Detailed hepatic arterial anatomy and its variations has its significance in liver surgeries and interventional hepatic procedures, relative to the hepatic lobe involved [Table 3].[5]
Table 2.
Hepatic arterial variants and liver transplantation[5]
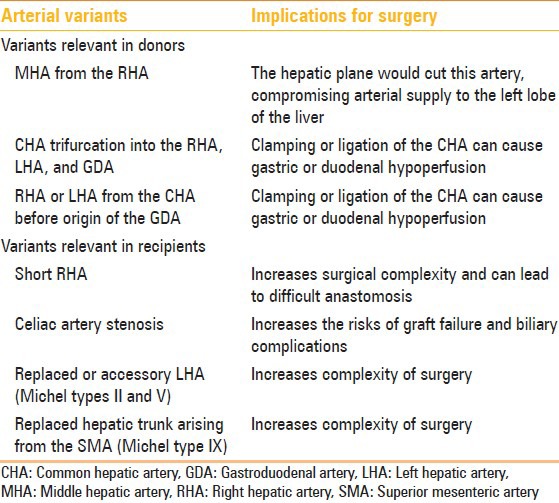
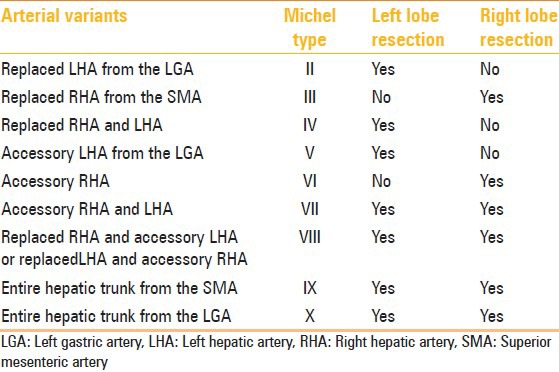
Table 3.
Hepatic arterial variants and relevance for tumor resection[5]
The purpose of this study was to study the anatomic variations in the origin and course of celiac axis, common hepatic artery and its branches – right, left, middle hepatic, and gastroduodenal arteries.
Materials and Methods
Patients
Approval for the study was obtained from the ethical board of the Institution. Multidetector CT (MDCT) abdominal angiography scans were obtained in patients sent for various liver and other abdominal pathologies in which biphasic contrast-enhanced CT was indicated. The time period of the study was from January 2012 to February 2013. A total of 600 patients were evaluated. The study population comprised 413 men and 187 women (mean age 39.8 years). We excluded patients with a history of major upper abdominal surgery.
CT examination protocol
MDCT angiography was performed on Philips Brilliance 40-slice after injecting maximum of 120 mL of nonionic iodinated contrast material (iodine concentration, 370 mg/mL) through an 18-20-gauge antecubital intravenous cannula at a rate of 5-7 mL/sec. The detailed multidetector CT angiography technique is summarized in Table 4.
Table 4.
Protocol for multidetector CT angiography
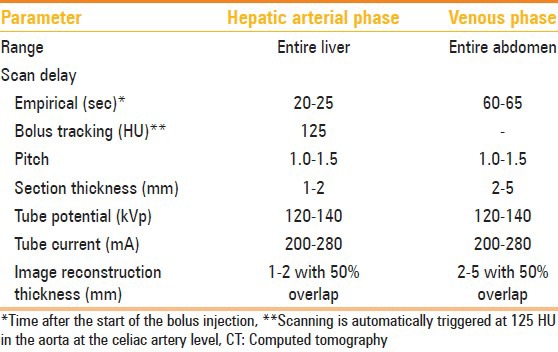
Image interpretation
The raw imaging data obtained from MDCT were processed on a commercially available workstation for multiplanar reformation as well as 3D reconstruction with maximum intensity projection (MIP) and volume rendering. The images were analyzed independently by three radiologists Radiologist 1 (B.S.) with 5 years, Radiologist 2 (M.S.) with 20 years and Radiologist 3 (M.K.M) with 24 years of experience in interpreting CT scans. The abbreviations used in this study are listed in Table 5. We analyzed the celiac axis anatomy and origin of the four major arteries – the common hepatic, splenic, left gastric, and superior mesenteric arteries. We also studied the origin of right hepatic artery (RHA), left hepatic artery (LHA), middle hepatic artery (MHA), and gastroduodenal artery (GDA). The course and relationship of common hepatic artery (CHA) to the surrounding structures (portal vein, pancreas head, or uncinate process) was also analyzed. We used some key definitions described by various authors for evaluating these arteries.
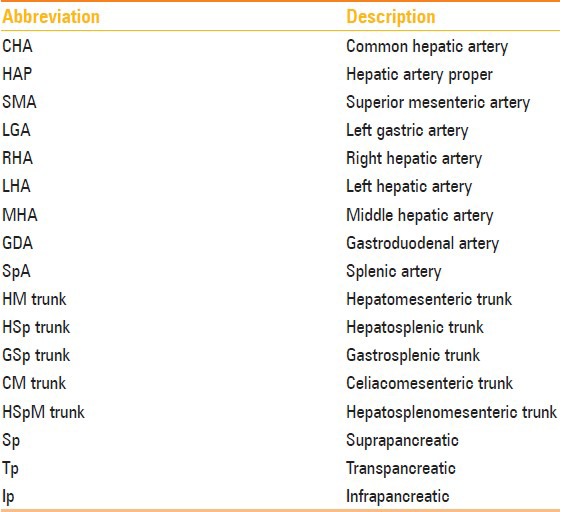
Table 5.
Abbreviations used in this study
Key definitions
CHA[4] : Artery giving rise to RHA or LHA and the GDA, irrespective of its origin and course.
Ambiguous celiac axis[4] : Congenital absence of CHA or congenital presence of an anastomotic channel connecting the celiac axis and superior mesenteric artery (SMA) or anastomotic channel connecting the CHA to the celiac axis and the SMA.
Typical celiac axis[4] : Arterial trunk that gives rise to the common hepatic, left gastric, and splenic arteries.
Replaced[6] : Replaced origin of hepatic arteries refers to the arterial blood supply from an ectopic location.
Accessory[6] : Accessory origin of hepatic arteries refers to the arterial blood supply from typical as well as ectopic branch.
Double hepatic arteries[6] : Defined when there is early branching of the RHA and/or LHA off the celiac axis or when one or both hepatic arteries arise directly from the aorta or when no CHA with GDA originates from either hepatic artery.
Late origin of GDA[6] : GDA originates from one hepatic artery after the division of the CHA into the RHA and LHA.
Early branching of RHA/LHA[6] : RHA/LHA originating before GDA.
Trifurcation[6] : CHA divides into GDA, RHA, and LHA.
Quadrifurcation[6] : CHA divides into GDA, RHA, LHA, and MHA.
Suprapancreatic[4] : CHA running along the upper border of pancreas.
Transpancreatic[4] : CHA passing through pancreatic parenchyma.
Infrapancreatic[4] : CHA coursing inferior to the pancreatic head and uncinate process.
Preportal[4] : Hepatic artery anterior to the main portal vein.
Retroportal[4] : Hepatic artery posterior to the main portal vein.
MHA[7] : Hilar artery that primarily supplies hepatic segment 4.
Results
Celiac axis variations
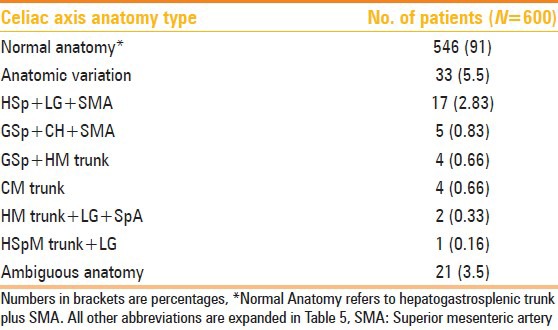
A total of 546 (91%) of the 600 patients had a normal celiac axis anatomy, i.e. hepatogastrosplenic trunk and SMA originating separately from the aorta. Six types of celiac axis anatomic variations were identified in our study [Table 6]. These were seen in 33 (5.5%) of the patients. Ambiguous anatomy was seen in 21 (3.5%) patients.
Table 6.
Celiac axis variations in 600 patients
CHA variations
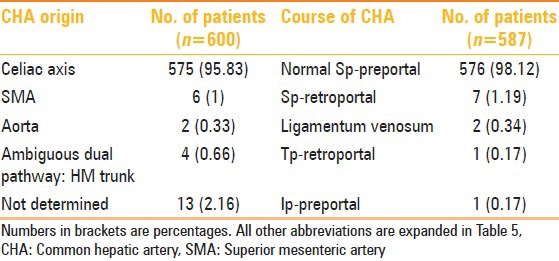
CHA originated from the celiac axis in 575 (95.83%) of the patients. Variations in anatomic origin of CHA were seen in eight patients (origin from SMA in six and from aorta in two patients). Ambiguous dual pathway (hepatomesenteric trunk) was seen in 4 (0.66%) patients. The origin of CHA could not be determined in 13 (2.16%) of the patients.
Normal suprapancreatic (Sp)-preportal course of CHA was identified in 576 (98.12%) of the cases. Sp-retroportal course was seen in seven patients, transpancreatic (Tp)-retroportal in one, infrapancreatic (Ip)-preportal in one, and through ligamentum venosum in two patients. Detailed findings of CHA variations are summarized in Table 7.
Table 7.
CHA anatomy and course in 600 patients
RHA, LHA, MHA, and GDA variations
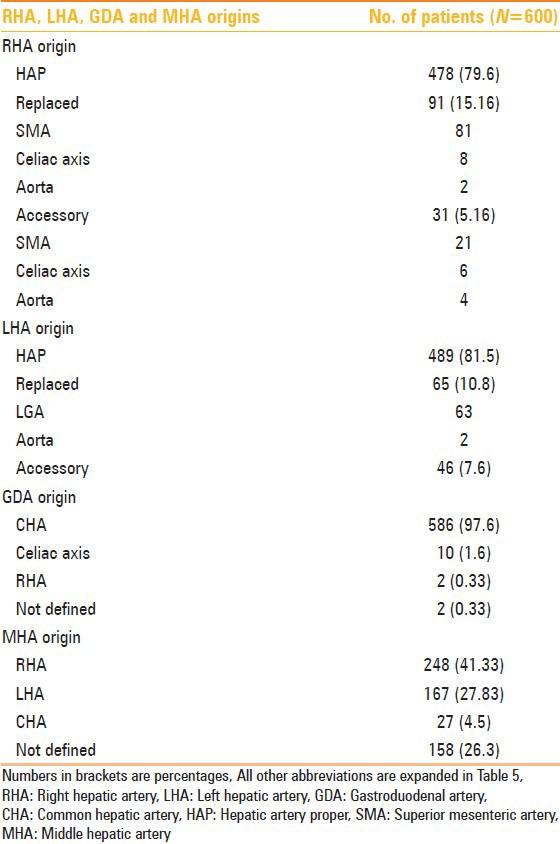
Normal origin of RHA from hepatic artery proper (HAP) was seen in 478 (79.6%) patients. Replaced origin of RHA was seen in 91 (15.16%) cases and accessory origin of RHA was seen in 31 (5.16%) cases. LHA originated from HAP in 489 (81.5%) patients. Replaced LHA origin was seen in 65 (10.8%) cases and accessory LHA origin seen in 46 (7.6%) cases. MHA originated from RHA in 248 (41.33%) patients, from LHA in 167 (27.83%), and from CHA in 27 (4.5%) cases. Origin of MHA could not be defined in 158 (26.3%) of the patients. GDA originated from CHA in 586 (97.6%) of the patients, from celiac axis in 10 (1.6%), and from RHA in 2 (0.33%) patients. Origin of GDA could not be determined in two cases. Detailed anatomic variations of RHA, LHA, MHA, and GDA are summarized in Table 8.
Table 8.
RHA, LHA, GDA, and MHA origins
Other variations
Trifurcation of CHA was seen in 43 (7.16%) and quadrifurcation of CHA in 13 (2.16%) of the patients. Early branching of RHA was seen in 5 (0.83%) and early branching of LHA in 3 (0.5%) of the patients. Double hepatic arteries were seen in 14 (2.33%) patients. Late origin of GDA was seen in two patients.
Discussion
By using clear definitions pertaining to the origin and course of these vessels as already mentioned, we performed systematic analysis of CT angiography images and were able to identify and classify the comprehensive spectrum of celiac axis, CHA, RHA, LHA, MHA, and GDA variations, including their variant origins and 3D anatomic courses. According to Tandler et al.,[8] four primitive ventral branches arise from the abdominal aorta that are interconnected by an anastomotic channel during embryogenesis. It is the persistent overgrowth or regression of these primitive vascular channels which leads to the development of variations of the celiac axis and the SMA. On the basis of this hypothesis, 15 types of celiac axis variation are possible.[9] Six of these 15 types were found in this study. According to Michels, the prevalence of a normal celiac axis anatomy is 89%. In our study, normal celiac axis anatomy was seen in 91% [Figure 1]. The most common anatomic variation was of hepatosplenic trunk with separately originating left gastric artery (LGA) and SMA (2.83%) [Figure 2]. This result was consistent with the results obtained by Song et al.[4] The next common anatomic variation in our study was that of gastrosplenic trunk with separate origins of CHA and SMA (0.83%) [Figure 3], followed by that of gastrosplenic trunk and a common hepatomesenteric trunk. This variation was seen more frequently in our study as compared to that observed by Song et al.[4] Ambiguous celiac axis anatomy was identified in 21 (3.5%) of the patients. Ambiguous anatomy due to persistent anastomotic channel was seen in 8 patients [Figure 4] and due to absent CHA was seen in 13 patients [Figure 5]. This persistent anatomic channel is also known as the Arc of Buehler. The Arc of Buehler is a remnant of a ventral anastomosis that interconnects embryonic ventral segmental arteries.[8,10,11]
Figure 1.
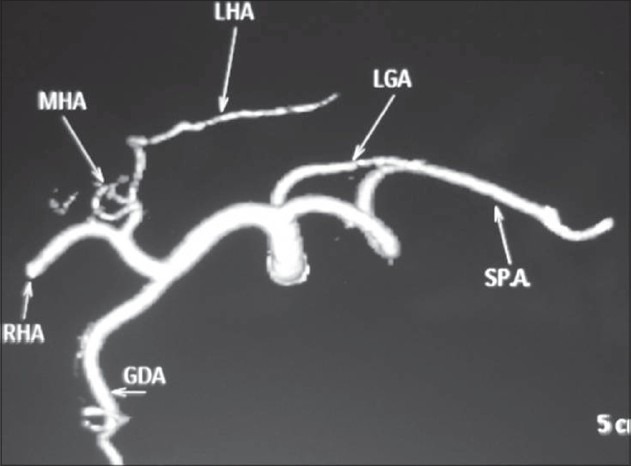
Volume-rendered images showing normal anatomy of the celiac axis and its branches
Figure 2.
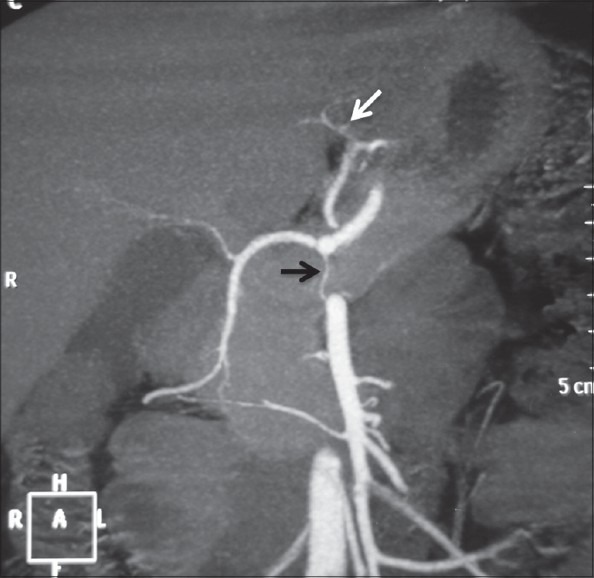
Volume-rendered image showing anatomic variation of the celiac axis (LG + HSp + SMA) with LGA (white arrow) arising from aorta, hepatosplenic trunk (arrowhead), and separate origin of SMA (black arrow)
Figure 3.
Volume-rendered image showing anatomic variation of celiac axis (GSp + CH + SMA) in the form of gastrosplenic trunk (black arrow), common hepatic (white arrow), and SMA (arrowhead) origin separately from aorta
Figure 4.
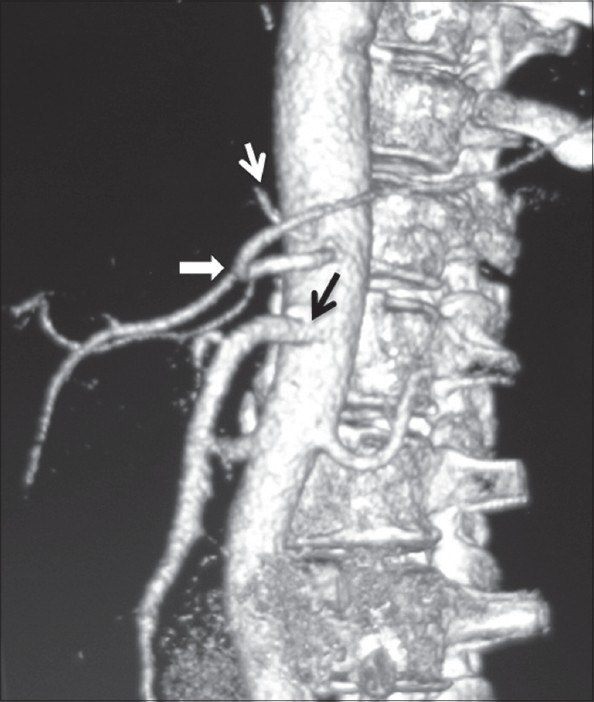
Coronal maximum-intensity projection images showing ambiguous celiac axis due to vertical anastomotic channel (black arrow) between celiac axis and SMA. Replaced LHA from LGA (white arrow) is also noted
Figure 5.
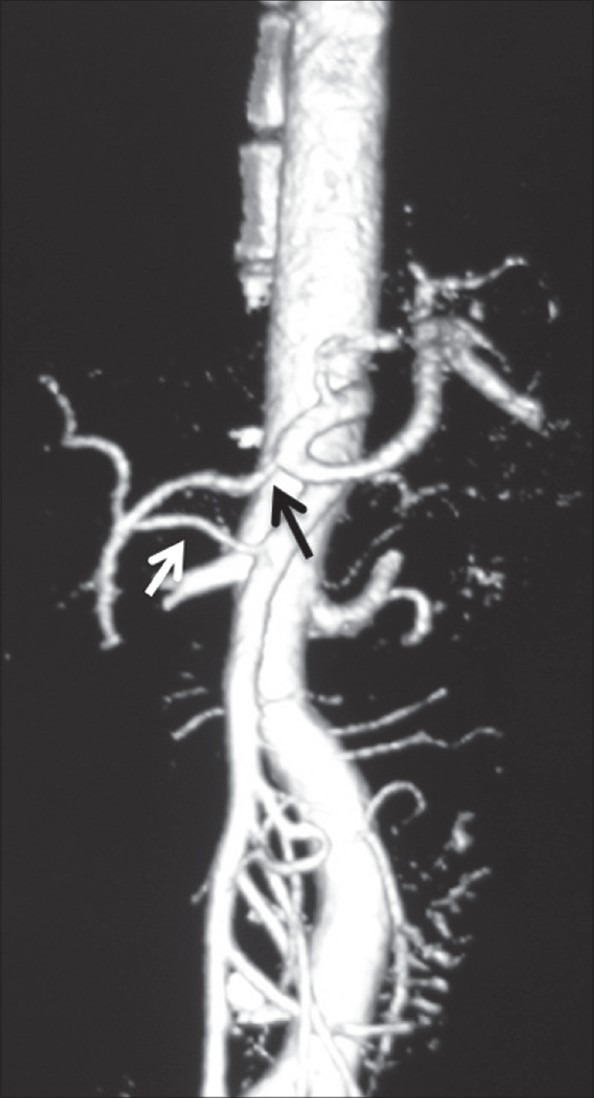
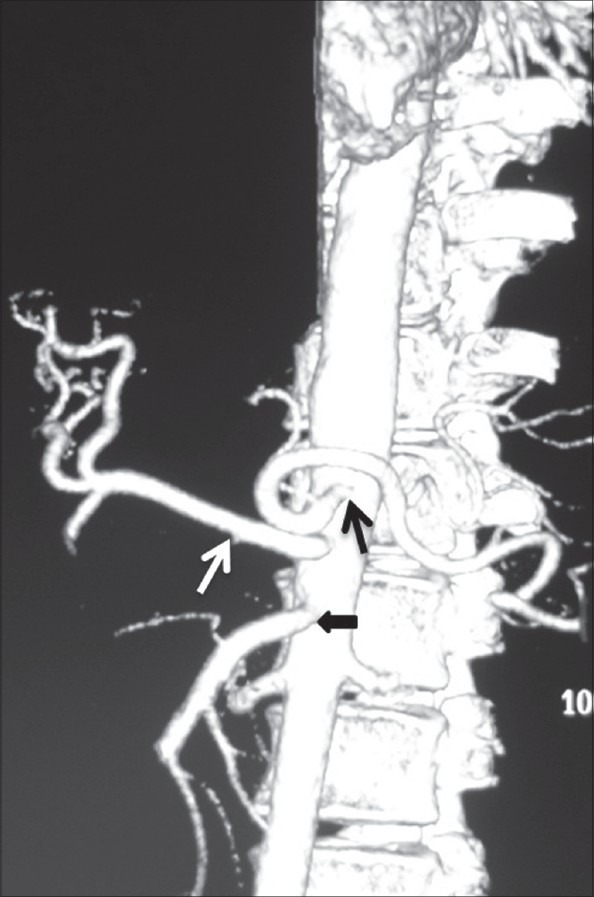
Volume-rendered image showing replaced origin of RHA (white arrow) from SMA and ambiguous celiac axis due to absent CHA and origin of GDA (black arrow) from celiac axis
Normal CHA origin was seen in 575 (95.83%) of the cases. Normal Sp-preportal course of CHA was seen in 576 (98.12%) patients. The SMA has been known as the most common origin site of a variant CHA. In our study also, we found similar findings with six cases of CHA arising from the SMA. CHA, after arising from the SMA, has three levels of pathways relative to the pancreatic head and the uncinate process – supra-, trans-, and infrapancreatic courses – as described by Song et al. The Sp-retroportal course was the most common type of variant CHA that arose from the SMA. In our study, four cases of CHA arising from the SMA had Sp-retroportal course, one case had Tp-retroportal course [Figure 6], and one had Ip-preportal course. A separate origin of the CHA from the aorta is a rare variation, and the prevalence of this variation in our study was only 0.33%. The reported prevalences of this anomaly in two previous dissection studies were 0.2% and 0.7%, respectively.[12,13]
Figure 6.
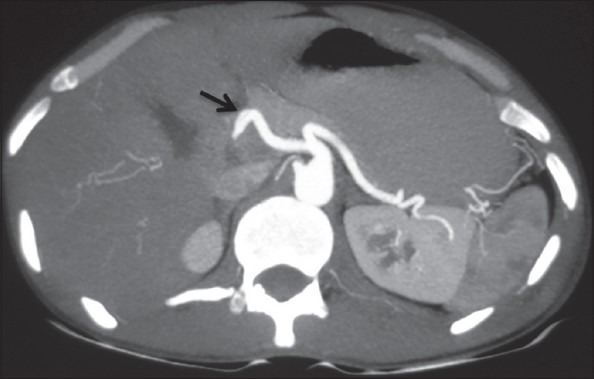
Axial maximum-intensity projection image showing transpancreatic course of CHA (arrow)
RHA origin from HAP was seen in 478 (79.6%) patients. Replaced origin of RHA was seen in 91 (15.16%) of the cases. The most common site of origin of replaced RHA was from SMA (13.49%) [Figure 7] followed by celiac axis (1.33%) and aorta (0.33%) [Figure 8]. Accessory origin of RHA was seen in 31 (5.16%) of the cases. The most common site of origin of accessory RHA was from the SMA followed by celiac axis and aorta [Figure 9]. LHA origin from HAP was seen in 489 (81.5%) patients. Replace origin of LHA was seen in 10.8% cases, with the most common site being from the LGA (10.46%) followed by aorta (0.33%). Accessory origin of LHA was seen in 7.6% cases, with all of them arising from the LGA in our study. In our study, all replaced and accessory RHA had retroportal course and replaced/accessory LHA had course through the ligamentum venosum.
Figure 7.
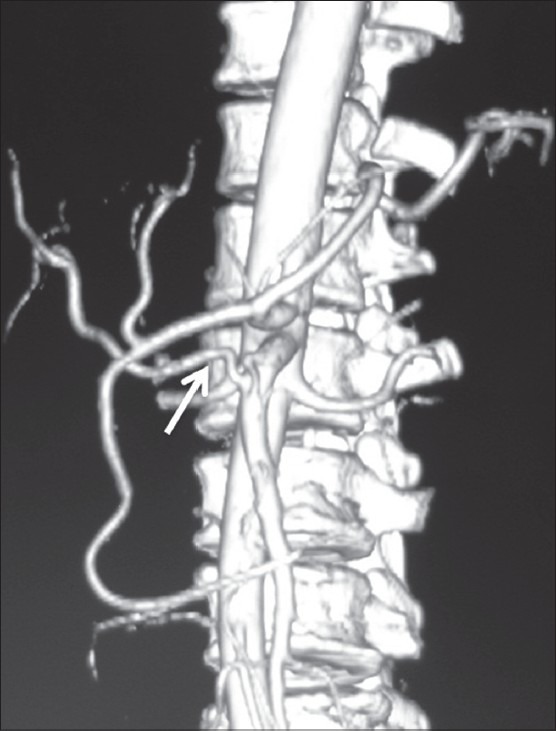
Volume-rendered image showing replaced origin of RHA (arrow) from SMA
Figure 8.
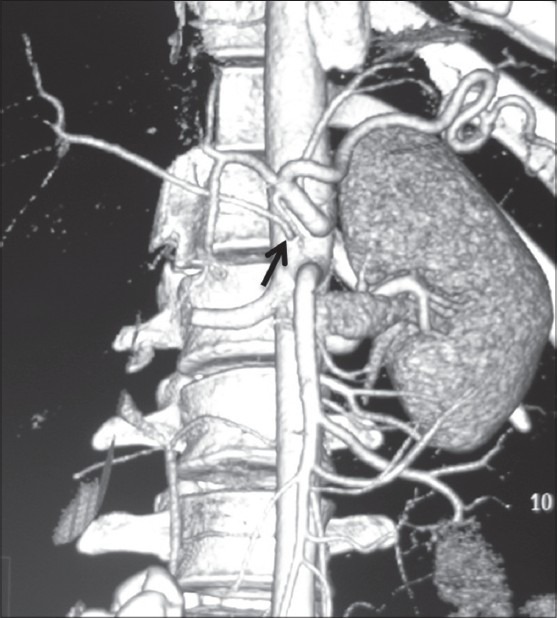
Volume-rendered image showing replaced origin of RHA (arrow) from aorta
Figure 9.
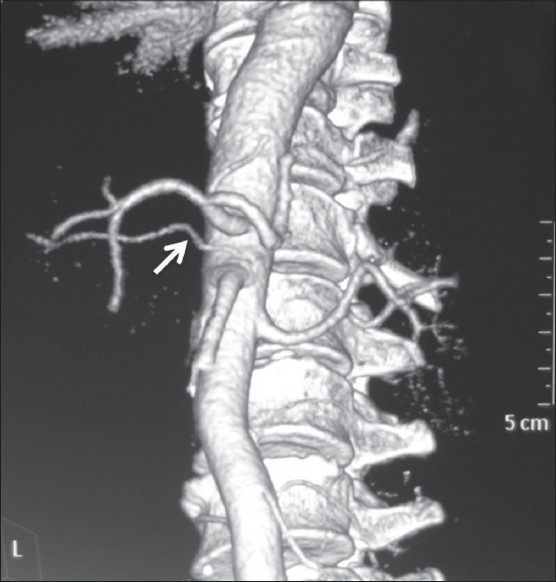
Volume-rendered image showing accessory origin of RHA (arrow) from aorta
Origin and course of MHA has not been given much importance in many textbooks of anatomy or surgery. However, MHA origin has relevance in liver transplant surgery while evaluating liver donors. Michels[14] characterized the MHA as an artery in the umbilical fossa that supplies hepatic segment 4. In our study, MHA originated from RHA in 248 (41.33%) patients [Figure 10], from LHA in 167 (27.83%) [Figure 11], and from CHA in 27 (4.5%) cases. MHA could not be defined in 158 (26.3%) cases. There was no instance of MHA originating from an accessory hepatic artery in our study. Interestingly, in almost all the cases in which replaced LHA origin from LGA was seen, MHA originated from the RHA, and in cases in which replaced RHA origin from SMA or celiac axis was identified, MHA originated from the LHA. Five types of MHA have been defined according to Wang et al., which are as follows:[7]
Figure 10.
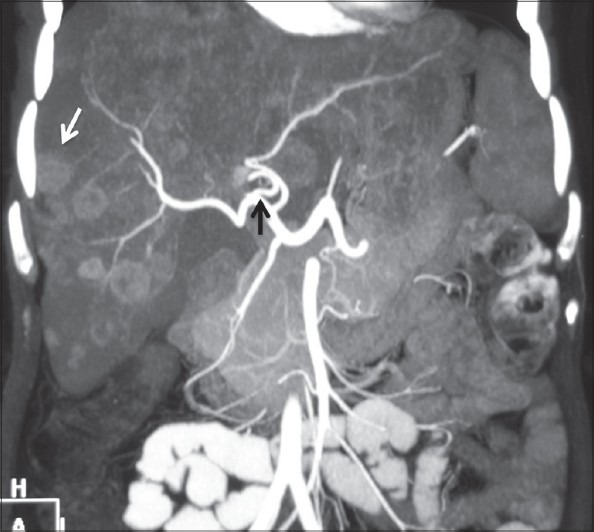
Coronal maximum-intensity projection image showing origin of MHA (black arrow) from RHA. Multiple liver metastases are also seen (white arrow)
Figure 11.
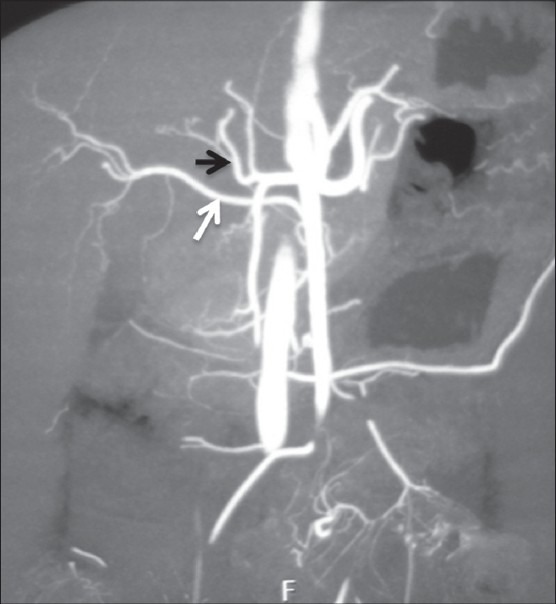
Coronal maximum-intensity projection image showing replaced origin of RHA (white arrow) from SMA and origin of MHA (black arrow) from LHA
Type I MHA: Origin from RHA in patients with a normal hepatic arterial configuration
Type II MHA: Origin from LHA in patients with a normal hepatic arterial configuration
Type III MHA: Origin from RHA in the presence of a replaced LHA
Type IV MHA: Origin from LHA in the presence of a replaced RHA
Type V MHA: Origin from CHA, HAP, and the right anterior hepatic artery (RAHA)
Normal origin of GDA was seen in 586 (97.6%) of the cases. In 10 cases, GDA origin was directly from the celiac axis and in 2 cases, the origin was from RHA [Figure 12]. GDA was not defined in two cases. Late origin of GDA was seen in two cases. Double hepatic artery is differentiated from late origin of the GDA by the presence of a CHA. In the case of a double hepatic artery, there is no CHA and the GDA may originate from either hepatic artery. In case of a distal origin of the GDA, GDA originates from one hepatic artery after the division of the CHA into the RHA and LHA.
Figure 12.
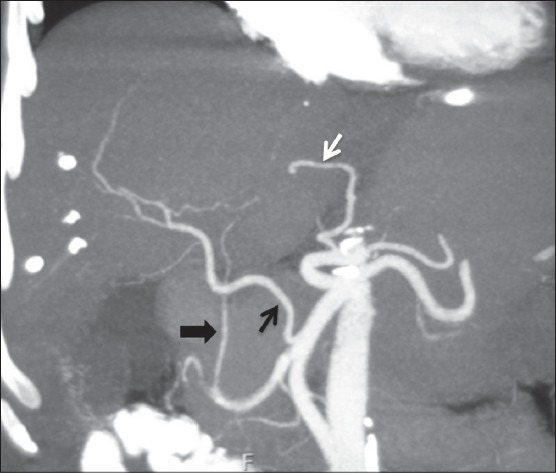
Coronal maximum-intensity projection image showing replaced RHA (black arrow) from SMA and replaced LHA (wh ite arrow) from LGA. Ambiguous celiac axis is seen due to absent CHA and origin of GDA (arrowhead) from RHA
In our study, double hepatic arteries were seen in 14 patients, trifurcation of CHA [Figure 13] was seen in 43 (7.16%), and quadrifurcation of CHA in 13 (2.16%) of the patients [Figure 14]. Early branching of RHA [Figures 15 and 16] was seen in 5 (0.83%) and early branching of LHA in 3 (0.5%) of the patients [Figure 17]. The importance of these anatomic variations has its relevance in advanced intervention procedures like transcatheter arterial chemoembolization (TACE), transarterial radionuclide therapy (TART), and placement of infusion pumps. When placed surgically, these catheters are typically placed in the GDA in a retrograde direction with the catheter tip in the proximal GDA or in the HAP. The GDA distal to the catheter insertion site and the right gastric artery and branches supplying blood to the stomach and duodenum are ligated to prevent nontarget infusion.[15] In this position, the catheter perfuses the RHA and LHA in a patient with standard anatomy. When placed percutaneously, catheters are typically positioned in the HAP to infuse both hepatic arteries. In cases of a double hepatic artery and trifurcation or quadrifurcation of the CHA, an alternate catheter position or more than one catheter must be considered preoperatively to ensure adequate tumor perfusion.[15,16]
Figure 13.
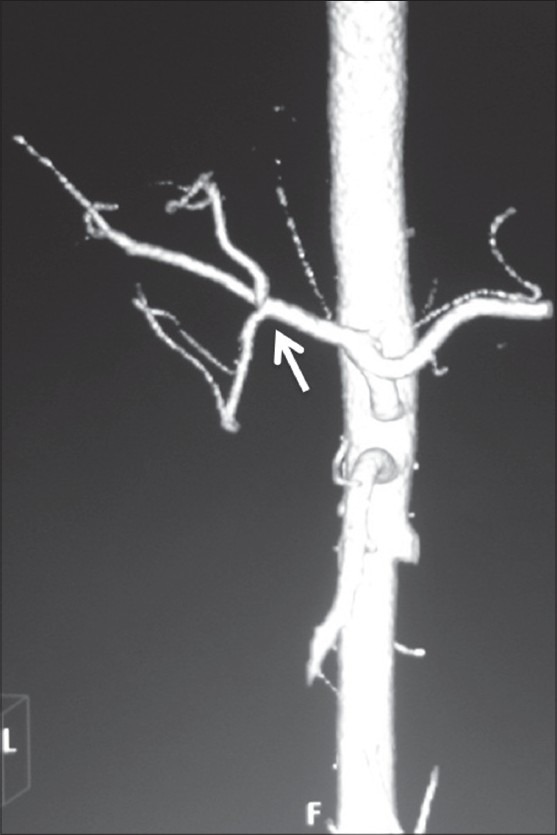
Volume-rendered image showing trifurcation of CHA (arrow) into RHA, LHA, and GDA
Figure 14.
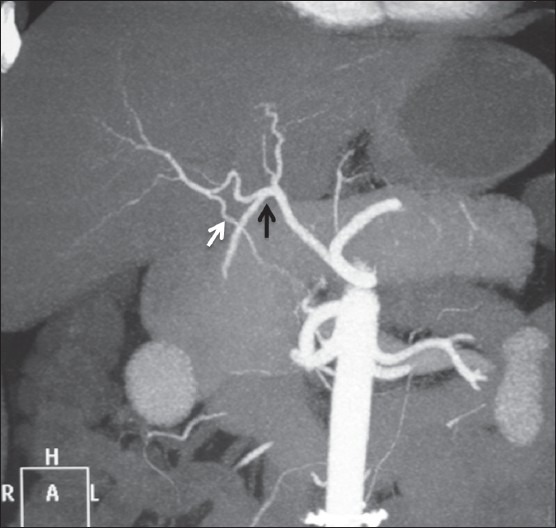
Coronal maximum-intensity projection images showing quadrifurcation of CHA (black arrow) and accessory RHA (white arrow) from SMA
Figure 15.
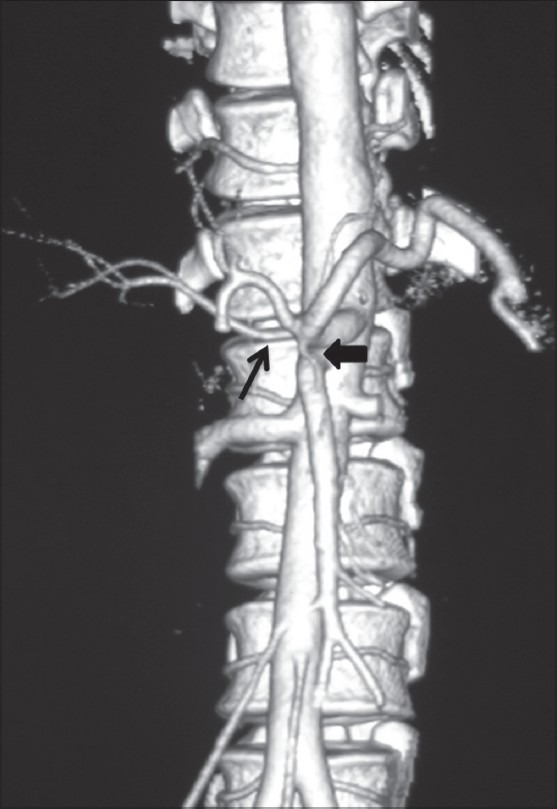
Volume-rendered images showing early branching of RHA (arrow) with ambiguous celiac axis due to vertical anastomotic channel (arrowhead) between celiac axis and SMA
Figure 16.
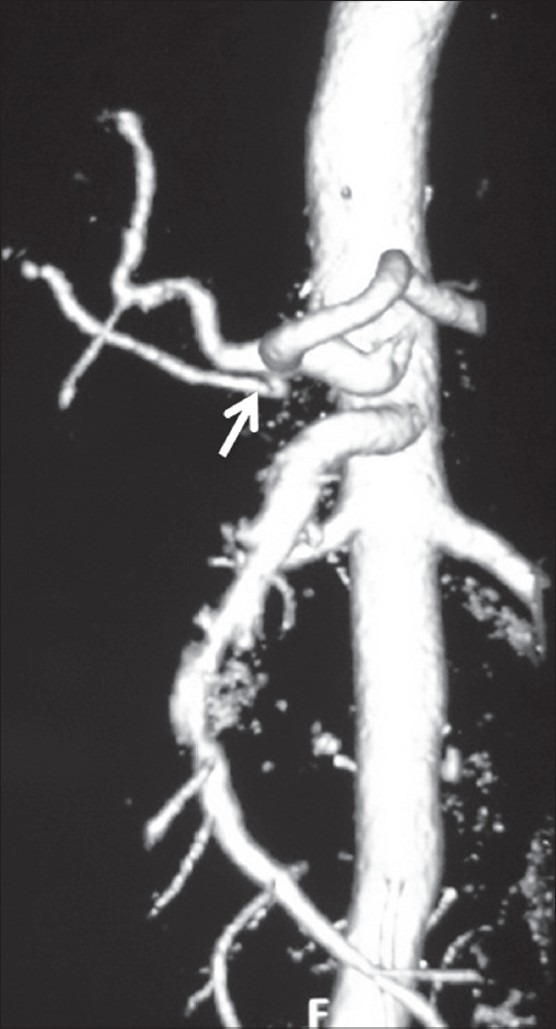
Volume-rendered image showing early branching of RHA (arrow)
Figure 17.
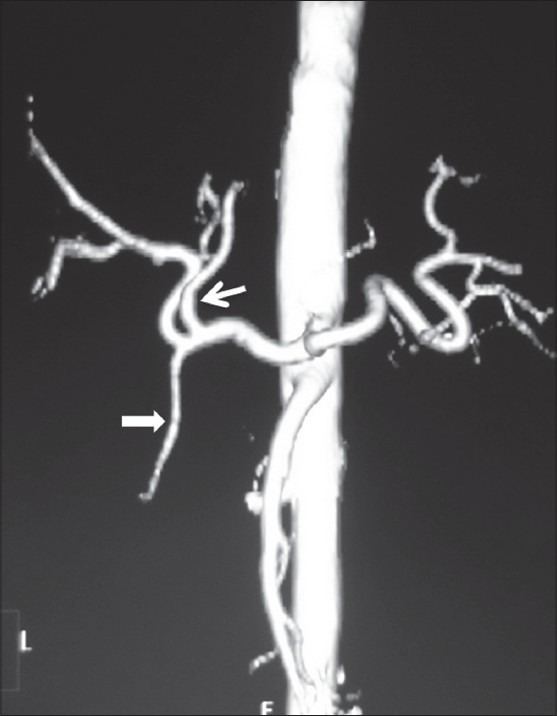
Volume-rendered image showing early branching of LHA (arrow) before GDA (arrowhead)
There are three main types of liver transplantation: cadaveric, live donor liver transplantation, and split-liver grafting. The right lobe of liver is resected and the left lobe left behind in living adult donors, while in children, the left lobe is resected. The incision for hemihepatectomy plane is close to Cantlie line, which is a relatively avascular plane running 1 cm to the right of the middle hepatic vein connecting the gall bladder and the inferior vena cava. Hepatic arterial variants that need extra caution, in liver transplant donors, include the MHA arising from the RHA, as the hepatectomy plane would cut this artery, trifurcation of CHA, and early branching of RHA/LHA as ligation of the CHA can cause gastric or duodenal hypoperfusion. Similarly, arterial variants relevant for transplant recipients are short RHA, celiac artery stenosis, replaced or accessory LHA, and replaced hepatic trunk arising from the SMA, as these variations increase the complexity during the surgery. Since the entire native liver is resected in the recipient, MHA variants are not of significance for these patients. Excessive manipulation of RHA during surgery, which occurs usually due to its anomalous course, can result in biliary complications like stricture and cholangitis, ultimately leading to graft failure. This can be prevented with accurate preoperative knowledge of the arterial anatomy. In patients with normal arterial anatomy, the chemotherapy pump is placed in the hepatic artery after the origin of the gastroduodenal artery to avoid turbulence within the hepatic artery and maintain long-term patency. Adequate assessment of the arterial anatomy prior to placement of the infusion pumps reduces the chances of complications like extrahepatic misperfusion and chemotoxicity, chemical cholangitis, bleeding, and duodenitis.
Ishigami et al.,[17] studied the significance of variant hepatic artery anatomy in liver transplant recipient patients and found that a small-caliber CHA often coexists with variant hepatic artery anatomy. Diameter of the CHA was measured near the origin of the GDA. The diameter of CHA was smaller in patients (mean 5.8 mm) with variant hepatic artery anatomy compared to those with classic hepatic artery anatomy (mean 6.3 mm). They concluded that variant hepatic artery anatomy in a liver transplant recipient increases the risk of post-transplantation hepatic artery complications like stenosis and thrombosis due to coexisting small caliber of CHA in these patients. Such patients can benefit from meticulous preoperative evaluation of the arterial anatomy and careful surgical planning. Accessory hepatic artery is less likely to increase the risk of such complications than a completely replaced hepatic artery because the caliber of the CHA is usually normal in cases of accessory hepatic artery.
Arterial anatomy of the celiac axis and SMA is of great value in pancreatic and hepatobiliary surgeries as direct visualization of the surgical field is limited during surgeries, especially in obese and patients with prior history of surgery. Thus, preoperative knowledge of anatomic variations would help surgeons to avoid extensive dissection and vascular damage. Identification of a replaced RHA is important as it usually courses posterior to the portal vein, and knowledge of such anatomy prevents complications such as bleeding during surgery. In surgeries for malignancies of head/uncinate process of the pancreas, information on the presence or absence of a replaced RHA is critical. Since these patients are jaundiced, utmost care needs to be taken to preserve this artery if it is not involved, as hepatic necrosis is more common in these patients if the arterial flow is also compromised.[18] Anomalous origin of RHA from the celiac axis or aorta is more prone to iatrogenic injury. An accessory LHA needs to be ligated separately in surgeries where blood supply in the porta hepatis is occluded. Anomalous course of CHA should be mentioned in pancreatico-duodenal surgeries as it may be inadvertently injured during resection.
The present study had some limitations. First, since our study was based on the analysis of CT images, description of fine arterial networks could have been missed and was beyond the capability of CT examination. Second, the new nomenclature system described by Song et al., Covey et al., and Wang et al., has not been assessed on the interventional and surgical procedures by us. However, we have tried to describe detailed 3D anatomy of the variations observed by us.
This study is the first of its kind which comprehensively describes variations of celiac axis and hepatic artery branches. In today's scenario, the role of MDCT angiography in preoperative evaluation of patients undergoing hepatobiliary surgeries and advanced interventional procedures cannot be underestimated in order to avoid life-threatening complications.
Acknowledgement
We express our sincere gratitude to our colleagues Dr. Varun, Dhirendra and the faculty of the Department of Radiodiagnosis, for their valuable contribution and constant support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Michel NA. Blood supply and anatomy of the upper abdominal organs, with a descriptive atlas. Philadelphia, Pa: Lippincott; 1955. Observatons on the blood supply of the liver and gallbladder (200 dissections) pp. 64–9. [Google Scholar]
- 2.Vandamme JP, Bonte J, Van der Scheueren G. A reevaluation of hepatic and cystic arteries: The importance of aberrant hepatic branches. ActaAnat. 1969;73:192–209. doi: 10.1159/000143296. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Nakayasu A, Kawabe K, Takeda H, Honjo I. Surgical significance of anatomic variations of the hepatic artery. Am J Surg. 1971;122:505–12. doi: 10.1016/0002-9610(71)90476-4. [DOI] [PubMed] [Google Scholar]
- 4.Song SY, Chung JW, Yin YH, Jae HJ, Kim HC, Jeon UB, et al. Celiac axis and common hepatic artery variations in 5002 patients: Systematic analysis with spiral CT and DSA. Radiology. 2010;255:278–88. doi: 10.1148/radiol.09090389. [DOI] [PubMed] [Google Scholar]
- 5.Catalano OA, Singh AH, Uppot RN, Hahn PF, Ferrone CR, Sahani DV. Vascular and biliary variants in the liver: Implications for liver surgeryRadioGraphics. 2008;28:359–78. doi: 10.1148/rg.282075099. [DOI] [PubMed] [Google Scholar]
- 6.Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited: Digital subtraction angiography performed in 600 patients. Radiology. 2002;224:542–7. doi: 10.1148/radiol.2242011283. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, He X, Li Z, Peng Z, Tam NL, Sun C, et al. Characterization of the middle hepatic artery and its relevance to living donor liver transplantation. Liver Transpl. 2010;16:736–41. doi: 10.1002/lt.22082. [DOI] [PubMed] [Google Scholar]
- 8.Tandler J. Über die Varietäten der Arteria coeliaca und deren Entwickelung. AnatHefte. 1904;25:473–500. [Google Scholar]
- 9.Morita M. Reports and conception of three anomalous cases in the area of the celiac and superior mesenteric arteries [in Japanese] Igaku Kenkyu. 1935;9:1993–2006. [Google Scholar]
- 10.Nebesar RA, Kornblith PL, Pollard JJ, Michels NA. Celiac and superior mesenteric arteries: A correlation of angiograms with dissections Boston Little, Brown. 1969 [Google Scholar]
- 11.Gordon DH, Martin EC, Kim YH, Kutcher R. Accessory blood supply to the liver from the dorsal pancreatic artery: an unusual anatomic variant. Cardiovasc Radiol. 1978;1:199–201. doi: 10.1007/BF02552033. [DOI] [PubMed] [Google Scholar]
- 12.Browne EZ. Variations in origin and course of the hepatic artery and its branches. Surgery. 1940;8:424–45. [Google Scholar]
- 13.Daseler EH, Anson BJ, Hambley WC, Reimann AF. The cystic artery and constituents of the hepatic pedicle: A study of 500 specimens. Surg Gynecol Obstet. 1947;85:47–63. [PubMed] [Google Scholar]
- 14.Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337–47. doi: 10.1016/0002-9610(66)90201-7. [DOI] [PubMed] [Google Scholar]
- 15.Zanon C, Grosso M, Clara R, et al. Percutaneous implantation of arterial port-acath via trans-subclavian access. Anticancer Res. 1999;19:5667–71. [PubMed] [Google Scholar]
- 16.Blumgart L, Fong Y. New York, NY: Saunders; 2000. Surgery of the liver and biliary tract; pp. 1625–8. [Google Scholar]
- 17.Ishigami K, Zhang Y, Rayhill S, Katz D, Stolpen A. Does variant hepatic artery anatomy in a liver transplant recipient increase the risk of hepatic artery complications after transplantation? AJR Am J Roentgenol. 2004;183:1577–84. doi: 10.2214/ajr.183.6.01831577. [DOI] [PubMed] [Google Scholar]
- 18.Thomas PG, Baer HU, Matthews JB, Gertsch P, Blumgart LH. Post-operative hepatic necrosis due to reduction in hepatic arterial blood flow during surgery for chronic biliary obstruction. Dig Surg. 1990;7:31–5. [Google Scholar]


