Abstract
Cesarean scar pregnancy (CSP) and cesarean scar dehiscence (CSD) are the most dreaded complications of cesarean scar (CS). As the incidence of CS is increasing worldwide, so is the incidence of CSP, especially in cases with assisted reproduction techniques. It is of utmost importance to diagnose CSP in the early first trimester, as it can lead to myometrial rupture with fatal outcome. On the other hand, CSD may be encountered during pregnancy or in the postpartum period. CSD in the postpartum period is very rare and can cause secondary postpartum hemorrhage (PPH) leading to increased maternal morbidity or even death if not diagnosed and managed promptly. Both complications can be diagnosed on ultrasonography (USG) and confirmed on magnetic resonance imaging (MRI). These two conditions carry high morbidity and mortality. In this article, we highlight the role of imaging in the early diagnosis and management of these conditions.
Keywords: Cesarean scar dehiscence, cesarean scar pregnancy, magnetic resonance imaging, ultrasonography
Introduction
Cesarean scar pregnancy (CSP) is very rare form of ectopic pregnancy, reported in only 0.15% of pregnant women with prior cesarean deliveries.[1,2] It involves early myometrial invasion and, therefore, carries a high risk of uterine rupture and surgical intervention that could compromise future fertility. It is more life-threatening than placenta previa or accreta because of early myometrial invasion in the first trimester itself. It carries a high risk of uterine rupture and maternal complications. Cesarean scar dehiscence (CSD) is life-threatening and can lead to increased morbidity, postpartum hemorrhage (PPH), and even death, if not treated promptly. Both conditions require an early diagnosis which can be achieved by transabdominal USG (TAS) or transvaginal USG (TVS) and confirmed on magnetic resonance imaging (MRI). The following two cases describe the salient features of these two entities on imaging and guided intervention in their early management.
Case Report
Case I
A 38-year-old gravida 3, para 2 with history of in vitro fertilization and a positive pregnancy test was admitted with vaginal spotting. The patient had undergone cesarean delivery 5 years ago. TAS revealed anteverted uterus with a 38-mm gestational sac, 5-mm yolk sac, and a live embryo with crown–rump length 13.3 mm corresponding to 7 weeks and 6 days in the lower uterine cavity at the scar site. TVS showed an empty endometrial cavity and endocervical canal. The gestational sac was implanted in the anterior myometrium with myometrial thinning (maximum thickness 5.2 mm) between the gestational sac and the urinary bladder [Figure 1A and B]. The hypoechoic cesarean scar (CS) was visualized adjacent to the gestational sac. On Color Doppler USG, mild increased peritrophoblastic flow was seen around the sac. MRI confirmed the presence of a gestational sac in the lower uterine segment at the scar site, with very thin (4 mm) myometrium between the gestational sac and the urinary bladder. The endometrial cavity and endocervical canal were empty and were displaced by the gestational sac posteriorly [Figure 2]. However, no bladder invasion was seen. The medical termination was then performed with fetal intracardiac potassium chloride injection under USG guidance.
Figure 1A.
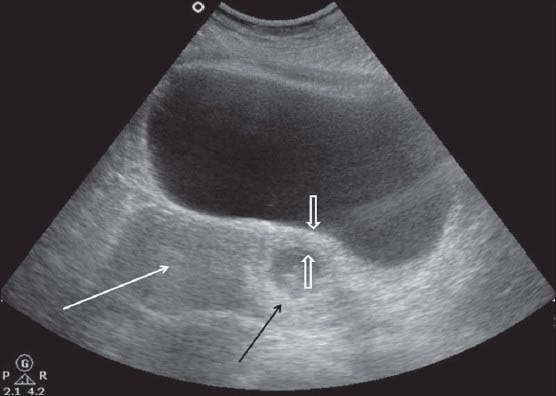
TAS showing the gestation sac (black arrow) with embryo in the lower uterine segment and myometrial thinning (open arrows). Note the endometrial cavity (white arrow) is empty
Figure 1B.
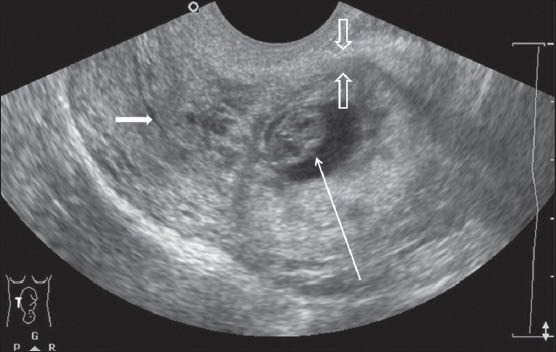
TVS showing the gestation sac (long white arrow) in the lower uterine segment with thinning of the anterior myometrium (open arrows). Note the empty endometrial cavity which is displaced posteriorly (small white arrow)
Figure 2.
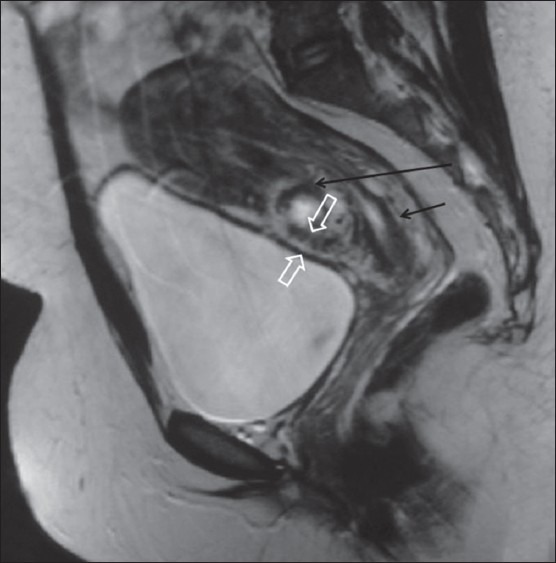
Sagittal T2WI MRI showing the relationship of the gestation sac (long black arrow) with the empty endometrial cavity (short black arrow) and urinary bladder. Open arrows show myometrial thinning between the urinary bladder wall and gestational sac
Case II
A 32-year-old gravida 3, para 2 with a previous lower segment cesarean section (LSCS) 3 years back had an elective LSCS at 40 weeks. She had no existing medical or antenatal problems. LSCS was done. A healthy baby was delivered, but she had severe pain in the lower abdomen for next 4 days along with spotting. TAS showed 10.2 × 5.3 × 6.2 cm hypoechoic collection along the anterior aspect of uterus. However, limited evaluation was possible due to abdominal tenderness and pain on probe compression. TVS was performed and it showed a large heterogeneously hypoechoic collection anterior to the uterus, which was communicating with the endometrial cavity with myometrial defect in the region of the CS [Figure 3]. MRI was done to confirm CSD. MRI showed a 10.5 × 8 × 6.6 cm hematoma appearing as heterogeneously hyperintense on T1-weighted image (T1WI) and T2-weighted image (T2WI), anterior to uterus [Figure 4A and B]. This hematoma was communicating with the endometrial cavity through a defect in the anterior wall of myometrium in the region of CS. Mild free fluid was seen in the peritoneal cavity [Figures 4A and B]. The patient was started on antibiotics and managed conservatively. Under USG guidance, a 10-French pigtail catheter was inserted into the collection which yielded thick hemorrhagic blood. Regular flushing and reaspiration was done. The hematoma resolved and the patient's clinical condition improved with antibiotics. On follow-up after 6 months, there was almost complete resolution of the hematoma and the CS had healed.
Figure 3.
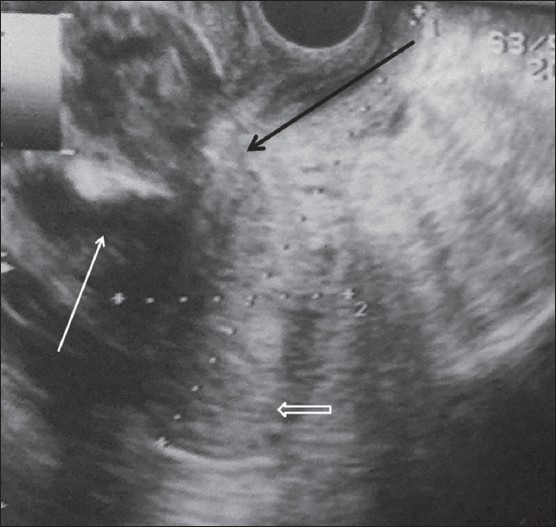
TVS showing the heterogeneous hypoechoic collection (white arrow) anterior to the uterus (open arrow). It is communicating with the endometrial cavity through myometrial defect (black arrow)
Figure 4A.
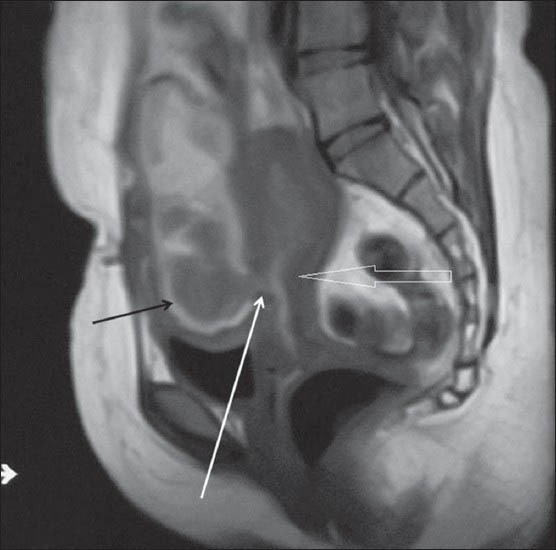
Sagittal T1WI MRI shows a heterogeneous hyperintense collection (black arrow) anterior to the uterus (open arrow). It is communicating with the endometrial cavity through myometrial defect (white arrow) which is better seen on MRI
Figure 4B.
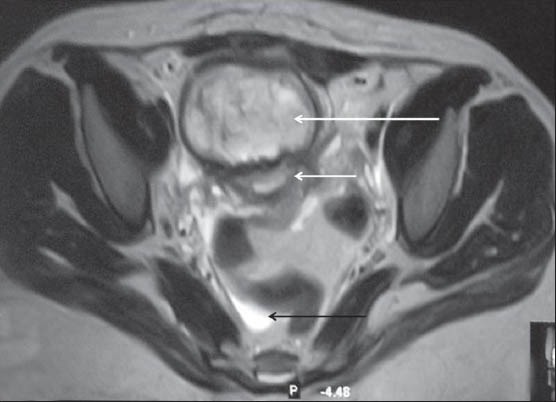
Axial T2WI MRI shows heterogeneous hyperintense collection (long white arrow) anterior to the uterus (short white arrow). Note mild free fluid in the peritoneal cavity (black arrow)
Discussion
CSP and CSD are rare obstetric events and carry high maternal morbidity and mortality. Usually the predisposing risk factors are prior uterine trauma, myomectomy, assisted reproductive technique and previous dilation and curettage.[3]
Diagnosis of CSP
Patients of CSP present usually with vaginal bleeding and abdominal pain, or are asymptomatic in one-third of cases. TAS is the initial investigation, followed by TVS. For TAS, the patient is examined with full bladder to assess the pelvis, uterus, and to see the position of the gestational sac, fetal pole, myometrial thickness, and its relation to the bladder wall. This is followed by TVS for detailed relationship of gestational sac in relation to the scar and endometrial cavity. The hypoechoic CS is usually visualized adjacent to the gestational sac. The diagnostic features of CSP seen on TVS are as follows:
The uterus is empty with normal endometrium
The cervix is empty with normal endocervical canal
The gestational sac is seen in lower uterine segment in the anterior portion
The gestational sac is seen in the region of the CS, with myometrial thinning between the bladder wall and the gestational sac (usually <5 mm)
The endometrial cavity and endocervical canal are empty and may be displaced by the gestation sac
On CD, increased peritrophoblastic flow is seen with high-velocity flow (>20 cm/s), a resistive index of less than 0.5, and a peak systolic to diastolic blood flow ratio of less than 3.
TVS is the earliest modality used in diagnosing CSP, but in inconclusive or equivocal cases or cases with precious pregnancy, MRI is done to confirm the diagnosis before any intervention. Sagittal, coronal, and transverse sections of T1- and T2-weighted MRI sequences are used to confirm the presence of gestational sac in the lower uterine segment at the scar site. The endometrial cavity and endocervical canal are empty and are displaced by the gestational sac which can be well appreciated on sagittal and axial sections. MRI better assesses the myometrial thinning between the sac and the urinary bladder.
Diagnosis of CSD
The CS is the most common site of uterine dehiscence and rupture as it is atrophic and inelastic.[4] Usually, the LSCS ruptures after labor and the classic scars rupture before labor. Patient presents with abdominal pain, swelling, and in some cases, massive secondary PPH. If undiagnosed, it can lead to maternal morbidity and even death. TAS and TVS are the earliest investigations to be done. Usually, TAS evaluation is limited due to abdominal pain on probe compression and overlying sutures and bandages. TVS can be done to further assess the patient.
The common findings seen on TAS and TVS are as follows:
Hypoechoic hematoma/collection anterior to the uterus
Focal defect in the anterior myometrium in the region of CS
Communication of collection with the endometrial cavity
Mild free fluid or hemoperitoneum if present
CD can be done to rule out any pseudoaneurysm or arteriovenous (AV) fistula formation.
But sometimes due to debris or air artifacts (if the collection gets infected), it is difficult to visualize the myometrial defect and its communication with the endometrial cavity. In these cases, and to further confirm the diagnosis, MRI can be done. It is definitely a better modality as it provides excellent soft-tissue detail, can image in any plane, and offers a comprehensive assessment of the uterine wall and the peritoneal cavity.[5,6]
The common findings seen on MRI are as follows:
Hematoma/collection can appear as hypo-/hyperintense on T1WI and hyperintense on T2WI (depending on blood degradation products)
The collection may be seen to communicate with the endometrial cavity
The extent of uterine rupture
The extent of hematoma in the pelvis and abdomen and its relation with various organs
The status of pelvic organs, peritoneal cavity, pleural cavity for any hemoperitoneum/free fluid/effusions
Myometrial thinning and scar thickness are better assessed on MRI.
Management
In cases of CSP, early termination by conservative methods in the first trimester is preferred, as it carries increased risk of complications and loss of fertility. Due to nonavailability of clear-cut guidelines, the management of CSP varies. In young patients who want to preserve fertility, medical management or minimally invasive surgeries like local injection, hysteroscopy, laparoscopy, open removal, or surgical aspiration are preferred. Methotrexate (MTX) local, systemic, and combined treatments can be given. Intracardiac injection of potassium chloride, intra-amniotic instillation of MTX, or direct traumatic punctures can be performed under USG guidance for feticide if cardiac activity is present in the embryo.[7] Surgical option is needed for those cases in which there is no need to preserve fertility.
CSD can be managed conservatively with proper antibiotic cover and drainage of the hematoma. To preserve fertility, vaginal or laparoscopic resuturing can be done. In cases with severe bleeding, urgent intervention may be required and patient may be taken up for uterine artery embolization or surgery. If scar wound is infected or abscess formation occurs, hysterectomy is preferred.[8]
In conclusion, as nowadays the trend and incidence of cesarean sections is increasing, so are the complications of CS. Though rare, CSP and CSD can lead to increased patient morbidity and mortality. Prompt diagnosis and image-guided intervention helps in early patient rehabilitation and preserves the fertility of the patient.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Larsen JV, Janowski K, Krolilowski A. Secondary post partum haemorrhage due to uterine wound dehiscence. Cent Afr J Med. 1995;41:294–6. [PubMed] [Google Scholar]
- 2.Seow KM, Huang LW, Lin YH, Yan-Sheng Lin M, Tsai YL, Hwang JL. Cesarean scar pregnancy: Issues in management. Ultrasound Obstet Gynaecol. 2004;23:247–53. doi: 10.1002/uog.974. [DOI] [PubMed] [Google Scholar]
- 3.Khalifa Y, Redgment CJ, Yazdani N, Taranissi M, Craft IL. Pregnancy. Intramural pregnancy following difficult embryo transfer. Hum Reprod. 1994;9:2427–8. doi: 10.1093/oxfordjournals.humrep.a138463. [DOI] [PubMed] [Google Scholar]
- 4.Navaratnam K, Ulaganathan P, Akhtar MA, Sharma SD, Davies MG. Posterior uterine rupture causing fetal expulsion into the abdominal cavity: A rare case of neonatal survival. Case Rep Obstet Gynecol 2011. 2011 doi: 10.1155/2011/426127. 426127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maldjian C, Milestone B, Schnall M, Smith R. MR appearance of uterine dehiscence in the post-cesarean section patient. J Comput Assist Tomogr. 1998;22:738–41. doi: 10.1097/00004728-199809000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Donnez O, Jadoul P, Squifflet J, Donnez J. Laparoscopic repair of wide and deep uterine scar dehiscence after cesarean section. Fertil Steril. 2008;89:974–80. doi: 10.1016/j.fertnstert.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Hung TH, Shau WY, Hsieh TT, Hsu JJ, Soong YK, Jeng CJ. Prognostic factors for an unsatisfactory primary methotrexate treatment of cervical pregnancy: A quantitative review. Hum Reprod. 1998;13:2636–42. doi: 10.1093/humrep/13.9.2636. [DOI] [PubMed] [Google Scholar]
- 8.Rivlin ME, Carroll CS, Sr, Morrison JC. Infectious necrosis with dehiscence of the uterine repair complicating cesarean delivery: A review. Obstet Gynecol Surv. 2004;59:833–7. doi: 10.1097/01.ogx.0000146198.55363.5a. [DOI] [PubMed] [Google Scholar]


