Abstract
Background
Human leucocyte antigens (HLAs) modulate immunity to polyomavirus BK (BKV). Identification of HLAs that alter the course of infection will facilitate risk stratification, and customization of pre-emptive intervention strategies.
Methods
We performed a retrospective cohort study with 998 kidney transplant patients with BKV infection status confirmed by polymerase chain reaction (PCR). Clinical parameters and donor–recipient matching for specific HLAs were examined in relation to occurrence of viremia. An emphasis was placed on donor–recipient matching rather than the actual frequency of specific HLA-alleles, since a successful immune response requires sharing of HLAs between a virus-infected target cell and the anti-viral effector cell.
Results
Using multivariate statistics, low risk of BK viremia was associated with matching of HLA-A2 [hazard ratio (HR) 0.51, 95% confidence interval (CI) 0.28–0.85], HLA-B44 (HR 0.31, 95% CI 0.076–0.85) and HLA-DR15 (HR 0.35, 95% CI 0.084–0.93) (P < 0.05), whereas high risk of viremia was associated with male gender (HR 2.38, 95% CI 1.46–4.09, P < 0.001).
Conclusions
HLAs that associated with a lower predisposition to the development of BK viremia have been identified. Evaluation of donor–recipient mismatching for these HLAs could potentially be used to (i) fine tune virus screening strategies for BKV in individual patients and (ii) facilitate discovery of major histocompatibility complex (MHC) class I and II binding peptides that can elicit clinically meaningful BKV-specific immunity.
Keywords: genotype, HLA mismatch, polymerase chain reaction (PCR), screening, viremia
INTRODUCTION
Polyomavirus BK (BKV) commonly infects human populations in early childhood, and 50–90% of adults are seropositive [1–4]. Primary infection is usually asymptomatic, following which the virus establishes latent infection in the genitourinary tract. Reactivation may occur under conditions associated with impaired immunity and is seen in 10–70% of kidney transplant recipients. Infection commences as viruria, which progresses to viremia and histologic nephropathy. In the late 90s, graft loss due to BKV nephropathy ranged from 50 to >80% of cases [5, 6]. More recently, widespread screening using urine cytology or polymerase chain reaction (PCR) has permitted early diagnosis at the stage of viruria or viremia, and current graft loss rates have fallen to 20% [7].
The major histocompatibility complex (MHC) is intimately involved in the generation of an immune response to viral infection. Viral peptides presented by MHC class I molecules interact with CD8-positive T-cells to generate virus-specific memory T-cells. In contrast, MHC class II molecules interact with CD4-positive T-cells to generate effector T-cells that may provide help to (i) CD8-positive T-cells for generation of more efficient cell-mediated immunity and (ii) B-cells for production of virus-specific antibodies. While CD8-positive T-cells dominate the immune response to some viral infections, CD4-positive T-cells are well documented in human BKV infection [8–10]. For this reason, it was considered important to evaluate both class I and class II antigens.
From a clinical perspective, identification of MHC-encoded human leukocyte antigens (HLAs) that influence the course of BKV infection will permit risk stratification in individual patients. Thus, it may allow for a prediction of the likelihood of spontaneous resolution of viruria or of progression to viremia and nephropathy. In turn, this will facilitate customization of BKV screening and pre-emptive intervention strategies, with more frequent virus testing being performed in patients at most risk of developing disease. Existing studies on the role of HLAs in BKV biology have largely focused on enumerating global mismatches among major classes of HLA molecules, particularly, HLA-A, B and DR. The number of patients studied is limited, different phases of infection have not been examined, and analyses at the level of individuals or antigenic epitopes have not been performed. The current study examined 998 kidney transplant patients with a post-transplant follow-up period of 1449 ± 652 days to identify HLAs associated with decreased or increased susceptibility to BK viremia.
SUBJECTS AND METHODS
Study population
The Thomas E. Starzl Transplant Institute performed 1708 kidney transplants in the period January 2002 to April 2010. From this population, we were able to identify 998 patients who had both urine and plasma polymerase chain reaction (PCR) for BKV DNA performed on at least four occasions. PCR testing policy was not uniform during this time: initially only patients with graft dysfunction were tested for BKV infection, but in 2006 a deliberate screening policy was implemented. Data collected retrospectively for this study indicated that samples obtained every 1–3 months for 1-year were available for analysis in 709 of 998 patients. Beyond the 1 year time point, most samples were collected from those subjects who had either earlier positive samples or had developed late graft dysfunction. Data from these 998 patients were analyzed for the purpose of this study. All study-related activities were performed according to a protocol approved by the University of Pittsburgh Institutional Review Board (Institutional Review Board protocol # 0602155).
Categorization of viral status
In 515 of 998 patients (51.6%), urine BKV DNA was below the detection threshold of <200 copies/mL established in our Molecular Diagnostic Laboratory. These subjects also tested negative for BKV in plasma. Viruria not complicated by viremia during the follow-up period was seen in 381 of 998 (38.2%) patients. We designated both the aforementioned subgroups of patients in the ‘non-viremia’ category. BK viremia was defined as detection of >1000 copies/mL in plasma on at least one occasion: this was observed in 102 of 998 (10.2%) patients. The first detection of viremia by PCR was observed at a median of 97 days (interquartile range: 37–216 days) post-transplantation. The viremia group includes patients with biopsy-proven BK virus nephropathy in 20 patients (20 of 998, 2.0%).
HLA matching procedures
HLA antigens in kidney transplant recipients and donors were typed using standard serological methods. Among all donors and recipients, the complete information of HLA-A, B and DR is available, whereas the data for HLA-C and HLA-DQ are missing in 7 and 1 cases in donors and 91 and 24 cases in recipients. For each donor–recipient combination, the degree of histocompatibility was determined by counting the number of HLA-A, HLA-B and HLA-DR antigens. We also investigated frequencies and genotype frequencies in each HLA serotype. HLA serotypes having >10% of allele frequency were used for further analysis.
Allograft pathology
Allograft biopsies were performed when patients showed an unexplained rise in serum creatinine. The diagnosis of T-cell-mediated acute rejection was based on Banff '97 criteria of renal allograft pathology [11]. In this study, acute rejection episodes were determined as an increase in serum creatinine of at least 15% above the baseline level with biopsy confirmation (Banff grade IA or higher). BKV nephropathy was defined as the presence of intratubular viral inclusions and demonstration of viral DNA within biopsy tissue. In situ hybridization for BKV DNA was performed on all biopsies taken concurrently with BK viruria. We investigated all biopsy-proven acute rejection for histopathologic type and temporal relationship with onset of viremia. In our analysis of rejection as a risk factor of viremia, rejection episodes that occurred after the viral clearance were excluded from consideration.
Statistical analysis
JMP version 8 (SAS Institute, Cary, NC) was used for all statistical analyses. Results were presented as mean ± SD. Student's t-test was used to compare the variables between groups. Fisher's exact probability test was used for categorical data when appropriate. Kaplan–Meier estimates and log-rank tests were used to evaluate the incidence of viremia. The frequency of matching of individual HLAs in patients with and without viremia was evaluated by a 2 × 2 chi-square test. The mean tacrolimus trough level was obtained 1, 3, 6, 9, 12, 18 and 24 months after transplantation, and the levels in two studied groups were compared with repeated measure analysis of variance (ANOVA). A univariate and multivariate Cox's proportional hazard model was used to determine which factors independently affected the development of viremia. The multivariate model included only those parameters that were associated with P < 0.1 in univariate analyses [12] in addition to recipient age, recipient gender and acute rejection episode, which have been previously reported to be associated with increased risk of progressive disease. Results were expressed as hazard ratio (HR) with respective 95% confidence intervals (CIs). P-values <0.05 were considered statistically significant in all analyses.
RESULTS
Demographic data
Demographic and clinical findings of the patients studied are summarized in Table 1. The percentage of male gender, HLA-B or HLA (A + B + DR) mismatched patients and the prevalence of acute rejection were significantly higher in viremic patients. The majority of patients (94%) in both groups received preconditioning with alemtuzumab (Campath 1H, humanized CD52 monoclonal antibody). For maintenance immunosuppression, tacrolimus monotherapy was less common in patients with viremia, and more patients in this group received adjuvant therapy with mycophenolate mofetil (MMF) or prednisolone (PSL). There was no significant difference in patient age, etiology of end-stage renal diseases (ESRDs), proportion of first grafts, type of donor, donor age, cold ischemia time or % panel-reactive antibodies (PRAs) between the study groups. The mean follow-up periods in non-viremia and viremia groups were 1456 and 1385 days, respectively. Twenty (20) patients with viremia developed nephropathy during the study period.
Table 1.
Demographic and clinical characteristics in patients with non-viremic and viremic patients
| Non-viremia | Viremia | P-value | |
|---|---|---|---|
| No. of patients | 896 | 102 | |
| Recipient gender (male) | 526 (58.7%) | 78 (76.5%) | <0.001 |
| Recipient age (years) | 49 ± 17 | 48 ± 18 | 0.61 |
| Etiology of ESRD | |||
| Glomerulonephritis | 179 (20.0%) | 18 (17.7%) | 0.87 |
| Hypertension | 119 (13.3%) | 15 (14.7%) | |
| Diabetes | 272 (30.4%) | 29 (28.4%) | |
| Others | 326 (36.3%) | 40 (39.2%) | |
| First graft | 713 (79.6%) | 76 (74.5%) | 0.23 |
| Cadaveric donor | 565 (63.1%) | 66 (64.7%) | 0.74 |
| Donor age (years) | 40 ± 16 | 38 ± 15 | 0.24 |
| Cold ischemic time (min) | 951 ± 755 | 1034 ± 859 | 0.30 |
| HLA mismatch HLA-A | 1.15 ± 0.76 | 1.29 ± 0.65 | 0.068 |
| HLA-B | 1.37 ± 0.71 | 1.54 ± 0.59 | 0.020 |
| HLA-DR | 1.16 ± 0.72 | 1.25 ± 0.67 | 0.25 |
| A + B + DR | 3.68 ± 1.74 | 4.09 ± 1.44 | 0.023 |
| PRA > 20% | 159 (17.8%) | 18 (17.7%) | 0.98 |
| Induction therapy | |||
| Alemtuzumab | 850 (94.9%) | 96 (94.1%) | 0.68 |
| Thymoglobulin | 21 (2.3%) | 2 (2.0%) | |
| Dacrizmab | 12 (1.4%) | 1 (1.0%) | |
| Basiliximab | 2 (0.2%) | 0 (0.0%) | |
| No antibody induction | 11 (1.2%) | 3 (2.9%) | |
| Maintenance immunosuppression | |||
| Tac monotherapy | 651 (72.7%) | 64 (62.7%) | 0.003 |
| Tac + MMF | 156 (17.4%) | 21 (20.6%) | |
| Tac + PSL | 50 (5.6%) | 12 (11.8%) | |
| Others | 39 (4.3%) | 5 (3.9%) | |
| Acute rejection episode | 236 (26.3%) | 44 (43.1%) | <0.001 |
| Follow-up period (days) | 1456 ± 648 | 1386 ± 685 | 0.30 |
ESRD: end-stage renal disease, Tac: tacrolimus, MMF: mycophenolate mofetil, PSL: prednisolone.
HLA matching in viremic and non-viremic groups
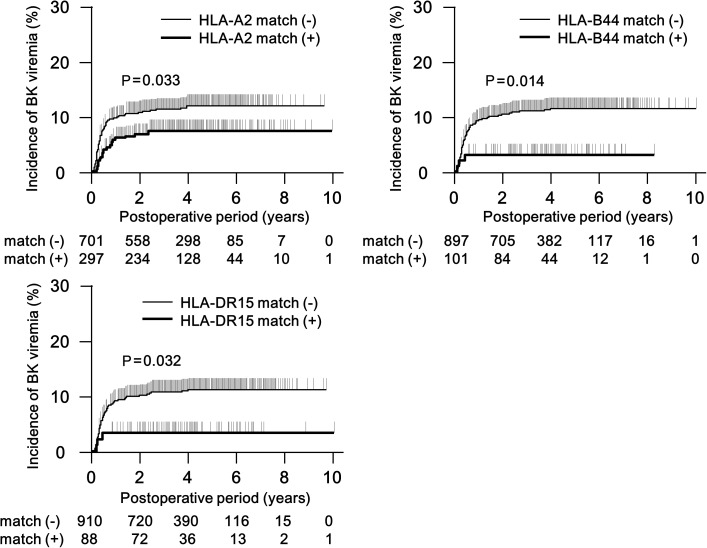
Table 2 shows HLA matching between donors and recipients for individual HLAs. The frequencies of HLA-A2, B44, C6, DR7, DR15 and DR51 matched transplants were significantly lower in viremic compared with viremia-free patients, while DQ7 matching was more common. Since HLA-DR7 and -DR15 show strong linkages with HLA-DR53 and -DR51, we used HLA-DR7 and DR15 for multivariate analyses. HLA-A2-, B44-, DR7- or DR15-matched patients showed a lower cumulative incidence of viremia in Kaplan–Meier plots (Figure 1). This effect was not seen in HLA-C6- and DQ7-matched patients.
Table 2.
Frequency of HLA-matched transplantation for individual HLAsa
| Non-viremia (n = 896) | Viremiab (n = 102) | P-value | |
|---|---|---|---|
| HLA-A1 match | 117 (13.1%) | 9 (8.8%) | 0.22 |
| A2 | 276 (30.8%) | 21 (20.6%) | 0.033 |
| A3 | 69 (7.7%) | 7 (6.9%) | 0.76 |
| B7 | 82 (9.2%) | 7 (6.9%) | 0.44 |
| B8 | 84 (9.4%) | 10 (9.8%) | 0.89 |
| B44c | 98 (10.2%) | 3 (2.9%) | 0.011 |
| C3 | 51 (6.2%) | 4 (4.8%) | 0.59 |
| C4 | 61 (7.5%) | 8 (9.5%) | 0.50 |
| C6 | 42 (5.2%) | 0 (0.0%) | 0.033 |
| C7 | 254 (31.1%) | 30 (35.7%) | 0.39 |
| DQ2 | 190 (21.7%) | 18 (18.6%) | 0.48 |
| DQ3 | 27 (3.1%) | 1 (1.0%) | 0.25 |
| DQ6 | 78 (8.9%) | 4 (4.1%) | 0.11 |
| DQ7 | 99 (11.3%) | 18 (18.6%) | 0.037 |
| DR4 | 142 (15.6%) | 14 (13.7%) | 0.58 |
| DR7 | 88 (9.8%) | 3 (2.9%) | 0.022 |
| DR11 | 79 (8.8%) | 11 (10.8%) | 0.51 |
| DR13 | 68 (7.6%) | 11 (10.8%) | 0.26 |
| DR15 | 85 (9.5%) | 3 (2.9%) | 0.027 |
| DR17 | 93 (10.4%) | 11 (10.8%) | 0.90 |
| DR51 | 93 (10.4%) | 3 (3.0%) | 0.017 |
| DR52 | 367 (41.0%) | 45 (44.6%) | 0.49 |
| DR53 | 249 (27.7%) | 20 (19.8%) | 0.086 |
All P-values correspond to the HLA in the corresponding row. Those further corroborated by Cox regression analysis are indicated in Table 3.
aData of HLA-C and DQ were not available in 96 and 24 transplants, respectively.
bFor the purposes of this table, even a single positive test in plasma was sufficient to include a patient in the viremia category. If analysis was restricted to the 93 patients who had sustained viremia (defined as two or more positive tests), P-values associated with DQ7 and DR15 fell to 0.064 and 0.092, respectively. Statistical significance of the remaining HLAs was not affected.
cAmong the 102 viremic patients, we identified a subset of 20 patients where the plasma level load exceeded 10 000 copies/mL, since some studies regard this viral load as a surrogate marker of BKV nephropathy. The frequency of B44 match in patients with viremia <10 000 versus >10 000 was 3.7 and 0.0%, respectively (P = 0.036). Thus, an association between B44 and lower risk of viremia remained statistically significant. Associations of HLA-DR7 and HLA-DQ7 with, respectively, lower and higher incidence of viremia became marginally significant (P = 0.064 and 0.058, respectively). Observed associations with other HLAs were lost, presumable because of the marked reduction in the number of observations available for analysis.
FIGURE 1:
Cumlative incidence of BK viremia in donor and recipient combinations with matching for the specified HLA. The P-values as indicated have been calculated by the log-rank test.
Acute rejection episodes in viremic and non-viremic groups
Among 102 viremic patients, 44 patients (43.1%) developed 99 episodes of acute rejection (0.97 episode per patient), whereas 236 out of 896 (26.3%) non-viremic patients developed 373 episodes (0.42 per patient). Thus, the overall incidence of rejection as well as the number of rejection episodes per patient was higher in viremic patients (P < 0.001 for incidence determined by Kaplan–Meier estimate, and P < 0.001 for the number of episodes). The high rejection rate does not reflect the overall incidence in our transplant program. Instead, it reflects the fact that patients with graft dysfunction had a greater chance of inclusion in the study. With respect to the timing of rejection in viremic patients, 23 of 99 episodes (23.2%) developed before the first positive plasma BKV PCR test, 12 episodes (12.1%) developed during viremia and 64 episodes (64.6%) after clearance of viremia. Thus, viremia could not be construed as the aftermath of acute rejection in most cases.
With regard to the severity of rejection in viremic patents, we documented Banff grade IA rejection in 50 biopsies (50.5%), grade IB in 44 biopsies (44.4%), grade IIA in 2 biopsies (2.0%) and antibody-mediated rejection in 3 biopsies (4.3%). In patients without viremia, the corresponding counts were 213 (57.3%) biopsies with grade IA, 123 with IB (33.1%), 23 with IIA (6.2%), 9 with grade IIB (2.4%), 4 with grade III (1.1%) and 1 with antibody-mediated rejection (0.3%).
Multivariate analysis for the factors associated with BK viremia
Recipient age, gender and clinical parameters associated with the development of viremia on univariate analyses, including maintenance immunosuppression (tacrolimus monotherapy), acute rejection episodes prior to viremia, HLA mismatch count (four to six mismatches) and donor–recipient matchings of HLA-A2, B44, C6, DQ7, DR7 and DR15 were further evaluated using multivariate Cox regression analysis (Table 3). DR51 and DR53 were not considered as these show a strong linkage with DR7 and DR51, respectively, and multicolinearity could be expected to confound the independent effects of the latter antigens. The adverse effect of recipient male gender remained statistically significant on multivariate analysis (HR 2.47, P < 0.001). In contrast, HLA-A2, B44 and DR7 matching conferred a low incidence of viremia (HR 0.51, P = 0.011, HR 0.31, P = 0.019 and HR 0.35, P = 0.034, respectively). Recipient age, maintenance immunosuppression, acute rejection and matching of HLA-DR7 were not associated with the development of viremia. Matchings of HLA-C6 and HLA-DQ7 showed a trend toward decreasing and increasing risk of viremia, respectively. Since excessive immunosuppression is another important risk factor of BK viral reactivation, we also investigated the mean tacrolimus trough levels in patients with and without viremia. The trough levels were comparable during the first 3-months post-transplant. However, subsequent levels were significantly lower in the viremia group, consistent with therapeutic reduction of tacrolimus dose (Supplementary data, Figure S1).
Table 3.
Multivariate Cox regression analysis for the factors associated with the development of BK viremiaa
| Models | HR | 95% CI | P-value |
|---|---|---|---|
| Recipient age (years)b | 0.77 | 0.26–2.40 | 0.65 |
| Recipient gender (male) | 2.38 | 1.46–4.09 | <0.001 |
| Maintenance IS (Tac monotherapy) | 0.68 | 0.44–1.07 | 0.094 |
| Acute rejection episodec | 0.96 | 0.58–1.56 | 0.89 |
| HLA mismatch (4–6) | 0.77 | 0.49–1.24 | 0.28 |
| HLA-A2 match | 0.51 | 0.28–0.85 | 0.011 |
| B44 match | 0.31 | 0.076–0.85 | 0.019 |
| C6 match | 0.24 | 0.013–1.12 | 0.075 |
| DQ7 match | 1.63 | 0.91–2.78 | 0.097 |
| DR7 match | 0.49 | 0.12–1.36 | 0.19 |
| DR15 match | 0.35 | 0.084–0.93 | 0.034 |
HR: hazard ratio, CI: confidence interval, IS: immunosuppression, Tac: tacrolimus.
aData in this table include all patients since 2002. Since routine screening for BKV infection was commenced only in 2006, we performed a sub-analysis based only on this subset of 709 patients. Male gender (HR 2.40, P = 0.002), HLA-A2 match (HR 0.51, P = 0.026), HLA-B44 match (HR 0.36, P = 0.048) and HLA-DR15 match (HR 0.26, P = 0.021) remained statistically significant.
bRecipient age, gender and specific variables associated with P-values <0.1 by univariate analysis with Student's t-test, chi-square test or Fisher's exact probability test (Tables 1 and 2) were entered in this multivariate analysis.
One hundred and five patients were excluded from this analysis because of the missing data in HLA-C and/or HLA-DQ.
cIn our analysis of rejection as a risk factor of viremia, rejection episodes that occurred after the viral clearance were excluded from consideration, however, the effect of male gender (HR 2.35, P < 0.001), HLA-A2 match (HR 0.51, P = 0.010), HLA-B44 match (HR 0.31, P = 0.019) and HLA-DE15 match (HR 0.35, P = 0.034) remained statistically significant even if this exclusion was not performed.
DISCUSSION
Initial studies seeking to understand the role of HLAs in the pathogenesis of BKV-associated diseases evaluated the total number of matches or mismatches between donor and recipient antigens. In one study of 40 patients with BKV nephropathy, mismatch for DR showed that the presence of DR antigens was a risk factor for disease. It was argued that HLA mismatch may thwart the development of effective HLA restricted anti-viral immunity. Additionally, one would expect that antigen mismatching would lead to more episodes of acute rejection and tissue injury followed by cellular regeneration created a milieu favorable for BKV replication [12]. Consistent with this notion, HLA mismatch was found to be a risk factor for BK viremia [13]. In our current analysis, HLA-B and HLA-DR mismatch correlated with the presence of viremia. In contrast, a number of studies have not confirmed any association between overall HLA mismatch and the occurrence of viremia [14] or BKV nephropathy [7, 15, 16]. Indeed, in one study, HLA mismatching was associated with lower incidence of graft loss in patients with BKVN [17]. These conflicting reports indicate that the effects of HLA on the evolution of BKV infection are bidirectional, and subject to over-riding factors such as the degree of immunosuppression, incidence of rejection, non-HLA-restricted immunopathology, cytokine gene polymorphisms and other elements of innate immunity [18].
Limited data are available on the role played by individual HLAs in modulating the course of BKV infection. One group of investigators noted that HLA A30 and DRw6 were associated with a high antibody titer to viral large T antigen, while lower titers were found in subjects who typed as HLA A1 and DR3 [19]. More recently, it was noted that 10 of 11patients with sustained viremia had an absence of HLA-C7 in the transplant recipients. Additionally, this antigen was absent in all 11 organ donors corresponding to these recipients [14]. However, a second study, which analyzed patients with biopsy-proven BKV nephropathy, could not confirm this [20]. Since a successful immune response against viral infection is characterized by MHC restriction, it could be argued that the frequency of particular antigens may not be as relevant as the frequency with which both the donor and recipient are matched for that antigen. This has been confirmed for several antigens as noted in Table 2. A lower incidence of viremia in donors and recipients matched for these HLA types suggests that these are protective antigens, but definitive proof would require prospective studies.
The mechanism of protection likely involves the presentation of BKV-derived peptides by donor or recipient antigen-presenting cells (APCs) to HLA-matched recipient effector T-cells. Direct antigen presentation by donor APCs may be operative in the early post-transplant period, whereas the indirect antigen presentation by recipient APCs may be more important at later time points. In prior work on the cell-mediated immune response to BKV, several large T-antigen-derived peptides were shown to bind or recognize HLA-A01, HLA-A03, HLA-A24, HLA-B07, HLA-B08, HLA-DRB1-01, HLA-DRB1-03 [21], HLA-DR01, HLA-DR07, HLA-DR-10 or HLA-DR15 [22, 23]. It is notable that from this list, only HLA-DR15 was identified as protective in our analysis. Thus, peptide binding with the corresponding HLA does not necessarily generate an effective immune response. The role of HLA-A2 matching (Table 2) is supported by clinical observations in patients with BKV nephropathy [24].
The HLA associations suggested by this work have potential clinical ramifications in two areas. First, the question may be raised whether patients carrying HLAs associated with lower risk of viremia need less intensive screening for BKV infection after transplantation. Reduction in HRs mentioned in Table 3 are all relatively modest, and it would be necessary to identify patients with a much lower risk of viral reactivation to relax current recommendations for viral screening. This could conceivably be achieved by developing multiscale predictive models, in which the effect of HLA matching is linked to other determinants of outcome such as sex, history of diabetes mellitus, use of ureteric stents, number of rejection episodes, intensity of immunosuppression, titers of anti-viral antibodies and frequency of BKV-sensitized T-cells in the circulation. Second, the observations made in this study could aid in the development of T-cell-based vaccines against BKV using bioinformatics approaches. For example, 9-mer peptides derived from amino acid sequences of BKV proteins could be evaluated in-silico for potential high-binding affinity to HLA A2, B44 and C6 antigens, all of which were associated with reduced risk of viremia. Peptides with confirmed high-binding affinity in biochemical assays could then be evaluated in vitro and in vivo for their ability to induce activation and differentiation of CD8-positive T-cytotoxic cells with activity against BKV-infected cells. Likewise 15-mer viral peptides binding to HLA-DR15 could be used as the starting point for discovery of MHC class II restricted epitope recognized by CD4-positive T-cells.
This study is limited by its retrospective nature. All patients are from a medical center that predominantly transplants Caucasian subjects. Molecular HLA typing was not available. Although discrepancies between serologic and molecular typing have been described for several HLA-A and HLA-B alleles, those identified as statistically significant in this study (A2, A3, B44) are not considered to be problematic in this regard [25, 26]. Similarly, the most common problem in HLA-Cw typing is believed to be false-negative results with relatively uncommon alleles for which no reliable serologic reagents are available [27]. A uniform protocol for BKV screening was not applied to all patients, although this should not invalidate the statistical associations that have emerged. Notwithstanding these limitations, evidence is provided that matching for several HLAs seems to affect the predisposition to develop BK viremia. These findings need to be confirmed by a prospective study in other medical centers.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGEMENTS
P.R. was supported by NIH grant RO1 AI 51227. K.M. was supported by The International Research Fund for Subsidy of Kyushu University School of Medicine Alumni.
REFERENCES
- 1.Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582–594. doi: 10.1111/j.1600-6143.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 2.Hariharan S. BK virus nephritis after renal transplantation. Kidney Int. 2006;69:655–662. doi: 10.1038/sj.ki.5000040. [DOI] [PubMed] [Google Scholar]
- 3.Ramos E, Hirsch HH. Polyomavirus-associated nephropathy: updates on a persisting challenge. Transpl Infect Dis. 2006;8:59–61. doi: 10.1111/j.1399-3062.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch HH, Randhawa P AST Infectious Disease Community of Practice. BK virus in solid organ transplant recipients. Am J Transplant. 2009;9:S136–S146. doi: 10.1111/j.1600-6143.2009.02904.x. [DOI] [PubMed] [Google Scholar]
- 5.Binet I, Nickeleit V, Hirsh HH, et al. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation. 1999;67:918–922. doi: 10.1097/00007890-199903270-00022. [DOI] [PubMed] [Google Scholar]
- 6.Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67:103–109. doi: 10.1097/00007890-199901150-00018. [DOI] [PubMed] [Google Scholar]
- 7.Wadei HM, Rule AD, Lewin M, et al. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN) Am J Transplant. 2006;6:1025–1032. doi: 10.1111/j.1600-6143.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 8.Trydzenskaya H, Sattler A, Müller K, et al. Novel approach for improved assessment of phenotypic and functional characteristics of BKV-specific T-cell immunity. Transplantation. 2011;92:1269–1277. doi: 10.1097/TP.0b013e318234e0e5. [DOI] [PubMed] [Google Scholar]
- 9.Hammer MH, Brestrich G, Andree H, et al. HLA type-independent method to monitor polyoma BK virus-specific CD4 and CD8 T-cell immunity. Am J Transplant. 2006;6:625–631. doi: 10.1111/j.1600-6143.2005.01221.x. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerer JM, Horne PH, Fiessinger LA, et al. Cytotoxic Effector Function of CD4-Independent, CD8+ T Cells Is Mediated by TNF-α/TNFR. Transplantation. 2012;94:1103–1110. doi: 10.1097/TP.0b013e318270f3c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 12.Awadalla Y, Randhawa P, Ruppert K, et al. HLA mismatching increases the risk of BK virus nephropathy in renal transplant recipients. Am J Transplant. 2005;4:1691–1696. doi: 10.1111/j.1600-6143.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 14.Bohl DL, Storch GA, Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant. 2005;5:2213–2221. doi: 10.1111/j.1600-6143.2005.01000.x. [DOI] [PubMed] [Google Scholar]
- 15.Ramos E, Drachenberg CB, Papadimitriou JC, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol. 2002;13:2145–2151. doi: 10.1097/01.asn.0000023435.07320.81. [DOI] [PubMed] [Google Scholar]
- 16.Vasudev B, Hariharan S, Hussain SA, et al. BK virus nephritis: risk factors, timing, and outcome in renal transplant recipients. Kidney Int. 2005;68:1834–1839. doi: 10.1111/j.1523-1755.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 17.Drachenberg CB, Papadimitriou JC, Mann D, et al. Negative impact of human leukocyte antigen matching in the outcome of polyomavirus nephropathy. Transplantation. 2005;80:276–278. doi: 10.1097/01.tp.0000165096.01034.15. [DOI] [PubMed] [Google Scholar]
- 18.Comoli P, Basso S, Azzi A, et al. Dendritic cells pulsed with polyomavirus BK antigen induce ex vivo polyoma BK virus-specific cytotoxic T-cell lines in seropositive healthy individuals and renal transplant recipients. J Am Soc Nephrol. 2003;14:3197–3204. doi: 10.1097/01.asn.0000096374.08473.e3. [DOI] [PubMed] [Google Scholar]
- 19.Noss G. Human polyoma virus type BK infection and T antibody response in renal transplant recipients. Zentralbl Bakteriol Mikrobiol Hyg [A] 1987;266:567–574. doi: 10.1016/s0176-6724(87)80239-0. [DOI] [PubMed] [Google Scholar]
- 20.Awadallah Y, Duquesnoy R, Randhawa P, et al. HLA susceptibility to BKV infection. Am J Transplant. 2006;6:640. doi: 10.1111/j.1600-6143.2005.01218.x. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Melenhorst J, Hensel N, et al. T-cell responses to peptide fragments of the BK virus T antigen: implications for cross-reactivity of immune response to JC virus. J Gen Virol. 2006;87:2951–2960. doi: 10.1099/vir.0.82094-0. [DOI] [PubMed] [Google Scholar]
- 22.Ramaswami B, Popescu I, Macedo C, et al. HLA-A01-, -A03-, and -A024-binding nanomeric epitopes in polyomavirus BK large T antigen. Hum Immunol. 2009;70:722–728. doi: 10.1016/j.humimm.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramaswami B, Popescu I, Macedo C, et al. The polyomavirus BK large T-antigen-derived peptide elicits an HLA-DR promiscuous and polyfunctional CD4+ T-cell response. Clin Vaccine Immunol. 2011;18:815–824. doi: 10.1128/CVI.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Trofe J, Gordon J, et al. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J Virol. 2006;80:3495–3505. doi: 10.1128/JVI.80.7.3495-3505.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu N, Ohashi M, Alosco S, et al. Accurate typing of HLA-A antigens and analysis of serological deficiencies. Tissue Antigens. 1997;50:380–386. doi: 10.1111/j.1399-0039.1997.tb02891.x. [DOI] [PubMed] [Google Scholar]
- 26.Bozón MV, Delgado JC, Selvakumar A, et al. Error rate for HLA-B antigen assignment by serology: implications for proficiency testing and utilization of DNA-based typing methods. Tissue Antigens. 1997;50:387–394. doi: 10.1111/j.1399-0039.1997.tb02892.x. [DOI] [PubMed] [Google Scholar]
- 27.Mytilineos J, Christ U, Lempert M, et al. Comparison of typing results by serology and polymerase chain reaction with sequence-specific primers for HLA-Cw in 650 individuals. Tissue Antigens. 1997;50:395–400. doi: 10.1111/j.1399-0039.1997.tb02893.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.