Abstract
Background and Purpose
Increased blood-brain barrier (BBB) permeability occurs in cerebral small vessel disease (SVD). It is not known if BBB changes predate progression of SVD.
Methods
We followed up patients with non-disabling lacunar or cortical stroke and BBB permeability MR imaging following their original stroke. About three years later, we assessed functional outcome (Oxford Handicap Score, OHS, poor outcome defined as 3-6), recurrent neurological events and white matter hyperintensity (WMH) progression on MRI.
Results
Amongst 70 patients, mean age 68 (SD±11) years, median time to clinical follow up was 39 months (IQR 30-45), median OHS was 2 (IQR 1-3); poor functional outcome was associated with higher baseline WMH score (p<0.001) and increased basal ganglia BBB permeability (p=0.046). Amongst 48 patients with follow-up MRI, WMH progression at follow-up was associated with baseline WMH (ANCOVA p<0.0001) and age (ANCOVA p=0.032).
Conclusions
Further long term studies to evaluate the role of BBB dysfunction in progression of SVD are required in studies that are large enough to account for key prognostic influences such as baseline WMH and age.
Keywords: Blood-brain barrier, leukoaraiosis, stroke, lacunar stroke
Introduction
The BBB becomes increasingly permeable with normal advancing age,1 particularly in patients with vascular dementia1 and small vessel disease (SVD, Figure 1).2-4 Cross-sectional studies do not determine whether increased BBB permeability predates progression of SVD and therefore might be causative. We followed up patients previously recruited in our cross-sectional study of BBB permeability changes in lacunar stroke2 to see if increased BBB permeability might predate progression of SVD.
Figure 1.
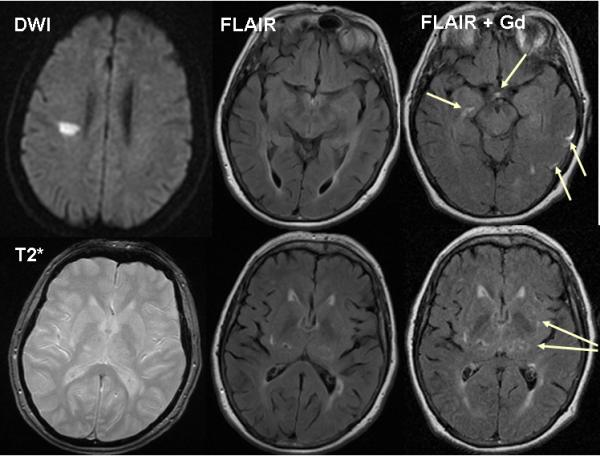
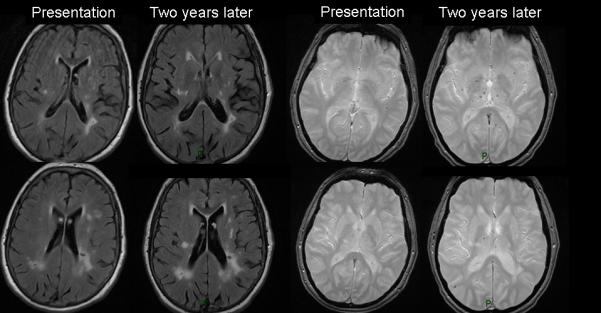
72-year-old patient with right hemisphere lacunar stroke. (a) two days after stroke MR DWI shows the acute right centrum semiovale lacunar infarct, T2* shows a few microbleeds, FLAIR shows numerous WMHs. BBB imaging six weeks later: FLAIR 30 minutes after iv Gadolinium (Gd) shows Gadolinium in cortical sulci and perivascular spaces (arrows). (b) FLAIR and T2* images two years later show increased WMHs and microbleeds (baseline FLAIR and T2* shown for comparison).
Methods
We originally recruited 97 previously independent patients with non-disabling ischaemic stroke,2 half lacunar, half cortical (controls). We assessed stroke severity (NIHSS score), performed diagnostic MR imaging (with DWI, T1, T2, FLAIR, T2*) to confirm stroke subtype and quantify white matter hyperintensities (WMH). We assessed BBB permeability in basal ganglia, cortex, white matter and lateral ventricular cerebrospinal fluid (CSF) 1-3 months after the index stroke with sequential MR T1 imaging before and after intravenous gadolinium (described previously2, 5). The study was approved by the Lothian Research Ethics Committee (2002/W/RA/03); all participants gave written informed consent.
About three years later, we followed-up all patients to determine functional status (Oxford Handicap Score, OHS,6 similar to the modified Rankin Score: poor functional outcome=OHS 3-6, dead=OHS 6), recurrent strokes and assess SVD progression on structural MR imaging. We cross-checked the patients’ histories with hospital records. Follow-up MR imaging was performed on the same 1.5T MR scanner (GE Signa LX, Milwaukee, WI, USA, 22 mTm−1 maximum strength gradients) using the same diagnostic sequences (T1, T2, FLAIR, T2*, DWI) described previously.2 We analysed imaging features blind, noting new infarcts or haemorrhages, WMH (Fazekas score7) and change in WMH (Prins score8). The intra-class correlation coefficient for WMH rating by the neuroradiologist was 0.964. We registered follow-up to baseline scans and measured intracranial, whole brain, and WMH volumes using a validated multispectral image analysis tool.9 We masked infarcts to avoid confounding WMH volume,10 normalised brain and WMH volumes to intracranial volume (ICV) and determined WMH volume change.
We performed univariate and multivariate analysis (binary logistic regression) to identify variables associated with functional status and WMH change at follow-up adjusted for duration of follow-up. We performed uni- and multi-variate linear regression to examine associations between BBB permeability at baseline, WMH progression and functional outcome. We analysed factors associated with WMH volume change by ANCOVA. All analyses were performed in Minitab version 15, State College, Pennsylvania, US or SAS 9.1 (www.sas.com).
Results
We obtained clinical follow-up on 70/97 (78%) and MR imaging on 48 (49% of 97) of the original study patients. The 70 patients with clinical follow-up did not differ from the 27 without clinical follow-up in baseline age (mean at original presentation 68+/−11 vs 63+/−12, p=NS), median NIHSS (both 2 IQR 1-3), hypertension (both 66%) or lacunar stroke (50% vs 43%). The median baseline Fazekas deep and periventricular WMH scores were each 1; periventricular and deep Fazekas scores were >2 in 20 and nine patients respectively.
At clinical follow-up at median 39 months (IQR 30-48), 25% had poor outcome (12 OHS 3-5, six dead). Twenty-five had clinical and/or imaging evidence of recurrent neurological events (36%): two, clinically-diagnosed recurrent stroke; 23, new focal weakness; four, new infarct on follow-up imaging. In univariate analyses, poor functional outcome was associated with increasing age, higher baseline WMH score and basal ganglia BBB permeability, but not baseline NIHSS, hypertension, diabetes or stroke subtype (Table 1). On multivariate logistic regression (adjusted for baseline age, WMH score, BBB permeability) predictors of poor outcome were baseline total WMH score (OR 4.8, 2.0-11.4, p<0.001) and basal ganglia BBB permeability (OR 2.2, 1.0-4.6, p=0.046).
Table 1.
Predictors of poor outcome (OHS 3-6) three years after lacunar or mild cortical stroke
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variable | OR/unit increase |
95% CI | P value | OR (95% CI)/ unit increase |
P value |
| Age (per year) | 1.09 | 1.03-1.17 | 0.007 | ||
| Male | 1.50 | 0.4-6.2 | 0.5 | ||
| Hypertension y/n | 1.00 | 0.3-3.1 | 1.0 | ||
| Diabetes y/n | 1.50 | 0.3-6.7 | 0.6 | ||
| Cortical v lacunar stroke |
1.67 | 0.55-5.05 | 0.364 | ||
| Baseline NIHSS | 1.10 | 0.7-1.9 | 0.6 | ||
| Cortical grey permeability |
1.30 | 0.6-2.7 | 0.5 | 1.37 (0.61–3.10)* | 0.4 |
| Basal ganglia permeability |
2.10 | 1.1-4.3 | 0.027 | 2.15 (1.01-4.57)* | 0.046 |
| White matter permeability |
0.9 | 0.2-5.5 | 0.9 | 1.5 (0.19–11.8)* | 0.7 |
| CSF permeability | 0.8 | 0.6-1.0 | 0.06 | 0.8 (0.6–1.1) * | 0.2 |
| Fazekas DWMH score (0-3) |
3.91 | 1.82-8.39 | <0.001 | 3.3 (1.4-7.5)# | 0.005 |
| Fazekas PVWMH score (0-3) |
4.09 | 1.90-8.78 | <0.001 | 3.5 (1.4-8.6)# | 0.006 |
| Fazekas WMH combined score (0-6) |
4.50 | 2.0-10.0 | <0.001 | 4.8 (2.0-11.4)*
+ 4.1 (1.6-10.3)# + |
<0.001 |
=corrected for baseline WMH;
=corrected for age;
per unit increase in WMH
Forty-eight patients had follow-up MR imaging, median 39 (IQR 30-45) months after index stroke; baseline age (67+/−10), median NIHSS (2, IQR 2-3), proportion hypertensive (65%), lacunar index stroke (48%) and WMH score were the same as for all 70 with clinical follow-up. 6/48 (13%) were OHS=3-5.
WMH score had increased in 33/48 and remained unchanged in 15/48 (according to Prins and Fazekas scores, Figure 1b). On univariate analyses, baseline WMH score, but not age or time since stroke, strongly predicted progression of WMH (OR 8.7, 2.1-35.3, p<0.001). Median WMH volume at baseline was 8.5 ml (IQR 5.9-15.2), at follow-up was 12.2 ml (IQR 8.6-19.7), median increase 3.2 ml (IQR 1.2-7.5, p<0.0001). The strongest predictors of increasing WMH volume at follow-up were baseline WMH volume (ANCOVA p<0.0001) and age (ANCOVA p=0.032). WMH volume at follow-up increased by: 1.80 times (1.56-2.08) per unit (mm3) of WMH volume and 1.01 times (1.00-1.02) per additional year of age at baseline.
Discussion
Poor functional outcome three years after lacunar or mild cortical ischaemic stroke was associated with having more WMH, being older and having increased BBB permeability in the basal ganglia. Worsening of WMH was dominated by having more WMH at baseline and older age. A third had clinical/imaging evidence of recurrent neurological events, although only two were formally diagnosed as recurrent stroke, consistent with recent recognition of WMH-associated subtle neurological symptoms.11 Long term poor functional outcome and WMH progression were not associated with vascular risk factors, baseline NIHSS or stroke subtype, possibly because the study was small, NIHSS very mild, or due to secondary prevention, although this does not control risk factors perfectly.
WMH increased in most patients at follow-up. Similar change in WMH scores (27-74% increase)12 or volume (1.1-2.4 ml) have been noted previously but the magnitude of effect of WMH or age on WMH progression, and the association with BBB permeability, are novel.1 These data on WMH progression, particularly the 95% confidence intervals (increase over three years of 1.56-2.08 times per additional mm3 of baseline WMH volume and 1.00-1.02 times per additional year of age at baseline), provide useful data for sample size calculations for studies wishing to use WMH change as a surrogate outcome measure.
The study strengths include testing clinical and imaging predictors of SVD progression, use of qualitative and quantitative WMH measures, careful blinding, validated imaging methods.5,9,10 Weaknesses include small sample size, limited power for multivariate testing, 22% loss to clinical follow-up (possibly reflecting the three-year time lapse).
Future studies assessing BBB permeability in SVD should: assess recurrent stroke, covert neurological symptoms, accumulating SVD features, vascular risk factors; track BBB permeability to new lesion formation;4 be powered to adjust for multiple overlapping covariates, particularly WMH load.
Acknowledgments
Funding: The Chief Scientist Office of the Scottish Executive (CZB/4/281), the Wellcome Trust (075611), the Row Fogo Charitable Trust, and the RCUK Centre for Cognitive Ageing and Cognitive Epidemiology (CCACE) Edinburgh, supported the study. The imaging was conducted in the Brain Research Imaging Centre, University of Edinburgh (www.bric.ed.ac.uk) a centre in the Scottish Imaging Network, A Platform for Scientific Excellence (SINAPSE) Collaboration.
Disclosures: The work was supported by research grants to the host institution but the authors have no conflict of interests. The authors had full access to the data and take responsibility for its integrity.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farrall AJ, Wardlaw JM. Blood brain barrier: ageing and microvascular disease - systemic review and meta-analysis. Neurobiol Aging. 2007;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Maniega SM, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- 3.Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. JNNP. 2010;81:192–197. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- 4.Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42:2158–2163. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armitage PA, Farrall AJ, Carpenter TK, Doubal FN, Wardlaw JM. Use of dynamic contrast-enhanced MRI to measure subtle blood-brain barrier abnormalities. Magn Reson Imaging. 2011;29:305–314. doi: 10.1016/j.mri.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bamford J, Sandercock P, Warlow C, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1989;20:828. doi: 10.1161/01.str.20.6.828. [DOI] [PubMed] [Google Scholar]
- 7.Fazekas F. Magnetic resonance signal abnormalities in asymptomatic individuals: their incidence and functional correlates. Eur Neurol. 1989;29:164–168. doi: 10.1159/000116401. [DOI] [PubMed] [Google Scholar]
- 8.Prins ND, van Straaten ECW, van Dijk EJ, Simoni M, van Schijndel RA, Vrooman HA, et al. Measuring progression of cerebral white matter lesions on MRI. Visual rating and volumetrics. Neurology. 2004;62:1533–1539. doi: 10.1212/01.wnl.0000123264.40498.b6. [DOI] [PubMed] [Google Scholar]
- 9.Valdes Hernandez MC, Ferguson KJ, Chappell FM, Wardlaw JM. New multispectral MRI data fusion technique for white matter lesion segmentation: method and comparison with thresholding in FLAIR images. Eur Radiol. 2010;20:1684–1691. doi: 10.1007/s00330-010-1718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Valdes-Hernandez MC, Doubal FN, Wardlaw JM. The effects of infarcts on the assessment of brain atrophy in longitudinal studies. Cerebrovasc Dis. 2011;31:148. [Google Scholar]
- 11.Windham BG, Griswold ME, Shibata D, Penman A, Catellier DJ, Mosley TH., Jr Covert neurological symptoms associated with silent infarcts from midlife to older age: the Atherosclerosis Risk in Communities study. Stroke. 2012;43:1218–1223. doi: 10.1161/STROKEAHA.111.643379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalieri M, Schmidt R. New developments in diagnosis of vascular cognitive impairment. J Neurol Sci. 2010;299:11–14. doi: 10.1016/j.jns.2010.08.031. [DOI] [PubMed] [Google Scholar]


