Abstract
Background
The therapeutic effect of tonsillectomy for immunoglobulin A nephropathy (IgAN) has been widely recognized, but the mechanism by which tonsillar immunity leads to glomerulonephritis has been unclear. We investigated subtypes and localization of dendritic cells (DCs) in tonsils and looked for relationships between the tonsillar DCs and the clinical features and renal histological changes of patients with IgAN.
Methods
We examined tonsils from 33 IgAN patients, using as control tonsillar specimens from subjects without glomerulonephritis. Five distinct markers of DCs (CD303, CD1c, CD209, CD208 and CD1a) were analyzed by immunohistochemistry and flow cytometry. The mRNA levels of these DC markers were evaluated using real-time polymerase chain reaction. The clinical data and histological results obtained evaluating renal biopsy tissues were statistically compared with immunological data.
Results
Of the five subtypes of DCs, CD208+ DCs were significantly increased in the tonsils of IgAN patients compared with that of controls. Furthermore, the number of CD208+ DCs in the tonsils was positively and linearly correlated with the proportion of crescentic glomeruli in renal biopsy tissues and with the urinary protein level. Only few CD208+ cells, however, were found in the kidney biopsy specimens of IgAN patients.
Conclusions
These observations suggest that increased CD208+ DCs in tonsils may play a directive role in the pathogenesis of IgAN. The present results support the therapeutic significance of tonsillectomy for IgAN patients.
Keywords: CD208, dendritic cells, glomerular crescent, IgA nephropathy, tonsillectomy
INTRODUCTION
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis in the world [1, 2] and has been found to have a worse renal prognosis than had originally been expected [3]. There is, therefore, a need for the effective treatment, which results in remission of the disease in its early stage.
Hotta (an author of this manuscript) et al. [4] first advocated the effects of treating IgAN patients with a combination of tonsillectomy and steroid pulse therapy not only on reducing hematuria/proteinuria, but also on long-term remission regarding renal function. A recent prospective randomized controlled study also showed 2-year favorable effects of the combination of tonsillectomy and steroid pulse therapy on proteinuria and renal function [5]. Moreover, efficacy of tonsillectomy for the recurrence of IgAN after kidney transplantation has been reported [6, 7]. Although these clinical observations strongly suggest a relationship between the tonsillar immune system and the pathogenesis of IgAN, the mechanism by which tonsillar immunity leads to glomerulonephritis is unclear.
Dendritic cells (DCs) are the most potent antigen-presenting cells, and their participation in the pathogenesis of IgAN has been suggested in some reports. Deterioration of renal lesions was observed after mucosal activation of DCs in the animal models of IgAN [8], and the expression of B-cell activation factor on DCs has been reported to have been significantly higher in IgAN patients than that in controls [9]. These reports indicate that DCs play key roles in the pathogenesis of IgAN.
Different subtypes of human DCs have been classified according to their phenotypic characteristics. All DCs derive from hematopoietic stem cells, which differentiate into various subtypes of DCs along two main branches: a series of lymphoid precursors and a series of myeloid precursors. The lymphoid precursors give rise to the plasmacytoid DCs (pDCs) expressing blood DC antigen 2 (BDCA2), alias CD303 [10], whereas the myeloid precursors give rise to conventional DCs expressing blood DC antigen 1 (BDCA1), alias CD1c, monocyte-derived DCs (moDCs) expressing DC-specific intercellular adhesion molecule 3 grabbing, non-integrin (DC-SIGN), alias CD209, and Langerhans cells expressing CD1a (mainly localized in skin) [11, 12]. Interdigitating DCs (iDCs), expressing DC lysosome-associated membrane glycoprotein (DC-LAMP), alias CD208, mainly localize in the interfollicular area of lymphoid tissues. CD208+ DCs are thought to be derived mainly from moDCs [13].
So far, it has not been shown which subtypes of DCs take part in the pathogenesis of IgAN. We therefore investigated the subtypes and their localization in the tonsils of patients with IgAN and looked for correlations between the types of tonsillar DCs and the patients' clinical and renal histological parameters.
MATERIALS AND METHODS
Patients and controls
The study protocols were approved by the Ethical Committee of National Defense Medical College.
Thirty-three of the biopsy-proven IgAN patients who had undergone tonsillectomy from January 2008 to September 2012 in the National Defense Medical College Hospital participated in this study. They were selected by excluding patients tonsillectomized >1 year after renal biopsy, patients suffering from diabetes, neoplasm, inflammatory diseases or other systemic diseases and patients who had received corticosteroids and/or other immunosuppressive agents. Also participating were nine control patients who had no history of renal, liver, systemic diseases and active infectious diseases. All control specimens were taken from the chronic tonsillitis patients without glomerulonephritis (without proteinuria or renal dysfunction). Written informed consent was obtained from each patient in accordance with the principles of the Declaration of Helsinki. Characteristics of both the IgAN and control patients are listed in Table 1.
Table 1.
Characteristics of IgAN patients and control patients
| Controls | IgA nephropathy | |
|---|---|---|
| Number of patients | 9 | 33 |
| Sex (male/female) | 1/8 | 9/24 |
| Age | 41.7 ± 20.4 | 33.9 ± 17.7 |
| Serum Cr (mg/dL) | 0.61 ± 0.18 | 0.73 ± 0.22 |
| Urinary protein (g/gCr) | <0.1 | 0.70 ± 0.98 |
Histological evaluation
Sections (3 μm thick) of formalin-fixed, paraffin-embedded tonsillar tissues were stained with hematoxylin and eosin (HE). For the immunostaining of CD209 and CD208, two-step indirect immunoperoxidase staining was performed using the secondary antibody conjugated with the horseradish peroxidase-labeled polymer (Envision; DAKO Corp. Carpinteria, CA, USA) and developed with 3,3′-diaminobenzidine reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA). We performed immunostaining of CD208 with the use of antigen retrieval process of proteinase K (DAKO Corp.) digestion for 10 min and subsequent heating at 95°C in citrate buffer (pH 6.0) for 20 min. The counterstaining was performed with Mayer's hematoxylin (Wako, Osaka, Japan).
Direct immunofluorescence (IF) staining for CD303, CD1c or CD1a was performed on acetone-fixed, frozen tonsillar sections (5 μm thick) using fluorescein isothiocyanate (FITC)-conjugated primary antibodies. Triple IF staining for CD208, CD3 and HLA-DR and for CD20, CD3 and IgA were also performed on frozen tonsillar sections of several patients with IgAN. In the process of triple staining, staining for IgA was performed with a direct IF staining method, and staining for CD208, CD3, HLA-DR and CD20 were performed with an indirect IF staining method using secondary antibodies; Alexa Fluor 350-labeled goat antirabbit immunoglobulin G (IgG; Molecular Probes, Eugene, OR, USA), Alexa Fluor 594-labeled donkey antimouse IgG (Molecular Probes) or Alexa Fluor 488-labeled donkey antirat IgG (Molecular Probes). Indirect IF staining for CD208 was performed on frozen sections (3 μm thick) of renal tissues of patients with IgAN (n = 17) using secondary antibody; Alexa Fluor 488-labeled goat antimouse IgG (Molecular Probes). Primary antibodies used in this study are listed in Table 2.
Table 2.
Antibodies used in this study
| Antibody type | Clone | Labeling | Supplier |
|---|---|---|---|
| CD303 | AC144 | FITC | Miltenyi Biotec, Bergisch Gladbach, Germany |
| CD1c | AD5–8E7 | FITC | Miltenyi Biotec |
| CD209 | 120507 | Unlabeled, PE | R&D Systems, Minneapolis, MN, USA |
| CD208 | 104.G4 | Unlabeled, PE | Beckman Coulter, Brea, CA, USA |
| CD1a | HI149 | FITC | Biolegend, San Diego, CA, USA |
| Human Lineage Marker/HLA-DR Antibody Mix (hLMAX) | – | Lineage; FITC, HLA-DR; PerCP-Cy5.5 | Imgenex, San Diego, CA, USA |
| HLA-DR | MEM-12 YE2/36HLK | PE Unlabeled | Exbio, Prague, Czech Republic; GeneTex, Irvine, CA, USA |
| CD3 | Polyclonal | Unlabeled | DAKO Corp., Carpinteria, CA, USA |
| CD20 | L26 | Unlabeled | DAKO Corp. |
| IgA | 106G | FITC | MBL, Nagoya, Japan |
FITC, fluorescein isothiocyanate; PE, phycoerythin; PerCP-Cy5.5, peridinin chlorophyll protein complex with cyanin-5.5.
To quantify the area of germinal centers of tonsils, we assessed HE-stained sections under light microscopy. Photographs of a random field were obtained using a digital camera (magnification; ×10), and germinal center area per total tonsillar area was measured by using the image analysis software (Lumina Vision Ver.2.04; Mitani Corp., Fukui, Japan). For the quantification of the number of each subtype of DCs, positive cells in the photographs of four consecutive fields (magnification; ×200) were counted and expressed as positive cell numbers per square millimeter. Photographs of triple IF staining were taken with a confocal laser scanning microscopy (LSM510; Carl Zeiss Co., Oberkochen, Germany).
Flow cytometry
Isolated cells of tonsils from four IgAN patients and three controls were analyzed by using flow cytometry as we reported [14]. The fresh tonsillar tissues were minced into small pieces. After degrading them by adding 0.5 mg/mL collagenase (Wako), tissue debris was removed using mesh and they were incubated with various antibodies listed in Table 2. Double staining was performed with antibodies for HLA-DR and CD303, CD1c or CD1a. Triple staining was also performed with antibodies for Human Lineage Marker/HLA-DR Antibody Mix and either CD209 or CD208. The lineage marker, which includes antibodies for CD3, CD14, CD16, CD19, CD20 and CD56, was used to exclude cell populations positive for these markers from DCs in the analysis of CD208 and CD209, because there have been reports that a low level of DC-LAMP (CD208) mRNA expression was observed upon activation in other antigen-presenting cells such as monocytes, macrophages and B-cells [13], and that CD209 was also expressed on CD14+ cells [15]. The hemolysis procedure was done using a Whole Blood Lysing Reagent Kit (Beckman Coulter, Brea, CA, USA). Intracellular staining procedures for the CD208 staining were performed using a BD Cytofix/Cytoperm Fixation/Permeabilization Kit (Becton Dickinson and Company, Franklin Lakes, NJ, USA) after staining cellular surface markers according to the manufacturer's instructions. Stained cells were analyzed by using the EPICS XL ADC System (Beckman Coulter). Data were analyzed using an EXPO32ADC software (Beckman Coulter). Antibodies used for flow cytometry are also listed in Table 2.
Real-time reverse transcription–polymerase chain reaction
Extraction of total RNA, reverse transcription into complimentary DNA (cDNA) and real-time polymerase chain reaction (PCR) using the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) were performed as previously described [16]. Total RNA from the tonsils of 23 IgAN patients and 8 controls was analyzed. We used TaqMan Gene Expression Assays with primer/probe sets for human CLEC4C (CD303), CD1c, CD209, LAMP3 (CD208), CD1a and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Applied Biosystems). The relative amount of mRNA was calculated using comparative Ct (▵▵Ct) methods. All specific amplification products were normalized against GAPDH mRNA, which for internal control was amplified in the same reaction.
Clinical and renal histological data
Two pathologists evaluated the renal biopsy specimens independently. The diagnosis of IgAN was based on the demonstration by direct IF staining of mesangial immune deposits that stain dominantly for IgA, on mesangial proliferation evident by light microscopy and on the presence of mesangial electron-dense deposits evident by electron microscopy. Correlations between the number of DCs in the tonsils and clinical data such as levels of proteinuria, levels of hematuria, serum creatinine and serum IgA at tonsillectomy were assessed. Renal histological data such as proportions (to total glomeruli) of crescentic glomeruli, sclerotic glomeruli, glomeruli with adhesion and glomeruli with mesangial proliferation were also assessed in each renal biopsy, and their relations with the levels of tonsillar DCs were evaluated. Renal biopsy evaluation and tonsillar tissue evaluation were done independently and in blind to each other.
Statistics
All data were analyzed by using the JMP10 statistics software (SAS Institute, Inc., Cary, NC, USA). Statistical analyses were performed using Student's t-test to compare the means of two groups and Pearson's correlation coefficient to show the correlations between two variables. A P-value of <0.05 was considered statistically significant. Data are shown as mean ± SE.
RESULTS
Histological characteristics of tonsillar tissues in IgAN
Light microscopic observation of HE-stained tonsillar tissues revealed that the interfollicular areas were characteristically expanded, while germinal centers were reduced in tonsils of IgAN patients compared with that of controls (Figure 1A and B). This finding was quantified in HE-stained tonsillar tissues by using an image analysis software to calculate the proportion of germinal center area to total tonsillar area. The proportion of germinal center area to total tonsillar area was significantly lower in IgAN patients than that in controls (Figure 1C).
FIGURE 1:

Histological structure of tonsils. Original magnification; ×10. (A and B) Representative photomicrograph of HE-stained tonsillar tissue. (A) A patient with chronic tonsillitis. (B) A patient with IgA nephropathy (IgAN). Enlarged interfollicular areas and small germinal centers are characteristic in the tonsils of patients with IgAN. (C) Germinal center area and total tonsil area were measured, and proportions of germinal center area to total tonsillar area were calculated. Results are means ± SE for each group. The proportion of germinal center to total tonsillar area in IgAN patients was significantly smaller than that in controls. *P < 0.01 versus controls. Control: n = 9, IgAN: n = 33.
Immunohistochemical stainings
The distribution of subtypes of DCs in tonsils did not basically differ between the IgAN patients and controls. In both, CD303+ cells were found sparsely in the under-epithelial and interfollicular areas (Figure 2A). CD1c+, CD209+ and CD208+ cells were mainly found in the interfollicular area (Figure 2B–D). CD208+ cells were clustered in the interfollicular area (Figure 2D). CD1a+ cells were localized in the under-epithelial area (Figure 2E).
FIGURE 2:
Various subtypes of DCs in tonsils (A–E) and kidneys (F) of patients with IgAN. Original magnification; A–C, E and F: ×200, D: ×100. (A) IF staining for CD303 (under-epithelial area). The arrows show epithelium. (B) IF staining for CD1c (interfollicular area). (C) Immunoperoxidase staining for CD209 (interfollicular area). The arrow shows a follicle. (D) Immunoperoxidase staining for CD208 (interfollicular area). The arrow shows a follicle. CD208+ cells are clustered in the interfollicular area. (E) IF staining for CD1a (under-epithelial area). The arrows show epithelium. (F) IF staining for CD208 in the renal tissue of an IgAN patient. CD208+ cells are observed in the renal interstitium. Photograph shows CD208+ cells around a globally sclerotic glomerulus.
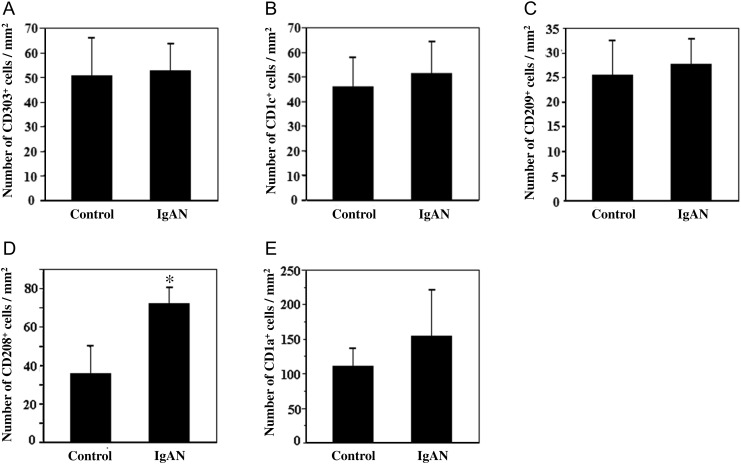
As shown in Figure 3D, the number of CD208+ cells in the tonsils of IgAN patients was significantly greater than that in the tonsils of controls. The levels of tonsillar CD303+, CD1c+, CD209+ or CD1a+ cells, however, did not differ between IgAN patients and controls (Figure 3A–C and E).
FIGURE 3:
Quantification analysis of different markers of subtypes of DCs in tonsillar tissues from controls and IgAN patients. Results are means ± SE for each group. (A) CD303+, (B) CD1c+, (C) CD209+, (D) CD208+ cells. The number of CD208+ cells in the tonsils of IgAN patients was significantly greater than that in the tonsils of controls. *P < 0.05 versus controls. (E) CD1a+ cells. Control: n = 9, IgAN: n = 33.
Triple staining for CD208, CD3 and HLA-DR revealed that CD208+ cells often existed in touch with CD3+ T-cells. Furthermore, around CD208+ cells, there were some activated T-cells that were double positive for CD3 and HLA-DR (Figure 4A–D). In addition, IgA+ cells mainly existed in the T-cell abundant interfollicular area adjacent to the follicles (Figure 4E–H).
FIGURE 4:
Triple IF staining of tonsils of patients with IgAN. (A–D) Triple IF staining for CD208 (Alexa Fluor 594: red), CD3 (Alexa Fluor 350: blue) and HLA-DR (Alexa Fluor 488: green). (A) CD208, (B) CD3, (C) HLA-DR. HLA-DR is known to be expressed on DCs, macrophages, B-cells and activated T-cells. (D) Merged image of A–C. The arrows indicate activated T-cells (CD3+ HLA-DR+). (E–H) Triple IF staining for CD20 (B-cells; Alexa Fluor 594: red), CD3 (T-cells; Alexa Fluor 350: blue) and IgA (FITC: green). (E) CD20. The arrows indicate follicles. (F) CD3, (G) IgA and (H) merged image of E–G.
Because we found that the increase in CD208+ cells was the most characteristic feature in tonsils of IgAN patients, we investigated the CD208+ cells in renal biopsy tissues. The renal biopsy specimens of all the 17 IgAN patients whose specimens were stained for CD208 had CD208+ cells in the interstitium, but in all samples the number of these was very small (Figure 2F). There was no correlation between the number of CD208+ cells in the kidneys and that in the tonsils.
Flow cytometry analysis
To evaluate DC markers on isolated cells, we examined fresh tonsillar specimens by flow cytometry. Figure 5A–F shows representative results of flow cytometry of tonsils from a patient with IgAN, whereas Figure 5G shows a result from a control subject. For CD303, CD1c and CD1a, cells that were double positive for HLA-DR and DC markers were identified to be DCs (Figure 5A–C). For CD209 and CD208, a lineage marker-negative population (presented as lin− in Figure 5D) was analyzed and is shown in Figure 5E–G. A population which was a lineage marker− and HLA-DR+ and DC marker+ was identified to be DCs.
FIGURE 5:
Flow cytometry analysis of a patient with IgAN (A–F) and a control subject (G). (A–C) Isolated cells were double-stained with antibodies for HLA-DR and either CD303, CD1c or CD1a. The HLA-DR-positive population was analyzed and is shown in graphs. HLA-DR+ and DC-marker+ cells in each staining were identified to be DCs. (A) HLA-DR and CD303 staining. (B) HLA-DR and CD1c staining. (C) HLA-DR and CD1a staining. (D–G) Isolated cells were triple-stained with antibodies for HLA-DR, lineage marker and either CD209 or CD208. The lineage marker-negative population (indicated lin− in graph D) was analyzed and is shown in E–G. Lineage marker−, HLA-DR+ and DC-marker+ cells in each staining were identified to be DCs. (E) HLA-DR and CD209 staining in the lineage marker− population in a patient with IgAN. (F) HLA-DR and CD208 staining in the lineage marker− population in a patient with IgAN. (G) HLA-DR and CD208 staining in the lineage marker− population in a control subject. (H–L) Quantification analysis results for the different subtypes of DCs in cells isolated from tonsils of IgAN patients and controls. (H) CD303+ DCs, (I) CD1c+ DCs, (J) CD209+ DCs, (K) CD208+ DCs. The percentage of HLA-DR+ cells in the lineage marker− population that were CD208+ was significantly greater in the tonsils of IgAN patients than that in the tonsils of controls. *P < 0.05 versus control. (L) CD1a+ DCs. Results are expressed as means ± SE for each group. Control: n = 3, IgAN: n = 4.
As shown in Figure 5K, the percentage of CD208+ cells in the lineage marker− and HLA-DR+ population was significantly greater in the tonsils of IgAN patients than that in the tonsils of controls. The percentage of CD303+, CD1c+, CD209+ or CD1a+ DCs did not differ between the tonsils of controls and those of IgAN patients (Figure 5H–J and L).
Real-time reverse transcription–PCR
The levels of CD208 and CD1c mRNA in the tonsils of IgAN patients were significantly greater than that in the tonsils of controls (Figure 6B and D). The mean value of CD303, CD209 and CD1a mRNA tended to be higher in IgAN patients than in controls, but the differences were not statistically significant (Figure 6A, C and E).
FIGURE 6:
mRNA expression for various markers of DCs. (A) CD303 mRNA, (B) CD1c mRNA, (C) CD209 mRNA, (D) CD208 mRNA and (E) CD1a mRNA. The levels of CD208 and CD1c mRNA in the tonsils of IgAN patients were significantly greater than that in the tonsils of controls. Results are expressed as means ± SE for each group. *P < 0.05 versus controls. Control: n = 8, IgAN: n = 23.
Positive correlation of DCs with clinical and renal histological features
The numbers of CD208+ cells in the tonsils were significantly and positively correlated with the proportion of crescentic glomeruli in the renal biopsy tissues (R = 0.49, P < 0.05) and with the urinary protein levels (R = 0.38, P < 0.05) (Figure 7A and B). No correlation was found between the number of CD208+ cells in tonsils and the level of hematuria, serum creatinine, serum IgA level or the proportions of sclerotic glomeruli, glomeruli with adhesion or glomeruli with mesangial proliferation to the total glomeruli. CD303, CD1c, CD209 and CD1a had no correlation with clinical or renal histological data.
FIGURE 7:
Correlation between the number of tonsillar CD208+ DCs and clinical findings. (A) A significant and positive correlation between the number of CD208+ cells in the tonsils and the proportion of crescentic glomeruli to total glomeruli in the renal biopsy tissues of patients with IgAN (n = 33). (B) A significant and positive correlation between the number of CD208+ cells in the tonsils and the urinary protein level (n = 33). CD303, CD1c, CD209 and CD1a had no correlation with clinical or renal histological data.
DISCUSSION
In this study, we found that the number of CD208+ DCs was significantly increased in the tonsils of IgAN patients. The number of CD208+ DCs in the tonsils was significantly and positively correlated with the proportion of crescentic glomeruli in the renal biopsy tissue and with the urinary protein level.
Histologically, tonsillar tissues consist of three microcompartments: crypt epithelium, interfollicular area and lymphoid follicle [17, 18]. Immune responses against foreign antigens arise in crypt epithelium with the uptake of the antigen by epithelial cells. The antigen is then processed and presented by DCs to CD4+ T-cells under the epithelium. The activated CD4+ T-cells then activate B-cells to induce differentiation of antigen-specific B-cells in the follicles. B-cells finally differentiate into immunoglobulin-secreting plasma cells in the interfollicular area [19]. Thus, the interfollicular area is an important place where the antigen-specific T-cell activation and subsequent T-cell–B-cell interaction takes place. Hotta, Takechi et al. [20] and Kawaguchi et al. [21] previously reported that the enlarged T-cell nodules in the interfollicular areas of tonsils were characteristic of IgAN patients. In this study, interfollicular areas were characteristically expanded, while areas of germinal centers were reduced in the tonsils of IgAN.
In the present study, CD1a+ DCs were found under the epithelial area, CD303+ DCs were found sparsely in both the epithelial area and the interfollicular area. CD1c+, CD209+ and CD208+ DCs were found mainly in the interfollicular area. We therefore speculate that CD1c+, CD209+ and CD208+ DCs play important roles in the expanded interfollicular area in the tonsils of IgAN patients. CD208+ DCs were the most abundant of these DC subtypes and are the characteristic cell type in the interfollicular area of the tonsils of IgAN patients.
CD208, also known as DC-LAMP, is a member of the LAMP family and known as a specific marker of iDCs [13, 22]. It is specifically expressed by human DCs upon activation and maturation, and CD208+ DCs are generally located in the interfollicular area of lymphoid tissues. In human DCs, CD208 is expressed in the intracellular major histocompatibility complex (MHC) Class II compartment just before the translocation of MHC Class II molecules to the cell surface [13]. So, it is suggested that CD208 participates in processing foreign antigens in the early stage. In addition to the close relation with MHC Class II molecules, CD208+ DCs strongly express costimulatory molecules such as CD80, CD83 and CD40, which are associated with the adhesion and activation of T-cells [23, 24]. Furthermore, the expression of CD208 is correlated with that of other maturation markers, such as CD86 [24]. We therefore speculate that CD208+ DCs have a strong ability to activate T-cells. Our observation of an increased number of CD208+ DCs accompanied by surrounding activated T-cells in an enlarged interfollicular area suggests that T-cell activation is accelerated in the tonsils of patients with IgAN. We think that the reduction of relative germinal center areas is a result of the expansion of interfollicular areas (T-cell areas) that is brought about by the chronically increased number of CD208+ DCs having a strong ability to activate T-cells.
Previous reports have shown that, in IgAN, the predominant subset of IgA in mesangial deposits is IgA1, the O-glycan chains in the hinge region of glomerular and serum IgA1 are highly underglycosylated [25, 26] and the IgA1 molecules produced by tonsillar lymphocytes are also underglycosylated [27]. In the present study, IgA+ cells were found in enlarged interfollicular areas near follicles. We suspect that, in the development of IgAN, CD208+ DCs may activate the immune system and lead to aberrant IgA1 production in tonsils.
Increases in CD208+ DCs in the lesions associated with dermal diseases such as psoriasis [28] and in various types of cancer, such as colorectal cancer or uterine cervical cancer, have been reported [29, 30]. In the present study, we found that the number of tonsillar CD208+ DCs was positively correlated with the proportion of crescentic glomeruli in the kidney and with the level of proteinuria. We, however, found few CD208+ cells in the renal biopsy tissues of IgAN patients. We suspect that the increased CD208+ DCs in the tonsils do not themselves migrate to the kidney, but may play a directive role by activating T-cells. Then, the activated T-cells themselves and/or subsequently produced immunoglobulins from activated B-cells may eventually cause kidney injury in IgAN. The alternative possibility is that some CD208+ DCs may migrate from tonsils to the kidney and cause inflammation there, but they can hardly be identified by the usual histochemical staining of renal biopsy tissues, because there are so few of them in the kidney.
Several reports suggest that toll-like-receptors (TLRs), especially TLR9, play important roles in the pathogenesis of IgAN. One study reported a correlation between the tonsillar TLR9 expression level and the therapeutic efficacy of tonsillectomy and steroid pulse therapy [31]. Another study reported that the expression of TLR9 and that of its ligand, MyD88, in spleen were correlated with the glomerular injury in animal models of IgAN [8]. Because the CD303+ DC (pDC) is the main DC that expresses TLR9 [32], it had been expected that CD303+ DCs would be increased in the tonsils of patients with IgAN. In the present study, however, we did not find such an increase. Since TLR9 is also on B- and T-cells besides CD303+ DCs in human [33, 34], in IgAN it may be overexpressed on these cells rather than CD303+ DCs.
The numbers of myeloid-related DCs in nasal-associated lymphoid tissue were previously reported to be increased in patients with IgAN: the numbers of CD1a+ DCs and CD209+ DCs were increased in the nasal biopsy samples of patients with IgAN [35]. We, however, did not find increases of CD1a+ or CD209+ DCs in the tonsils of IgAN patients. This is probably due to the difference between the DC populations of tonsillar tissues, which are lymphoid tissues, and nasal mucosa.
In summary, CD208+ DCs were significantly increased in the tonsils of patients with IgAN, and the number of tonsillar CD208+ DCs was positively correlated with the proportion of glomerular crescent formation and the level of proteinuria. The present results suggest the possibility that increased myeloid-related mature DCs, CD208+ DCs, reinforce the innate and adaptive immunity, thereby resulting in deterioration of renal lesions. The present results, thus, may support the therapeutic significance of tonsillectomy for IgAN patients. Further studies are required to find out how tonsillar CD208+ DCs induce renal lesion in patients with IgAN.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
We are grateful to our colleagues, Ms Tatsuyo Harasawa (at the Central Research Laboratory of National Defense Medical College) and Ms Sachiko Iwama (a technologist in our Department) for excellent technical assistance and to Dr Shuhji Seki, Dr Hiroyuki Nakashima (Department of Immunology and Microbiology, National Defense Medical College), Dr Kensuke Joh (Department of Pathology, Sendai Shakaihoken Hospital) and Dr Hiroki Sato (Department of Preventive Medicine and Public Health, National Defense Medical College) for valuable advice and discussion.
REFERENCES
- 1.Emancipator SN, Lamm ME. IgA nephropathy: pathogenesis of the most common form of glomerulonephritis. Lab Invest. 1989;60:168–183. [PubMed] [Google Scholar]
- 2.Levy M, Berger J. Worldwide perspective of IgA nephropathy. Am J Kidney Dis. 1988;12:340–347. doi: 10.1016/s0272-6386(88)80021-0. [DOI] [PubMed] [Google Scholar]
- 3.Manno C, Strippoli GF, D'Altri C, et al. A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. Am J Kidney Dis. 2007;49:763–775. doi: 10.1053/j.ajkd.2007.03.013. doi:10.1053/j.ajkd.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Hotta O, Miyazaki M, Furuta T, et al. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis. 2001;38:736–743. doi: 10.1053/ajkd.2001.27690. doi:10.1053/ajkd.2001.27690. [DOI] [PubMed] [Google Scholar]
- 5.Komatsu H, Fujimoto S, Hara S, et al. Effect of tonsillectomy plus steroid pulse therapy on clinical remission of IgA nephropathy: a controlled study. Clin J Am Soc Nephrol. 2008;3:1301–1307. doi: 10.2215/CJN.00310108. doi:10.2215/CJN.00310108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennoki T, Ishida H, Yamaguchi Y, et al. Proteinuria-reducing effects of tonsillectomy alone in IgA nephropathy recurring after kidney transplantation. Transplantation. 2009;88:935–941. doi: 10.1097/TP.0b013e3181b75374. doi:10.1097/TP.0b013e3181b75374. [DOI] [PubMed] [Google Scholar]
- 7.Ushigome H, Suzuki T, Fujiki M, et al. Efficacy of tonsillectomy for patients with recurrence of IgA nephropathy after kidney transplantation. Clin Transplant. 2009;23:17–22. doi: 10.1111/j.1399-0012.2009.01003.x. doi:10.1111/j.1399-0012.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- 8.Kajiyama T, Suzuki Y, Kihara M, et al. Different pathological roles of toll-like receptor 9 on mucosal B cells and dendritic cells in murine IgA nephropathy. Clin Dev Immunol. 2011;2011:819646. doi: 10.1155/2011/819646. doi:10.1155/2011/819646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goto T, Bandoh N, Yoshizaki T, et al. Increase in B-cell-activation factor (BAFF) and IFN-gamma productions by tonsillar mononuclear cells stimulated with deoxycytidyl-deoxyguanosine oligodeoxynucleotides (CpG-ODN) in patients with IgA nephropathy. Clin Immunol. 2008;126:260–269. doi: 10.1016/j.clim.2007.11.003. doi:10.1016/j.clim.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. doi:10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 11.Colonna M. Skin function for human CD1a-reactive T cells. Nat Immunol. 2010;11:1079–1080. doi: 10.1038/ni1210-1079. doi:10.1038/ni1210-1079. [DOI] [PubMed] [Google Scholar]
- 12.Noessner E, Lindenmeyer M, Nelson PJ, et al. Dendritic cells in human renal inflammation—part II. Nephron Exp Nephrol. 2011;119:e91–e98. doi: 10.1159/000332032. doi:10.1159/000332032. [DOI] [PubMed] [Google Scholar]
- 13.de Saint-Vis B, Vincent J, Vandenabeele S, et al. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. doi:10.1016/S1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 14.Hyodo T, Oda T, Yamamoto K, et al. Voltage-gated potassium channel Kv1.3 blocker as a potential treatment for rat anti-glomerular basement membrane glomerulonephritis. Am J Physiol Renal Physiol. 2010;299:F1258–F1269. doi: 10.1152/ajprenal.00374.2010. doi:10.1152/ajprenal.00374.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angel CE, Chen CJ, Horlacher OC, et al. Distinctive localization of antigen-presenting cells in human lymph nodes. Blood. 2009;113:1257–1267. doi: 10.1182/blood-2008-06-165266. doi:10.1182/blood-2008-06-165266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada M, Oda T, Higashi K, et al. Involvement of epimorphin in the repair of experimental renal fibrosis in mice. Lab Invest. 2010;90:867–880. doi: 10.1038/labinvest.2010.50. doi:10.1038/labinvest.2010.50. [DOI] [PubMed] [Google Scholar]
- 17.Nave H, Gebert A, Pabst R. Morphology and immunology of the human palatine tonsil. Anat Embryol (Berl) 2001;204:367–373. doi: 10.1007/s004290100210. doi:10.1007/s004290100210. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y, Chen X, Nishi S, et al. Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int. 2004;65:1135–1144. doi: 10.1111/j.1523-1755.2004.00486.x. doi:10.1111/j.1523-1755.2004.00486.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoefakker S, van 't Erve EH, Deen C, et al. Immunohistochemical detection of co-localizing cytokine and antibody producing cells in the extrafollicular area of human palatine tonsils. Clin Exp Immunol. 1993;93:223–228. doi: 10.1111/j.1365-2249.1993.tb07970.x. doi:10.1111/j.1365-2249.1993.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takechi H, Nagura H, Houzawa K, et al. Clinical and immunological study of the tonsils in IgA nephropathy. Contrib Nephrol. 2007;157:197. [Google Scholar]
- 21.Kawaguchi M, Sakai T, Sakamaki A, et al. Expanded primary T nodules in the palatine tonsils from patients with IgA nephropathy. Acta Otolaryngol Suppl. 1993;508:36–42. doi: 10.3109/00016489309130264. doi:10.3109/00016489309130264. [DOI] [PubMed] [Google Scholar]
- 22.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 23.Zhou LJ, Schwarting R, Smith HM, et al. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol. 1992;149:735–742. [PubMed] [Google Scholar]
- 24.Bjorck P, Flores-Romo L, Liu YJ. Human interdigitating dendritic cells directly stimulate CD40-activated naive B cells. Eur J Immunol. 1997;27:1266–1274. doi: 10.1002/eji.1830270531. doi:10.1002/eji.1830270531. [DOI] [PubMed] [Google Scholar]
- 25.Hiki Y, Odani H, Takahashi M, et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. doi:10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 26.Odani H, Hiki Y, Takahashi M, et al. Direct evidence for decreased sialylation and galactosylation of human serum IgA1 Fc O-glycosylated hinge peptides in IgA nephropathy by mass spectrometry. Biochem Biophys Res Commun. 2000;271:268–274. doi: 10.1006/bbrc.2000.2613. doi:10.1006/bbrc.2000.2613. [DOI] [PubMed] [Google Scholar]
- 27.Horie A, Hiki Y, Odani H, et al. IgA1 molecules produced by tonsillar lymphocytes are under-O-glycosylated in IgA nephropathy. Am J Kidney Dis. 2003;42:486–496. doi: 10.1016/s0272-6386(03)00743-1. doi:10.1016/S0272-6386(03)00743-1. [DOI] [PubMed] [Google Scholar]
- 28.Higaki M, Higaki Y, Kawashima M. Increased expression of CD208 (DC-LAMP) in epidermal keratinocytes of psoriatic lesions. J Dermatol. 2009;36:144–149. doi: 10.1111/j.1346-8138.2009.00609.x. doi:10.1111/j.1346-8138.2009.00609.x. [DOI] [PubMed] [Google Scholar]
- 29.Kanao H, Enomoto T, Kimura T, et al. Overexpression of LAMP3/TSC403/DC-LAMP promotes metastasis in uterine cervical cancer. Cancer Res. 2005;65:8640–8645. doi: 10.1158/0008-5472.CAN-04-4112. doi:10.1158/0008-5472.CAN-04-4112. [DOI] [PubMed] [Google Scholar]
- 30.Sandel MH, Dadabayev AR, Menon AG, et al. Prognostic value of tumor-infiltrating dendritic cells in colorectal cancer: role of maturation status and intratumoral localization. Clin Cancer Res. 2005;11:2576–2582. doi: 10.1158/1078-0432.CCR-04-1448. doi:10.1158/1078-0432.CCR-04-1448. [DOI] [PubMed] [Google Scholar]
- 31.Sato D, Suzuki Y, Kano T, et al. Tonsillar TLR9 expression and efficacy of tonsillectomy with steroid pulse therapy in IgA nephropathy patients. Nephrol Dial Transplant. 2012;27:1090–1097. doi: 10.1093/ndt/gfr403. doi:10.1093/ndt/gfr403. [DOI] [PubMed] [Google Scholar]
- 32.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. doi:10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Mansson A, Adner M, Cardell LO. Toll-like receptors in cellular subsets of human tonsil T cells: altered expression during recurrent tonsillitis. Respir Res. 2006;7:36. doi: 10.1186/1465-9921-7-36. doi:10.1186/1465-9921-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieg AM, Vollmer J. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol Rev. 2007;220:251–269. doi: 10.1111/j.1600-065X.2007.00572.x. doi:10.1111/j.1600-065X.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 35.Eijgenraam JW, Reinartz SM, Kamerling SW, et al. Immuno-histological analysis of dendritic cells in nasal biopsies of IgA nephropathy patients. Nephrol Dial Transplant. 2008;23:612–620. doi: 10.1093/ndt/gfm595. doi:10.1093/ndt/gfm595. [DOI] [PubMed] [Google Scholar]