Summary
Vitamin D has well-defined classical functions related to calcium metabolism and bone health but also has non-classical effects that may influence other aspects of health. There has been considerable recent interest in the role of vitamin D on outcomes related to pregnancy and young child health but few efforts have been made to systematically consolidate this evidence to inform the research and policy agenda for low income countries.
A systematic review was undertaken to identify intervention and observational studies of vitamin D supplementation, intake, or status (25-hydroxy-vitamin D) during pregnancy on perinatal and infant health outcomes. Data from trials and observational studies isolating the effect of vitamin D supplementation and intake were extracted and study quality was evaluated. Meta-analysis was used to pool effect estimates.
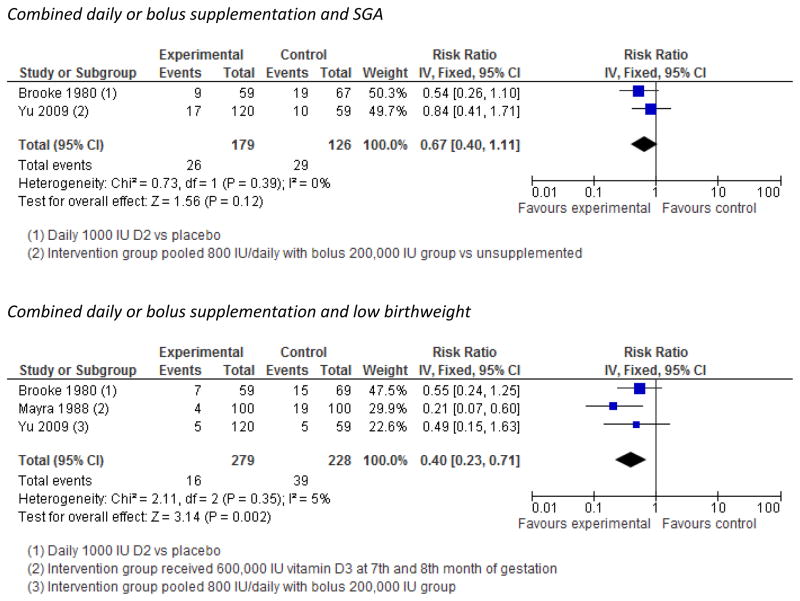
We identified 5 randomized trials with outcomes of relevance to our review. All had small sample size and dosage amount, duration, and frequency varied as did the ability to correct deficiency. Pooled analysis of trials using fixed effects models suggested protective effects of supplementation on low birthweight (3 trials, Risk ratio (RR)=0.40 [95% confidence interval (CI), 0.23, 0.71]) and non-significant but suggestive effects of daily supplementation on small-for-gestational age (SGA) (2 trials, RR=0.67, [0.40, 1.11]. No effect on preterm delivery (<37 weeks) was evident (2 trials, RR=0.77 [0.35, 1.66]).
Little evidence from trials exists to evaluate the effect of vitamin D supplementation during pregnancy on maternal, perinatal or infant health outcomes. Based on both trials and observational studies, we recommend that future research explore SGA, preterm delivery, pre-eclampsia, and maternal and childhood infections, as outcomes of interest. Trials should focus on populations with a high prevalence of vitamin D deficiency, explore the relevance of timing of supplementation, and the dosage used in such trials should be sufficient to correct deficiency.
Introduction
There is evidence of early interest in the relationship between vitamin D status and perinatal health outcomes. “Rachitic pelvis” was noted as a common obstetric complication in the early 20th century, and may have contributed to the development of caesarian section birthing procedures.1 In the 1930’s, Green and Mellanby speculated that vitamin D had little “anti-infective” activity but “might prove of significance in connexion with other obstetric difficulties, such as uterine inertia.”2 Associations between childhood rickets, sunlight, or cod-liver oil and risk of respiratory infections such as TB and pneumonia were recognized a century ago, although it has only been in recent years that the effects of vitamin D on immunity have been revisited and the underlying mechanisms behind its putative effects have started to become more clear.3
Vitamin D can be synthesized in the skin through exposure to ultraviolet (UV) light or can be obtained through dietary intake. Sunlight exposure is often the major influence on vitamin D status and is influenced by skin color, latitude, season, as well as lifestyle and cultural practices. In comparison, diet is often thought to exert a minor influence on vitamin D status although consumption of supplements can strongly influence status.4, 5
Globally, it has been estimated that a billion people may be affected by vitamin D deficiency or insufficiency.6 Generally, populations from countries near the equator (particularly agrarian and nomadic populations) are thought to have sufficient exposure to UV wavelengths required for optimal vitamin D status, and this has indeed been the case in some surveys.4, 7 Yet, childhood rickets, a condition caused vitamin D and/or calcium deficiency, has long been noted in certain equatorial populations, particularly those in which head covering or spending time indoors is common.4 Recent studies in Ethiopia and India have also found that more than 80% and 66% of pregnant women suffered from vitamin D deficiency using a cutoff of <50nmol/L 25(OH)D, indicating the need for more research on potential adverse effects and benefits of supplementation in developing country contexts.8, 9
Metabolism of vitamin D during pregnancy and maternal-fetal links
Once ingested or produced by the body, vitamin D3 is transported to the liver for hydroxylation to 25(OH)D, the main circulating form of vitamin D and best measure of vitamin D status, and then to the kidney where the active hormonal form of vitamin D, 1,25(OH)2D, is produced. Maternal 25(OH)D is thought to freely cross the human placenta as it does in rats.10 The placenta expresses vitamin D receptors (VDR) and also produces the enzyme CYP27B1 to convert 25(OH)D to its active form.11
The most widely appreciated role of vitamin D in the human body is to maintain normal levels of calcium and phosphate in the blood which in turn facilitate other essential processes such as bone mineralization, contraction of muscles, nervous system activities, and cellular function.12 Adequate vitamin D status is critically important for the neonate, with neonatal hypocalcemia and rickets being major consequences of deficiency.10 In areas where vitamin D deficiency is endemic, rickets may be diagnosed soon after birth10. In general, breast-milk is thought to be a relatively poor source of vitamin D, making maternal vitamin D status during pregnancy important for vitamin D status of the child during early infancy.10, 13, 14
There are a number of plausible biological pathways through which vitamin D could influence placental, fetal, and maternal health or growth during pregnancy. Vitamin D has important immune-modulating properties of vitamin D which may help to establish a proper maternal immune response to the placenta.15–17 It also regulates key target genes associated with proper implantation of the placenta.18 Vitamin D has a direct role in the production of antimicrobial peptides such as cathelicidin, which are produced upon activation of up-regulated vitamin D receptors, require 25(OH)D as a substrate for production, and may play an important role in preventing infection during pregnancy or early childhood.3, 19–22 In its active form, vitamin D regulates expression of human chorionic gonadotropin (hCG) in syncytiotrophoblasts and stimulates production of sex steroids.23, 24 In vitro and animal studies have also suggested that vitamin D has important roles in glucose and insulin metabolism which may affect availability of energy to the fetus.16, 25,25, 26 Vitamin D also influences musculoskeletal growth.27–30
Requirements for vitamin D
The recommended daily allowance (RDA) of vitamin D for women in the United States aged 19–50 years, including during pregnancy, was recently established at 600 IU per day.31 This recommendation was based on the amount of intake necessary to sustain blood levels of 25(OH)D above 50nmol/L for populations with minimal sunlight exposure and was developed solely based on outcomes related to skeletal health.31 This recommendation was contentious as many researchers have argued that insufficiency should be defined at thresholds of 75 nmol/L or even higher which would require a much higher intake to reach.32, 33
A number of recent reviews have covered a rapidly growing body of literature on vitamin D during pregnancy,4, 16, 34–37 although few have taken a systematic approach35 and only a couple have focused on the implications for low income country settings.4, 38 To address this gap, we undertook a systematic review of the literature on vitamin D during pregnancy and perinatal and infant health outcomes.
Methods
The primary aim of our literature search was to identify epidemiologic studies that isolated the effect of intake of vitamin D from supplements or diet on the outcomes presented in Table 1. Skeletal health related outcomes such as bone mineralization and rickets were not part of this review and have been detailed elsewhere.31, 39,10 Recognizing in advance that few supplementation trials had been published, we sought to also identify prospective observational studies with intake of vitamin D or maternal blood 25(OH)D levels during pregnancy as exposures. Using the search strings outlined in Table S1 we searched the NLM Pubmed database and Cochrane Library through June 2011 as well as the bibliographies of reviews and relevant studies.
Table 1.
Primary outcomes covered by this review
|
Indicates outcomes for which outcomes from more than one study could not be extracted for meta-analysis
An initial review of titles and abstracts was undertaken by one reviewer followed by an in-depth review of full papers that could not be initially excluded (ATL). We excluded (1) non-human studies (2) studies not in English, French, or Spanish (3) reviews, case reports and commentaries (4) topics unrelated to the review (5) studies that could not isolate the effects of vitamin D supplementation or intake (6) cross sectional and non-prospective case-control studies. A modified version of the Child Health Epidemiology Reference Group’s GRADE tool was used to evaluate intervention studies and observational studies of vitamin D intake for risk of bias, and those with a study quality of “Very Low” were excluded. Authors of recent trials presenting clinical outcomes were requested to provide additional data on other outcomes of relevance to the meta-analysis.
Study data were extracted using a standard spreadsheet. Review Manager 5.140 was used to generate pooled effect estimates for all outcomes with data from more than one eligible study. For studies with continuous outcomes we generated mean differences and for dichotomous outcomes we calculated relative risk or odds ratio estimates, each with 95% confidence intervals. Inverse variance weights were used to generate pooled effect estimates with a fixed effects (FE) model in absence of significant heterogeneity. Heterogeneity was explored through visual inspection of Forest plots as well as through I2 values and Chi-square tests for heterogeneity. In the presence of significant heterogeneity (p<0.10), we used a random effects (RE) model. For trials testing multiple intervention arms (of daily or bolus doses), we pooled data from intervention arms, calculated the pooled mean and standard deviation41 where appropriate, and also presented separate analysis for daily vs. bolus dosage. For observational studies, comparisons were made of extreme categories.
Results
Identification of studies
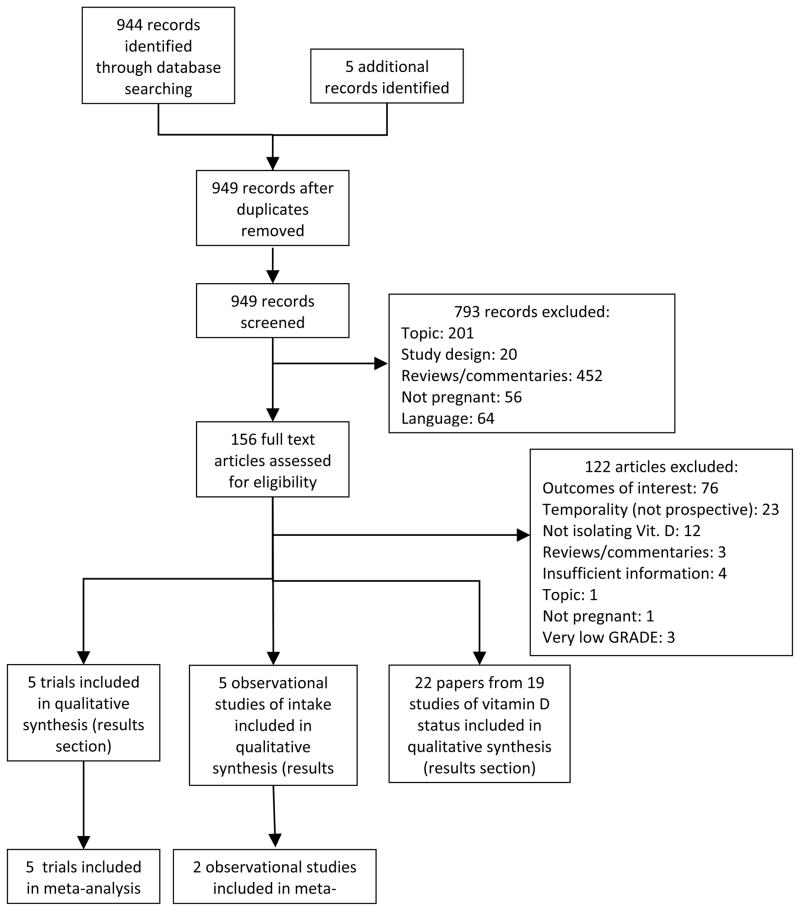
We identified 944 hits through our database searches and five additional studies through bibliographical searches (Figure 1). Five trials of vitamin D supplementation during pregnancy met our inclusion criteria, one of which did not provide sufficient data for inclusion in quantitative analysis42, and one study was excluded that ranked ‘Very low’ on GRADE criteria (Table 2 and Table S2).43 Additional data was received from the authors of two recently published trials (personal communication with Dr. Yu, Nov. 2011 and Drs. Hollis/Wagner, Jan. 2012). Six observational studies of vitamin D intake were identified of which two44, 45 ranked ‘Very Low’ on GRADE criteria. (Table S3). We also found 13 studies with prospective assessment of 25(OH)D prior to birth outcomes (Table S4) and four studies in which pre-eclampsia was reported as an outcome (Table S5).
Figure 1.
Study flow
Table 2.
Description of intervention studies of vitamin D supplementation and observational studies of vitamin D intake (including supplements) eligible for inclusion in meta-analyses
| Study ID | Country and latitudea | Population | Intervention or exposure | Comments and GRADE assessment of risk of bias |
|---|---|---|---|---|
| Intervention studies | ||||
| Brooke (1980)63, 70, 91 | UK (London) 51°N |
Affluent Asian immigrants in 3rd trimester. Excluded preterm deliveries, congenital malformations, and certain maternal illnesses. Mean 25(OH)D: 20.1 nmol/L at 28 weeks. | Randomized to a daily dose of 1) 1000 IU Vitamin D2 (n=59) or 2) Placebo (n=67) provided from 28–32 weeks gestation to delivery. | Double blind RCT. Small sample size GRADE: Moderate |
| Hollis (2011)5,b | US (South Carolina) 34°N |
Multiethnic population, mean age 27 years, women with pregnancy <16 weeks gestation at enrollment. All participants received prenatal multivitamin/mineral supplement. Mean baseline 25(OH)D by group: 58.2–61.6 nmol/L. | Randomized to daily dose of 1) 400 IU vitamin D3 (n=111) 2) 2000 IU vitamin D3 (n=122) 3) 4000 IU vitamin D3 (n=117) provided from 12–16 wks until delivery. Women with baseline 25(OH)D levels >100nmol/L randomized to group 1 or 2. | Double blind RCT. Loss to follow-up, potential selection bias due to randomization process. Grade: Moderate |
| Mallet (1986)88 | France (Northwest) 49°N |
Caucasian population, mean age 25 y, analysis included at-term infants born at term during Feb. and Mar. Mean maternal 25(OH)D in the unsupplemented group was 9.4 nmol/L. | Random assignment to (1) 1000 IU vitamin D2 daily for last 3 months of pregnancy (n=21) (2) One-time dose of 200,000 IU in 7th month of gest. (n=27) (3) No supplement (n=29). | Randomized non-placebo controlled trial; unclear whether women were randomized into the control group. GRADE: Moderate |
| Mayra (1988)47 | India (Rohtak) 28°N |
Singleton pregnancies aged 22–35 years attending antenatal clinic of a medical college. Complications including pre-eclampsia, haemorrhage or premature delivery excluded. 25(OH)D levels not assessed. | Random selection to (1) 2 doses of 600,000 IU vitamin D3, one each in month 7 and 8 of pregnancy (n=100) (2) No supplement (n=100). | Randomized non-placebo controlled trial; method of randomization unclear, no placebo, unclear dropout/number of exclusions. Grade: Low |
| Yu (2009)43,b | UK (London) 51°N |
Multi ethnic hospital population recruited at 27 weeks gestation Excluded women with pre-existing sarcoidosis, osteomalacia, renal dysfunction, TB. Median 25(OH)D≈25nmol/L at 27 weeks. | Random selection to (1) Single oral dose of 200,000 IU Vit. D (n=60), (2): Daily supp. with 800 IU Vitamin D from 27 weeks to delivery (n=60) (3) No treatment (n=60) | Randomized non-placebo controlled trial. Small sample size, no placebo. GRADE: Moderate |
| Observational studies | ||||
| Haugen (2009)76 | Norway (nationwide) 58°–71°N |
23,423 nulliparous pregnant women from the Norwegian Mother and Child Cohort study with singleton births recruited at first ultrasound exam. | FFQ uses to assess intake of vitamin D for first 4–5 months of pregnancy and supp. use assessed at week 30. | Prospective cohort study. Supplement use validated with good correlation against reported use and biomarkers. Large sample size. GRADE: Moderate |
| Scholl (2009)64 | U.S. (Camden, NJ) 39° N |
Multiethnic cohort, data from 2,215 pregnancies enrolled at entry to prenatal care. Excluded those with serious non-obstetric problems. | Dietary intake of vitamin D and supplement use assessed using 3 24 hour recalls. | Large sample size, controlled for ethnicity, BMI, energy intake, intake of key nutrients, and gestational duration for birthweight. GRADE: Moderate |
| Camargo (2007)60 | US (Massachusetts) 42° N |
1194 mother-child pairs from Project VIVA cohort. Excluded multiple gestations, non-English speakers, plans to move, and gestational age >22 weeks at initial appointment. | Dietary intake was collected using a validated semi-quantitative FFQ and intake from supplements was collected for 1st and 2nd trimesters | Large sample size, examination by quintiles of intake. Only presented unadjusted analysis for perinatal outcomes. GRADE: Low |
| Oken (2007)92 | US (Massachusetts) 42° N |
1178 women from the Project VIVA cohort recruited after median 10.4 weeks gestation. Excluded women with pre-existing hypertension. | Same as Camargo (2007) above | Large sample size, examination by quintiles of intake. GRADE: Low |
Approximate latitude estimated by authors
Additional unpublished or in-press data was also provided by author
All but one trial46 and four observational studies of 25(OH)D were conducted in high-income country settings, and most populations had either a presumed risk or high prevalence of deficiency at baseline (Table 1).9, 47–50 Dosing approaches in the trials varied: Two trials contained multiple intervention arms testing both daily supplementation (800 IU and 1000 IU D2) and high single dose supplementation (200,000 IU) in the third trimester. One recent trial tested both daily 2000 IU D3 and 4000 IU D3, the latter at the upper limit of the current U.S. recommendations and a study in India tested the effects of two bolus doses of 600,000 IU.31
Vitamin D supplementation appeared to improve maternal 25(OH)D levels in all four trials for which data was available although mean attained levels at term in two trials indicated that the dosage tested was not sufficient to alleviate deficiency in much of the population (Table S6).42, 51 In contrast, the results of trials by Hollis and Brooke indicate a substantial improvement in vitamin D status, and there is some speculation that the dose used in the latter trial may have been higher than reported.5, 36, 52
Birthweight, low birthweight, and crown-heel length at birth
The results of all meta-analyses are summarized in Table 3. Pooled analysis of all five trials with information on mean birthweight suggested no significant overall effect (Figure S1), although significant heterogeneity was apparent (I2=70%, p=0.0009) and appeared to originate primarily from the divergent results of studies testing a large bolus dose, one of which showed a significant increase and the other which showed a significant decrease in birthweight. Pooled results of observational studies indicated a significant 52g increase [3.5, 102] comparing the highest vs. lowest categories of intake (Figure S1). An observational study in New Zealand also found a significant 71g birth weight difference across quartiles of intake noting low median intakes of 68 IU/day in the population though data was insufficient for pooling.53
Table 3.
Summary of findings and overall assessment of quality of evidence for randomized trials and observational studiesa
| Quality Assessment | Summary of Findings | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. studies and study design | Heterogeneity of results? | Consistent size of effect? | Generalizable to population of interest? | Generalizable to intervention of interest? | Other sources of bias (e.g., major limitations in study design) | N | Statistical method | Effect estimate (95% CI) |
| Trials: Small-for-gestational age, Overall quality of evidence grade=Low | ||||||||
| 2 RCT’s43, 63 (1 with 2 arms) | Low (I2=0%), p=0.39 | Both protective | Both in the UK among deficient largely non-white populations | Similar dose; both in 3rd trimester | Both small studies | 305 | RR, IV Fixed effects |
0.67 (0.40, 1.11) |
| Trials: Weight gain during the third trimester (g), Overall quality of evidence grade=Low | ||||||||
| 2 RCT’s47, 91 | High (I2=65%, p=0.09) | Yes—both showed ↑ weight gain in vit D. group | India, Asian immigrants in the UK | Both in 3rd trimester; 1 w/daily 1000 IU vs. 1 with 600,000 IU at month 7 and 8. | Both small studies | 326 | MD, IV Random effects |
11.8 g/day (3.4, 20.2) |
| Trials and Observational studies: Mean birthweight, Overall quality of evidence grade=Low | ||||||||
|
Most trials null; 1 increased weight, 1 arm decreased weight. | All in deficient populations; 1 from India remaining from high income settings. | Wide variety of doses tested ranging from 800 IU daily to 4000 IU daily and large bolus doses up to 2X 600,000 IU | Only 2 had blinded control groups; all small sample size; high or unclear dropout in two studies | 932 1497 |
MD, IV Random MD, IV Fixed |
58.1 [−63.9, 180.0] 52.7 [3.5, 101.8] |
|
| Trials: Low birthweight (<2.5 kg), Overall quality of evidence grade=Low | ||||||||
| 3 RCT’s (1 with 2 int. arms).42, 46, 52 | Low (I2=5%, p=0.35) | RR ranged from .21 to .55 (only 1 significant) | 1 in India, others in London among Asian migrants and mixed pop. | Mix of daily sup. (800–1000 IU) and bolus dosing at high doses all in 3rd trimester | Only 1 study placebo controlled | 507 | RR (IV, Fixed, 95% CI) | 0.40 [0.23, 0.71] |
| Trials: Crown-heel length at birth (cm), Overall quality of evidence grade=Low | ||||||||
| 2 RCT’s47, 70 | High (I2=82%, p=0.02). | One null, one with sig greater weight gain | One in India, one among Asian immigrants to the UK | 1000 IU D2; Bolus 600,000 IU at month 7 and 8. | One unblinded | 317 | MD, IV. Random effects |
1.02 (−0.26, 2.30) cm |
| Trials: Preterm delivery (<37 weeks), Overall quality of evidence grade=Low | ||||||||
| 2 RCT’s 5, 43 (both had 2 int. arms pooled here) | Moderate (I2=46%, p=0.17) | Both null in opposite directions | Both in multi-ethnic populations in high income countries | (1) 4000 or 2000 IU vs. 400 IU (2) daily 800 or bolus 200,000 IU vs. nothing | One unblinded | 529 | RR, IV Fixed effects |
0.77 [0.35, 1.66] |
| Trials and observational studies: Mean gestational age at delivery (in weeks), Overall quality of evidence grade=Low | ||||||||
| 2 RCT’s 5, 43 with 2 int.arms 2 Obs. Studies60, 64 |
Low(I2=7%, p=0.30); None (I2=0%, p=0.75); |
All null | All in high income countries | Comparison of 4000/2000 IU vs. 400 IU and daily 800 IU vs. nothing; | One unblinded; 1 Obs. study did not adjust for confounding | 529 1497 |
MD, IV. Fixed effects | 0.17 [−0.16, 0.51] 0.08 [−0.12, 0.28] |
| Observational studies: Pre-eclampsia, Overall quality of evidence grade=Very Low | ||||||||
| 2 Obs. studies76, 92 | None (I2=0%, p=0.33) | Both null | Both in high income countries | 1 comparison of <200 vs. >800 IU; 1 based on continuous exposure | 12,626 | OR, IV, Fixed effects | 0.95 [0.86, 1.06] | |
Abbreviations used in table: RR=Risk ratio, MD=mean difference, IV=Inverse variance, pop.=populations, pos.=positive, neg.=negative, Obs.=observational, IU=international units, pop.=population, int.=intervention
Pooled analysis of three trials revealed that supplementation led to a significant 60% reduction in the risk of low birthweight (Figure 2), and disaggregated analysis of bolus high dose trials also suggested a strong benefit (2 trials, RR=0.25, [0.11, 0.61]) although daily supplementation trials did not show statistically significant benefit (2 trials, RR=0.56, [0.27, 1.13])(Figure S2).
Figure 2.
Trials of vitamin D supplementation and risk of low birthweight and small-for-gestational age
The majority of studies examining relationships between maternal vitamin D status and mean or low birthweight found no association.9, 30, 47, 49, 54, 55 However, two large studies, one from the Netherlands and the other from Australia both found significantly lower birthweight among infants born to women with vitamin D deficiency (using thresholds of <25 and 29 nmol/L).56, 57 Another study from Australia found no overall effect of low maternal 25(OH)D levels (<28 nmol/L) on mean birth weight, yet effect modification was found by infant vitamin D receptor (Fokl) genotype: offspring born to women in the with higher 25(OH)D levels were significantly heavier within the FF or Ff but not the ff genotypes.58
Pooled analysis of two supplementation trials showed no significant difference in mean crown-heel length associated with supplementation (Figure S3). None of the observational studies we identified reported a significant association between maternal 25(OH)D status and crown-heel length at birth.9, 30, 47, 54, 55, 59, 60
Small-for-gestational age and gestational weight gain
Pooled analyses indicated significantly greater average daily weight gain in the third trimester among women supplemented with vitamin D (2 trials, 11.8g/day [3.4, 20.2]) (Figure S4), and a non-statistically significant 33% decrease in the risk of SGA (Figure 2). However, an observational cohort study from Boston reported no difference in birth weight for gestational age by quartile of vitamin D intake in unadjusted analysis.61
Prospective observational studies of vitamin D status and SGA have had mixed findings (Table S4). Two studies, one of HIV-positive women in Tanzania and another among pregnant adolescents in the UK did not find statistically significant associations between maternal 25(OH)D levels and SGA.49, 62 However, the largest study to date, from the Netherlands, found that the risk of SGA among vitamin D deficient women (25(OH)D<30nmol/L), was nearly twice that of women with adequate status in multivariate adjusted models (AOR 1.9 [95% CI: 1.4, 2.7)60. A recent nested case-control study from Pittsburgh found evidence of a U-shaped relationship between vitamin D status and SGA among white women in the study: those with serum 25(OH)D levels less than 37.5 nmol/L had a 7.5-fold [95%: 1.8, 31.9] higher risk of SGA compared to those with levels between 37.5 and 75 nmol and those with levels exceeding 75nmol/L had a 2.1 times higher risk [1.2, 3.8].63 Effect modification by race was also evident as no statistically significant relationships between serum 25(OH)D levels and SGA risk were observed among black women included in the study.
Preterm birth
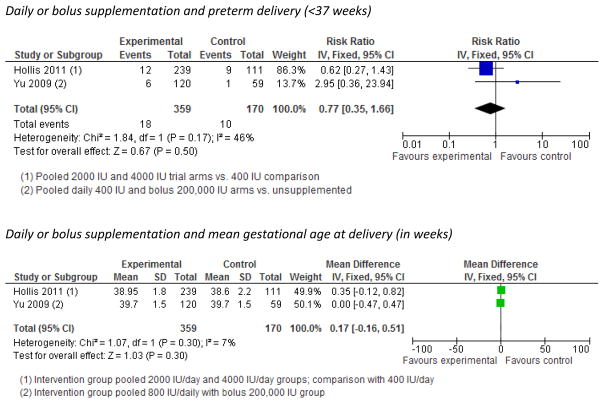
No significant association was evident between vitamin D supplementation and preterm delivery (<37 weeks) or mean duration of gestation in pooled analyses (Figure 3, Figure S5), or in two observational studies of intake of vitamin D and mean duration of gestation (Figure S6).5, 42, 64
Figure 3.
Trials of vitamin D supplementation and risk of preterm delivery and mean duration of gestation
Two studies examined relationships with blood levels of 25(OH)D during pregnancy and preterm birth. In Tanzania, no difference in risk of preterm birth (<37 weeks, RR=0.84 [0.55–1.28] or severe preterm birth (<34 weeks, RR=0.77 [0.50, 1.18] was observed among HIV-positive women using a cutoff of 80 nmol/L. Similarly, no difference in 3rd trimester 25(OH)D levels was found for adolescents in the UK delivering preterm vs. normal gestational length babies62.
Observational studies with serum 25(OH)D levels as an exposure did suggest longer gestational duration associated with higher 25(OH)D levels. In The Netherlands, women with serum levels >50nmol/L had a slightly longer gestational length of 40.2 weeks vs. 40.0 weeks vs. women in two categories of lower intake (P<0.001).57 An Australian study noted a significant 0.7 week shorter gestation [−1.3, −0.1] among women with blood 25(OH)D levels <28nmol/L compared to those with higher levels.30 A hospital-based study from Japan also found lower mean 25(OH)D levels among mothers with threatened premature delivery.65
Fetal loss
One trial reported a case of stillbirth in the non-intervention arm, but we found no other trials or observational studies of vitamin D intake with data on fetal loss.42 One study among HIV+ women from Tanzania found no significant difference in fetal loss among women with deficiency vs. those with sufficient status (RR=1.05: 95% CI (0.63, 1.74).49
Pre-eclampsia, Caesarian section, and maternal mortality
We did not find any published data from trials on the effects of vitamin D supplementation on risk of pre-eclampsia. Preliminary data shared with us by investigators from the trial in South Carolina indicates monotonic reductions in risk with increasing supplement dosage (400 IU, 2000 IU, 4000 IU) for a composite pre-eclampsia/eclampsia/gestational hypertension outcome which although not significant in intent-to-treat analysis, became significant after controlling for race (Hollis et al, in press).
Pooled analysis of a very large (n=23,423) study from Norway and a large (n=1,718) study from Massachusetts (Figure S7) revealed no significant difference in risk of pre-eclampsia between top and bottom categories of total vitamin D intake (OR=0.95; [95% CI: 0.86, 1.06]). While the comparison between >800 IU with <200 IU of total vitamin D intake in the Norwegian study was not statistically significant (OR=0.89 [0.75, 1.06), it is important to note that women with intake between 688 and 800 IU had significantly reduced risk of pre-eclampsia (Adjusted OR 0.77 [0.61–0.96]) compared with those consuming <200 IU/day. Interestingly, a heightened risk of gestational hypertension was associated with increased vitamin D intake in the study from Massachusetts (Adjusted OR=1.11 [1.01, 1.21]).
Observational studies of vitamin D status during pregnancy and risk of pre-eclampsia have not shown consistent associations (Table S5) Investigators of a study from Pittsburgh observed an inverse monotonic dose response relationship between 25(OH)D levels in early pregnancy and pre-eclampsia: for every 50 nmol/L decline in 25(OH)D levels, the risk of pre-eclampsia more than doubled (adjusted OR 2.4 [1.1, 5.4]).66 Similarly, a nested case control study from North Carolina reported that women with 25(OH)D levels <50 nmol/L had nearly a fourfold greater risk of severe preeclampsia compared with those with levels ≥75nmol/L.67 In contrast, a recent nested case control study conducted in Massachusetts found no statistically significant difference in risk of pre-eclampsia for women with 25(OH)D levels <37.5 nmol/L (AOR 1.35 [0.40, 4.50]).68 Another prospective cohort study of pregnancies at high risk for pre-eclampsia in Canada found no effect of 25(OH)D during early pregnancy on pre-eclampsia risk.69
In a recently published trial from South Carolina, no statistically significant differences in mode of delivery (Caesarian section vs. vaginal delivery) were noted by vitamin D supplementation group, although adjustment for race reportedly led to a statistical significant trend of increasing protection with higher dosage (Hollis et al, in-press).5 An observational study undertaken in Boston found that women with vitamin D levels 25(OH)D<37.5 nmol/L during pregnancy were found to have nearly a four times greater risk of caesarian delivery (AOR 3.84 [1.71, 8.62]).1 Similarly no association between maternal vitamin D status and mode of delivery was found in an Australian observational study.56
No trials or observational studies of vitamin D supplementation/intake and maternal mortality or morbidity during pregnancy were identified. However, a study from Tanzania found that pregnant women with 25(OH)D levels <80nmol/L had increased risk of HIV disease progression and that risk of all-cause mortality was significantly lower in the highest quintile of 25(OH)D compared with the lowest (Incidence RR=0.58 [ 0.40, 0.84]).48
Neonatal and infant growth
One supplementation trial in the UK reported significantly greater weight gain among infants born to women supplemented with vitamin D compared with the placebo group 3, 6, 9, and 12 months (12 month comparison: 6.39 kg vs. 5.92 kg in the control group (p<0.01)).70 Up to 6 months of age, there were no differences in attained height between groups, but by 1 year of age, those in the vitamin D group had grown 27.9 cm vs. 24.6 cm in the placebo group (p<0.001).
A large observational study in the Netherlands found no differences in multivariate adjusted mean weight-for-age z-scores by category of maternal vitamin D status during pregnancy at any measure taken during the first year of life. While infants born to mothers with 25(OH)D levels <30nmol/L had significantly lower mean length for age z scores at 1 month compared with those born to women adequate status (>50nmol/L), paradoxically they had higher z scores by 12 months with no differences observed at 3, 6, or 9 months.57 In another study conducted in the Gambia among a population with high vitamin D status, no significant relationships or trends were found by maternal 25(OH)D status taken at either week 20 or 36 of pregnancy and weight or height-for-age z scores at 2, 13, or 52 weeks.47 A cohort study of the effects of maternal vitamin D status during pregnancy on subsequent outcomes in Southampton, UK, found no differences in attained weight or length at 9 months of follow-up across quartiles of 25(OH)D status measured in late pregnancy (with cut-points at <30, 50, 75, and >75nmol/l).54 However, in a linear regression model examining predictors of height at 9 months that included height measured at birth, a significant association with maternal 25(OH)D was observed (p=0.02). 54, 71 A study in India found no difference in attained height at age 5 years between children of mothers with 25(OH)D levels above vs. below 50 nmol/L during pregnancy.50
Neonatal and infant morbidity and mortality
We did not identify any trials of vitamin D supplementation during pregnancy with outcomes related to infant or child mortality, though a death was noted in one trial.42 In a cohort study of 1,194 children from Massachusetts, no significant trend was found across quintiles of maternal vitamin D intake during pregnancy on risk of reported diagnosis by a health professional of respiratory infections up to 3 years of age in multivariate adjusted analysis.61 A study of 922 infants in New Zealand found significantly greater risk of respiratory infection (a composite variable of colds, cough, whooping cough, chest infections, and ear infections) by 3 months of age among infants with cord blood levels of 25(OH)D less than 25 nmol/L in adjusted analysis compared with levels at or above 75 nmol/L, OR=2.04 [1.13, 3.17].72 In contrast to these findings, a cohort study from Southampton, England which followed up children at age 9 months found that mothers in the top quartile of 25(OH)D status in late pregnancy were significantly more likely to report their children having been diagnosed with pneumonia or bronchiolitis, compared with those in the bottom quartile (unadjusted OR 4.80, [1.01, 22.73]), although no differences in risk for respiratory infections overall or for chest infections or bronchitis were observed. 54 The same study unexpectedly also found that children of mothers in the highest quartile of vitamin D status were more likely to have reported having one or more bouts of diarrhea vs. those in the bottom quartile (OR=1.87 [1.01, 3.46]. In a study of infants born to HIV+ mothers in Tanzania, vitamin D insufficiency during pregnancy (<80nmol/L) was associated with heightened risk of a composite endpoint of infant mortality/HIV infection (RR=1.49 [1.07, 2.09]).49
Discussion
Scientific understanding of the importance of vitamin D during pregnancy for perinatal and child health outcomes is growing rapidly, and our review consolidates a great deal of new information from trials and observational studies not incorporated in a prior meta-analysis on the topic.35 In addition to its classic functions related to calcium homeostasis and bone development, emerging evidence suggests that adequate maternal vitamin D status could play important roles in ensuring proper fetal and placental development and proper immune response and function during pregnancy, although the relative importance of its different putative effects on pathways leading to clinical outcomes is not well understood.16, 22, 66
It is important to interpret the findings of our pooled analyses with caution, particularly given the small number and small sample size of existing trials and the heterogeneity observed across a number of outcomes. Many trials to date were conducted among populations with evidence of high prevalence of deficiency, and provided supplementation during the third trimester at levels that were not sufficient to fully not correct deficiency in the population (Table S6). We also note that most studies to date have been conducted in populations located in northern climates with suspected high prevalence of vitamin D deficiency. Caution is therefore also needed when considering the generalizability of these findings to populations with greater potential exposure to sunlight, although it is also important to consider the role that cultural practices of head covering and urban lifestyles may have in contributing to deficiency even in tropical countries.
Despite the lack of a statistical significance of the effect observed in our pooled analysis of SGA, we found fairly consistent evidence from trials suggesting that vitamin D has the potential to influence IUGR among deficient populations, supported by significant effects observed in the meta-analysis of data on gestational weight gain and low birthweight. The outcome with the greatest number of trials was mean birthweight, for which the findings suggested either a null or modest effect. It is well accepted that mean birthweight is limited in its ability to capture fetal growth restriction, partly because it also reflects gestational age.63 We only found one observational study suggesting that risk of SGA might increase at higher levels of 25(OH)D, but many studies appeared to only examine 25(OH)D as a dichotomous categorical variable, reinforcing the importance for future observational studies to examine potential non-linear relationships across the entire distribution.63 Studies also suggest that further exploration of heterogeneity in effects by race and/or vitamin D receptor genotype may be important.58, 63
Some, but not all studies, suggested an effect of vitamin D on risk of pre-eclampsia. In addition to the studies we reviewed, a trial using cod liver oil containing vitamin D along with multi-micronutrient supplement found a significant 32% reduction in preeclampsia risk, and a trial of vitamin D and calcium from India indicated a non-significant benefit on preeclampsia.73, 74 It has been speculated that early pregnancy, when the placenta is still developing, might be the most etiologically relevant time period for vitamin D supplementation on pre-eclampsia but this hypothesis requires further testing and received limited support from the one observational study to explore the importance of timing of supplementation during pregnancy.75
We found little evidence to evaluate the effect of vitamin D on preterm birth or any of its obstetric precursors. Approximately 25–40% of all preterm births are thought to be associated with intrauterine infection.76 An influence of vitamin D status on bacterial infections during pregnancy is plausible and is supported by the consistency of findings of several cross-sectional observational studies in the U.S. suggesting inverse relationships between 25(OH)D and bacterial vaginosis during pregnancy.15, 77, 78 Larger observational studies or trials with more detailed outcome evaluation are needed to assess the potential for vitamin D to influence risk of preterm birth.
Both the observational study from Massachusetts and the trial from South Carolina provide support for an effect of vitamin D on risk of primary Caesarian-section.1,5 In apparent contrast to these findings, two studies excluded from our analysis due to their non-prospective nature suggest no association between mode of delivery or dystocia and maternal vitamin D status.79, 80 Heterogeneity in these associations is not entirely unexpected since decision-making related to Caesarian delivery may vary across settings. Given the strength of the association observed in the study from Massachusetts, the role of vitamin D in regulation of calcium (which may help to initiate labor and influence muscle function), and the potential role on pre-eclampsia, further exploration would be worthwhile.81
Neonatal and early childhood morbidity, mortality, and growth
Maternal serum levels of 25(OH)D during pregnancy are correlated with cord blood and neonatal levels of 25(OH)D and could be an important influence on neonatal and early childhood health and growth, particularly since breastmilk is a poor source of vitamin D in absence of high dose maternal supplementation.5 A growing number of studies suggest the importance of adequate vitamin D status during childhood to the prevention of respiratory infections. A study from Ethiopia found that children with rickets had a 13 fold higher risk of pneumonia compared with controls5 while a trial in Afghanistan found that vitamin D supplementation of children diagnosed with pneumonia significantly reduced both the risk of repeat episodes during 90 days of followup by 22% as well as increasing the time to repeat episode.82 Limitations of the studies we identified include reliance on maternal reports of doctor’s diagnosis, little specificity of outcomes, and in the case of the study from England showing increased risk associated with better vitamin D status, a lack of adjustment for potential confounders such as access to health care or education.54 Infections remain an important cause of neonatal and early childhood mortality in many countries, and trials of maternal vitamin D supplementation in such settings should also include follow-up of children for respiratory infections (and HIV in high prevalence settings).48, 49, 83
The importance of vitamin D to linear growth during childhood was considered at least as early the 1930’s.84, 85 The strongest support for a direct role of vitamin D supplementation on child growth comes from the trial in the UK which found beneficial effects on linear growth at 9 months but not prior to that time.70 It is difficult to explain the inconsistencies between these findings and observations from the Netherlands showing that children born to vitamin D deficient mothers appeared to have greater growth velocity, although residual confounding and vitamin D supplementation practices during early childhood in the latter study have been offered as explanations.57
Policy implications and future research directions
Despite growing interest in the relationships between vitamin D status during pregnancy and perinatal and infant health outcomes, the epidemiological evidence base remains weak for all of the outcomes we included in our review. Noting compelling evidence of a potential role of vitamin D on a number of perinatal and maternal health outcomes, more randomized trials of vitamin D supplementation during pregnancy in deficient populations are needed to evaluate the potential for vitamin D supplementation to prevent adverse outcomes. Such trials should be undertaken in settings in which vitamin D deficiency is likely to be a risk: latitude, cultural practices of head covering, urban lifestyle, poor dietary intake of vitamin D, and season are all factors that may be useful to consider when selecting populations for such trials.
Dosage for such trials should be guided by population 25(OH)D levels at baseline and desired target levels.86 The threshold used to define optimal serum 25(OH)D levels is contentious, though many researchers have argued that levels of at least 75–80nmol or even higher are desirable based on observational studies and estimates of circulating levels from living under UV-rich conditions similar to which humans evolved.32, 37. Recent research from the multi-dose trial of pregnant women suggests that serum levels of 25(OH)D exceeding 100nmol/L may be needed to optimize production of 1,25(OH)D.5
The fat soluble properties of vitamin D mean that intermittent (e.g. weekly or monthly) dosage is possible, which may be particularly useful in countries with weak health infrastructure, but also raises the need to consider potential adverse effects due to toxicity. The upper limit of intake above which adverse health outcomes such as hypercalcaemia during pregnancy ensue is uncertain. The US tolerable upper limit for women of reproductive age including pregnant women was recently set at 4000 IU/day though it has been argued that there is little evidence of toxicity of higher levels up to 10,000 IU/day.33 Dosages administered during recent trials without adverse effects include daily provision of up to 4,000 IU/day vitamin D3 throughout pregnancy in the trial from South Carolina5 (though women with high baseline serum levels were excluded from the highest dose group and supplementation began at 16 weeks), provision of a weekly dose of 35,000 IU D3 during the third trimester in Bangladesh87, and one time doses of 200,000 IU vitamin D3 in the third trimester of pregnancy,42, 51 Monitoring of adverse events in clinical trials using high intermittent dosing may also be an important consideration.88
Lastly, we note that the vast majority of studies published to date on vitamin D and perinatal health outcomes have been undertaken in high income countries. While populations in these settings may be at greater risk for vitamin D deficiency due to lower UV light exposure, more research is needed in populations from low income countries, which may have lower dietary intake, higher disease burden, and for which vitamin D deficiency may be a growing problem associated with urbanization.
Summary
In a systematic review of the literature we found only a low level of evidence relating vitamin D supplementation or intake during pregnancy on perinatal and infant health related outcomes. Emerging evidence suggesting plausible effects on intra-uterine growth restriction, pre-eclampsia, and both maternal and infant infections as important outcomes in need of further research in low income settings. Several trials have recently been completed or are underway including in Pakistan and Bangladesh that should help to clarify the importance of vitamin D supplementation as an intervention for perinatal, maternal, and infant health outcomes.5, 89 Vitamin D is an essential nutrient with well-established roles in in calcium metabolism and the prevention of rickets that are important for policymakers to consider alongside the outcomes reviewed here.
Supplementary Material
Figure S1. Trials and observational studies of vitamin D supplementation or intake and mean birthweight (in grams)
Figure S2. Trials of vitamin D supplementation and SGA and low birthweight, disaggregated by dosing approach
Figure S3. Trials of vitamin D supplementation and mean crown-heel length (in cm.)
Figure S4. Trials of vitamin D supplementation and mean weight gain during the 3rd trimester
Figure S5. Supplementation and preterm and mean gestational age at delivery, disaggregated by dosing approach
Figure S6. Observational studies of vitamin D intake and mean gestational age at delivery
Figure S7. Observational studies of vitamin D intake and pre-eclampsia risk
Table S1. Search strategy and keywords
Table S2. Evaluation of the quality of eligible trials for potential inclusion in the meta-analysis
Table S3. Evaluation of the quality of evidence of individual observational studies for potential inclusion in the meta-analysis
Table S4. Prospective observational studies of the relationship between maternal blood levels of 25(OH)D during pregnancy and measures of fetal growth, birth weight, and preterm birth
Table S5. Prospective observational studies of the relationship between maternal blood levels of 25(OH)D during pregnancy and pre-eclampsia
Acknowledgments
Acknowledgements and Funding Sources
We greatly appreciate the willingness of Dr. Christina Yu and Dr. Bruce Hollis and Dr. Carol Wagner to share additional data for this analysis. Andrew Thorne-Lyman is supported by NIH training grant T32 #DK 007703 and a Julius B. Richmond Fellowship from the Center on the Developing Child at Harvard University.
References
- 1.Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. Journal of Clinical Endocrinology and Metabolism. 2009;94:940–945. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green H, Pindar D, Davis G, Melanby E. Diet as a prophylactic agent against puerperal sepsis-with special reference to vitamin A as an anti-infective agent. British Medical Journal. 1931:595–598. doi: 10.1136/bmj.2.3691.595. ii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chesney RW. Vitamin D and The Magic Mountain: the anti-infectious role of the vitamin. Journal of Pediatrics. 2010;156:698–703. doi: 10.1016/j.jpeds.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Prentice A. Vitamin D deficiency: a global perspective. Nutrition Reviews. 2008;66:S153–164. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 5.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: Double blind, randomized clinical trial of safety and effectiveness. Journal of Bone and Mineral Research. 2011 doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. New England Journal of Medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Arabi A, El Rassi R, El-Hajj Fuleihan G. Hypovitaminosis D in developing countries-prevalence, risk factors and outcomes. Nat Rev Endocrinol. 2010;6:550–561. doi: 10.1038/nrendo.2010.146. [DOI] [PubMed] [Google Scholar]
- 8.Feleke Y, Abdulkadir J, Mshana R, Mekbib TA, Brunvand L, Berg JP, et al. Low levels of serum calcidiol in an African population compared to a North European population. European Journal of Endocrinology/European Federation of Endocrine Societies. 1999;141:358–360. doi: 10.1530/eje.0.1410358. [DOI] [PubMed] [Google Scholar]
- 9.Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. European Journal of Clinical Nutrition. 2009;63:646–652. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. American Journal of Clinical Nutrition. 2008;88:520S–528S. doi: 10.1093/ajcn/88.2.520S. [DOI] [PubMed] [Google Scholar]
- 11.Henry HL, Norman AW. Vitamin D: metabolism and biological actions. Annual Review of Nutrition. 1984;4:493–520. doi: 10.1146/annurev.nu.04.070184.002425. [DOI] [PubMed] [Google Scholar]
- 12.WHO (World Health Organization) Vitamin and minteral requirements in human nutrition. 2. Geneva: 2004. [Google Scholar]
- 13.Salle BL, Delvin EE, Lapillonne A, Bishop NJ, Glorieux FH. Perinatal metabolism of vitamin D. American Journal of Clinical Nutrition. 2000;71:1317S–1324S. doi: 10.1093/ajcn/71.5.1317s. [DOI] [PubMed] [Google Scholar]
- 14.Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. American Journal of Clinical Nutrition. 2004;80:1752S–1758S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- 15.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. Journal of Nutrition. 2009;139:1157–1161. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodnar LM, Simhan HN. Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstetrical and Gynecological Survey. 2010;65:273–284. doi: 10.1097/OGX.0b013e3181dbc55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewison M. Vitamin D and the immune system. Journal of Endocrinology. 1992;132:173–175. doi: 10.1677/joe.0.1320173. [DOI] [PubMed] [Google Scholar]
- 18.Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. Journal of the Society for Gynecologic Investigation. 2004;11:263–271. doi: 10.1016/j.jsgi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatric Research. 2009;65:106R–113R. doi: 10.1203/PDR.0b013e31819dba91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misawa Y, Baba A, Ito S, Tanaka M, Shiohara M. Vitamin D(3) induces expression of human cathelicidin antimicrobial peptide 18 in newborns. International Journal of Hematology. 2009;90:561–570. doi: 10.1007/s12185-009-0452-9. [DOI] [PubMed] [Google Scholar]
- 21.Bartley J. Vitamin D, innate immunity and upper respiratory tract infection. Journal of Laryngology and Otology. 2010;124:465–469. doi: 10.1017/S0022215109992684. [DOI] [PubMed] [Google Scholar]
- 22.Liu NQ, Kaplan AT, Lagishetty V, Ouyang YB, Ouyang Y, Simmons CF, et al. Vitamin D and the regulation of placental inflammation. Journal of Immunology. 2011;186:5968–5974. doi: 10.4049/jimmunol.1003332. [DOI] [PubMed] [Google Scholar]
- 23.Barrera D, Avila E, Hernandez G, Mendez I, Gonzalez L, Halhali A, et al. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reproductive Biology and Endocrinogy. 2008;6:3. doi: 10.1186/1477-7827-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrera D, Avila E, Hernandez G, Halhali A, Biruete B, Larrea F, et al. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. Journal of Steroid Biochemistry and Molecular Biology. 2007;103:529–532. doi: 10.1016/j.jsbmb.2006.12.097. [DOI] [PubMed] [Google Scholar]
- 25.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. Journal of Clinical Endocrinology and Metabolism. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maestro B, Molero S, Bajo S, Davila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3) Cell Biochemistry and Function. 2002;20:227–232. doi: 10.1002/cbf.951. [DOI] [PubMed] [Google Scholar]
- 27.Pawley N, Bishop NJ. Prenatal and infant predictors of bone health: the influence of vitamin D. American Journal of Clinical Nutrition. 2004;80:1748S–1751S. doi: 10.1093/ajcn/80.6.1748S. [DOI] [PubMed] [Google Scholar]
- 28.Pasco JA, Wark JD, Carlin JB, Ponsonby AL, Vuillermin PJ, Morley R. Maternal vitamin D in pregnancy may influence not only offspring bone mass but other aspects of musculoskeletal health and adiposity. Medical Hypotheses. 2008;71:266–269. doi: 10.1016/j.mehy.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Giuliani DL, Boland RL. Effects of vitamin D3 metabolites on calcium fluxes in intact chicken skeletal muscle and myoblasts cultured in vitro. Calcified Tissue International. 1984;36:200–205. doi: 10.1007/BF02405318. [DOI] [PubMed] [Google Scholar]
- 30.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. Journal of Clinical Endocrinology and Metabolism. 2006;91:906–912. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- 31.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 32.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. Journal of Bone and Mineral Research. 2011 doi: 10.1002/jbmr.328. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF. Vitamin D: A D-Lightful Solution for Health. Journal of Investigative Medicine. 2011 doi: 10.231/JIM.0b013e318214ea2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutrition Reviews. 2010;68:465–477. doi: 10.1111/j.1753-4887.2010.00306.x. [DOI] [PubMed] [Google Scholar]
- 35.Mahomed K, Gulmezoglu AM. Vitamin D supplementation in pregnancy. Cochrane Database Syst Rev. 2009:CD000228. doi: 10.1002/14651858.CD000228. [DOI] [PubMed] [Google Scholar]
- 36.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. American Journal of Clinical Nutrition. 2004;79:717–726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 37.Hollis BW. Vitamin D requirement during pregnancy and lactation. Journal of Bone and Mineral Research. 2007;22 (Suppl 2):V39–44. doi: 10.1359/jbmr.07s215. [DOI] [PubMed] [Google Scholar]
- 38.Dawodu A, Wagner CL. Mother-child vitamin D deficiency: an international perspective. Archives of Disease in Childhood. 2007;92:737–740. doi: 10.1136/adc.2007.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prentice A, Schoenmakers I, Laskey MA, de Bono S, Ginty F, Goldberg GR. Nutrition and bone growth and development. Proceedings of the Nutrition Society. 2006;65:348–360. doi: 10.1017/s0029665106005192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Nordic Cochrane Centre TCC. Review Manager (RevMan) Version 5.1. Copenhagen: 2011. [Google Scholar]
- 41.Headrick TC. Statistical Simulation: Power Method Polynomials and other Transformations. Boca Raton, FL: Chapman & Hall/CRC; 2010. [Google Scholar]
- 42.Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clinical Endocrinology. 2009;70:685–690. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- 43.Marya RK, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecologic and Obstetric Investigation. 1981;12:155–161. doi: 10.1159/000299597. [DOI] [PubMed] [Google Scholar]
- 44.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clinical and Experimental Allergy. 2009;39:875–882. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 45.Mannion CA, Gray-Donald K, Koski KG. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ. 2006;174:1273–1277. doi: 10.1503/cmaj.1041388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marya RK, Rathee S, Dua V, Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian Journal of Medical Research. 1988;88:488–492. [PubMed] [Google Scholar]
- 47.Prentice A, Jarjou LM, Goldberg GR, Bennett J, Cole TJ, Schoenmakers I. Maternal plasma 25-hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatrica. 2009;98:1360–1362. doi: 10.1111/j.1651-2227.2009.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehta S, Giovannucci E, Mugusi FM, Spiegelman D, Aboud S, Hertzmark E, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 2010;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. Journal of Infectious Diseases. 2009;200:1022–1030. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krishnaveni GV, Veena SR, Winder NR, Hill JC, Noonan K, Boucher BJ, et al. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: the Mysore Parthenon Study. American Journal of Clinical Nutrition. 2011;93:628–635. doi: 10.3945/ajcn.110.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstetrics and Gynecology. 1986;68:300–304. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. British Medical Journal. 1980;280:751–754. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson PE, McDonald BW. The association of maternal diet and dietary supplement intake in pregnant New Zealand women with infant birthweight. European Journal of Clinical Nutrition. 2010;64:184–193. doi: 10.1038/ejcn.2009.134. [DOI] [PubMed] [Google Scholar]
- 54.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. European Journal of Clinical Nutrition. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Makitie O, et al. Maternal vitamin D status determines bone variables in the newborn. Journal of Clinical Endocrinology and Metabolism. 2010;95:1749–1757. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 56.Bowyer L, Catling-Paull C, Diamond T, Homer C, Davis G, Craig ME. Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clinical Endocrinology. 2009;70:372–377. doi: 10.1111/j.1365-2265.2008.03316.x. [DOI] [PubMed] [Google Scholar]
- 57.Leffelaar ER, Vrijkotte TG, van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. British Journal of Nutrition. 2010:1–10. doi: 10.1017/S000711451000022X. [DOI] [PubMed] [Google Scholar]
- 58.Morley R, Carlin JB, Pasco JA, Wark JD, Ponsonby AL. Maternal 25-hydroxyvitamin D concentration and offspring birth size: effect modification by infant VDR genotype. European Journal of Clinical Nutrition. 2009;63:802–804. doi: 10.1038/ejcn.2008.55. [DOI] [PubMed] [Google Scholar]
- 59.Clifton-Bligh RJ, McElduff P, McElduff A. Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabetic Medicine. 2008;25:678–684. doi: 10.1111/j.1464-5491.2008.02422.x. [DOI] [PubMed] [Google Scholar]
- 60.Leffelaar ER, Vrijkotte TG, van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. British Journal of Nutrition. 2010;104:108–117. doi: 10.1017/S000711451000022X. [DOI] [PubMed] [Google Scholar]
- 61.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. American Journal of Clinical Nutrition. 2007;85:788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker PN, Wheeler SJ, Sanders TA, Thomas JE, Hutchinson CJ, Clarke K, et al. A prospective study of micronutrient status in adolescent pregnancy. American Journal of Clinical Nutrition. 2009;89:1114–1124. doi: 10.3945/ajcn.2008.27097. [DOI] [PubMed] [Google Scholar]
- 63.Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, et al. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. Journal of Nutrition. 2010;140:999–1006. doi: 10.3945/jn.109.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scholl TO, Chen X. Vitamin D intake during pregnancy: association with maternal characteristics and infant birth weight. Early Human Development. 2009;85:231–234. doi: 10.1016/j.earlhumdev.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Shibata M, Suzuki A, Sekiya T, Sekiguchi S, Asano S, Udagawa Y, et al. High prevalence of hypovitaminosis D in pregnant Japanese women with threatened premature delivery. Journal of Bone and Mineral Metabolism. 2011 doi: 10.1007/s00774-011-0264-x. [DOI] [PubMed] [Google Scholar]
- 66.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. Journal of Clinical Endocrinology and Metabolism. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baker AM, Haeri S, Camargo CA, Jr, Espinola JA, Stuebe AM. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. Journal of Clinical Endocrinology and Metabolism. 2010;95:5105–5109. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Powe CE, Seely EW, Rana S, Bhan I, Ecker J, Karumanchi SA, et al. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension. 2010;56:758–763. doi: 10.1161/HYPERTENSIONAHA.110.158238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shand A, Nassar N, Von Dadelszen P, Innis S, Green T. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG. 2010;117:1593–1598. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 70.Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. British Medical Journal (Clinical Research Ed) 1981;283:1024. doi: 10.1136/bmj.283.6298.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 72.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 73.Olsen SF, Secher NJ. A possible preventive effect of low-dose fish oil on early delivery and pre-eclampsia: indications from a 50-year-old controlled trial. British Journal of Nutrition. 1990;64:599–609. doi: 10.1079/bjn19900063. [DOI] [PubMed] [Google Scholar]
- 74.Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecologic and Obstetric Investigation. 1987;24:38–42. doi: 10.1159/000298772. [DOI] [PubMed] [Google Scholar]
- 75.Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P, et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20:720–726. doi: 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- 76.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. American Journal of Obstetrics and Gynecology. 2003;189:139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 78.Davis LM, Chang SC, Mancini J, Nathanson MS, Witter FR, O’Brien KO. Vitamin D insufficiency is prevalent among pregnant African American adolescents. Journal of Pediatric and Adolescent Gynecology. 2010;23:45–52. doi: 10.1016/j.jpag.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 79.Mandic Havelka A, Yektaei-Karin E, Hultenby K, Sorensen OE, Lundahl J, Berggren V, et al. Maternal plasma level of antimicrobial peptide LL37 is a major determinant factor of neonatal plasma LL37 level. Acta Paediatrica. 2010;99:836–841. doi: 10.1111/j.1651-2227.2010.01726.x. [DOI] [PubMed] [Google Scholar]
- 80.Brunvand L, Shah SS, Bergstrom S, Haug E. Vitamin D deficiency in pregnancy is not associated with obstructed labor. A study among Pakistani women in Karachi. Acta Obstetrica Gynecologica Scanddinavica. 1998;77:303–306. [PubMed] [Google Scholar]
- 81.Holick MF. Vitamin D: the other steroid hormone for muscle function and strength. Menopause. 2009;16:1077–1078. doi: 10.1097/gme.0b013e3181bd9804. [DOI] [PubMed] [Google Scholar]
- 82.Manaseki-Holland S, Qader G, Isaq Masher M, Bruce J, Zulf Mughal M, Chandramohan D, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Tropical Medicine and International Health. 2010;15:1148–1155. doi: 10.1111/j.1365-3156.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 83.Villamor E. A potential role for vitamin D on HIV infection? Nutrition Reviews. 2006;64:226–233. doi: 10.1301/nr.2006.may.226-233. [DOI] [PubMed] [Google Scholar]
- 84.Hypponen E, Fararouei M, Sovio U, Hartikainen AL, Pouta A, Robertson C, et al. High-dose vitamin d supplements are not associated with linear growth in a large finnish cohort. Journal of Nutrition. 2011;141:843–848. doi: 10.3945/jn.110.133009. [DOI] [PubMed] [Google Scholar]
- 85.Stearns G, Jeans P, Vandecar V. The effect of vitamin D on linear growth in infancy. Journal of Pediatrics. 1936;9:1–10. [Google Scholar]
- 86.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25- hydroxycholecalciferol response to extended oral dosing with cholecalciferol. American Journal of Clinical Nutrition. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 87.Roth D, Al-Mahmud A, Arifeen SE, Raqib R, Black R, Baqui A. Randomized Pilot Trial of Two Oral Vitamin D3 Supplementation Regimens during the Third Trimester of Pregnancy in Bangladeshi Women: Effects on Neonatal Vitamin D Status and Safety. Pediatric Academic Societies and Asian Society for Pediatric Research; Denver: 2011. [Google Scholar]
- 88.Roth DE. Vitamin D supplementation during pregnancy: safety considerations in the design and interpretation of clinical trials. Journal of Perinatology. 2011 doi: 10.1038/jp.2010.203. [DOI] [PubMed] [Google Scholar]
- 89.(NIH) USNIoH. Clinicaltrials.gov. 2011 [updated 2011; cited 2011 7/25/2011]; Available from: http://clinicaltrials.gov/
- 90.Maxwell JD, Ang L, Brooke OG, Brown IR. Vitamin D supplements enhance weight gain and nutritional status in pregnant Asians. British Journal of Obstetrics and Gynaecology. 1981;88:987–991. doi: 10.1111/j.1471-0528.1981.tb01686.x. [DOI] [PubMed] [Google Scholar]
- 91.Oken E, Ning Y, Rifas-Shiman SL, Rich-Edwards JW, Olsen SF, Gillman MW. Diet during pregnancy and risk of preeclampsia or gestational hypertension. Annals of Epidemiology. 2007;17:663–668. doi: 10.1016/j.annepidem.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shand AW, Nassar N, Von Dadelszen P, Innis SM, Green TJ. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG. 2010;117:1593–1598. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Trials and observational studies of vitamin D supplementation or intake and mean birthweight (in grams)
Figure S2. Trials of vitamin D supplementation and SGA and low birthweight, disaggregated by dosing approach
Figure S3. Trials of vitamin D supplementation and mean crown-heel length (in cm.)
Figure S4. Trials of vitamin D supplementation and mean weight gain during the 3rd trimester
Figure S5. Supplementation and preterm and mean gestational age at delivery, disaggregated by dosing approach
Figure S6. Observational studies of vitamin D intake and mean gestational age at delivery
Figure S7. Observational studies of vitamin D intake and pre-eclampsia risk
Table S1. Search strategy and keywords
Table S2. Evaluation of the quality of eligible trials for potential inclusion in the meta-analysis
Table S3. Evaluation of the quality of evidence of individual observational studies for potential inclusion in the meta-analysis
Table S4. Prospective observational studies of the relationship between maternal blood levels of 25(OH)D during pregnancy and measures of fetal growth, birth weight, and preterm birth
Table S5. Prospective observational studies of the relationship between maternal blood levels of 25(OH)D during pregnancy and pre-eclampsia