Summary
Vitamin A (VA) deficiency during pregnancy is common in low income countries and a growing number of intervention trials have examined the effects of supplementation during pregnancy on maternal, perinatal, and infant health outcomes.
We systematically reviewed the literature to identify trials isolating the effects of VA or carotenoid supplementation during pregnancy on maternal, fetal, neonatal and early infant health outcomes. Meta-analysis was used to pool effect estimates for outcomes with more than one comparable study. We used GRADE criteria to assess the quality of individual studies and the level of evidence available for each outcome.
We identified 23 eligible trials of which 17 had suitable quality for inclusion in meta-analyses. VA or beta-carotene (βC) supplementation during pregnancy did not have a significant overall effect on birthweight indicators, preterm birth, stillbirth, miscarriage, or fetal loss. Among HIV-positive women, supplementation was protective against low birthweight (<2.5 kg), RR=0.79, [95% CI 0.64, 0.99], but no significant effects on preterm delivery or small-for-gestational age were observed. Pooled analysis of the results of three large randomized trials found no effects of VA supplementation on neonatal/infant mortality, or pregnancy-related maternal mortality, random effects RR=0.86, [0.60, 1.24] although high heterogeneity was observed in the maternal mortality estimate[I2=74%, p=0.02]. VA supplementation during pregnancy was found to improve hemoglobin levels and reduce anemia risk (<11.0 g/dL) during pregnancy random effects RR=0.81 [0.69, 0.94], also with high heterogeneity (I2=52%, p=0.04). We found no effect of VA/βC supplementation on mother-to-child HIV transmission in pooled analysis, although some evidence suggests that it may increase transmission.
There is little consistent evidence of benefit of maternal supplementation with VA or βC during pregnancy on maternal or infant mortality. While there may be beneficial effects for certain outcomes, there may also be potential for harm through increased HIV transmission in some populations.
Introduction
It has been estimated that about 19 million pregnant women in low income countries each year are affected by vitamin A (VA) deficiency, the majority in South and Southeast Asia.1, 2 Nearly 10 million women suffer from night blindness during pregnancy, a condition often caused by VA deficiency and associated with a constellation of adverse health and nutritional conditions among mothers and infants.1, 3–5
Dietary VA is found as preformed retinol in animal sources (such as liver, eggs, and milk), as retinyl esters in fortified foods, and as provitamin A carotenoids found in green leafy vegetables and yellow/orange fruits such as mangos and papaya.6 Although many provitamin A carotenoids have been identified, β-Carotene (β-C) has the highest conversion rates to retinol and additionally has anti-oxidative properties. Lycopene is a carotenoid without provitamin A activity but is an efficient anti-oxidant.
The recommended safe intake of VA during pregnancy by FAO/WHO is 800 μg RE/day (2664 IU)7. VA is known to have teratogenic effects if consumed at high doses during early pregnancy, and the FAO/WHO advise limiting intake to a maximum of 3000μg retinol equivalents (RE) per day (10,000 IU) or weekly intakes of 25,000 IU to minimize risks.7 One approach used in some trials to try to meet the needs of highly deficient populations while minimizing risks of teratogenicity has been to include both VA and β-C in supplements.8, 9
VA deficiency is most often considered to be a problem of public health concern among preschool age children (age 6–59 months) as it increases risk of mortality from infectious disease, particularly measles, diarrhea, and malaria.6, 10 Findings that high dose supplementation with VA two to three times per year reduces the risk of all cause child mortality by an average of 24% form a solid evidence base for supplementation programs of this age group.11
In contrast, VA deficiency during pregnancy was, until recently, a largely unexplored area of public health interest2. Early work in the 1930’s by Mellanby and Green identified a potential role for VA (in the form of cod liver oil) to reduce puerperal septicaemia, although little follow-up work continued for the next half century.12–14 Four main developments contributed to a re-awakening of interest in the potential role of VA supplementation during pregnancy (1) Recognition that maternal supplementation during pregnancy and lactation could be an opportunity to improve the VA status and health of infants (2) Findings from a large trial in Nepal that VA and β-C supplementation during pregnancy was associated with a 40% reduction in risk of pregnancy-related mortality15 (3) Observations that low serum retinol levels were associated with increased risk of mother to child transmission of HIV, and the hypothesis that VA, by virtue of its role in epithelial integrity or immune-modulating properties might help to reduce transmission16 (4) Growing recognition that VA might have a role in improving hematologic status during pregnancy.17
The objective of our review was to consolidate knowledge about the effects of supplementation on multiple outcomes related to maternal, perinatal, and infant health to inform policy in low income countries and to identify research priorities.
Methods
We searched the literature to identify trials, intervention studies, and quasi experimental studies isolating the effect of supplementation with VA and/or carotenoids during pregnancy and outcomes shown in Table 1. We searched NLM Pubmed and the Cochrane Library using search strategies outlined in Supplementary Table 1 in November 2010 and updated the search in June, 2011. We also hand-searched reports from major micronutrient conferences as well as the bibliographies of relevant reviews and studies.
Table 1.
List of primary and sub-outcomes covered by this review
|
Indicates outcomes for which sufficient comparable outcomes could not be extracted from more than one study
An initial screening of all titles and abstracts was undertaken to exclude (1) non-human studies, (2) studies not in English, French, or Spanish (3) Reviews, case reports, commentaries and other papers not reporting primary data (4) Topics clearly not relevant to the exposure/outcome relationships (5) papers with research designs that clearly could not isolate effects of VA/carotenoids (for example studies of cod-liver oil). One reviewer (ATL) reviewed the original full papers of studies that could not be excluded based on title and abstract review.
Data were extracted using a standard spreadsheet developed for other reviews in this supplement using best available published data. The authors of one paper provided a clarification of data on birth outcomes in response to requests (personal communication, Anna Coutsoudis (4/11/2011).8 The quality and the potential for bias of each study was using a slightly modified version of the Child Health Epidemiology Reference Group’s GRADE tool.18
Meta-analysis was used to generate effect estimates for outcomes in which there was more than one study with sufficient data appropriate to enable pooling. We excluded studies that were classified as “Very low” using the GRADE criteria. Studies that randomized VA during both pregnancy and lactation were eligible for inclusion, recognizing that attribution of effects of VA supplementation specifically during pregnancy could not be made for post-partum outcomes.
Review Manager 5.1 was used for all data analysis.19 Dichotomous outcomes were expressed as risk ratios, and continuous outcomes were expressed as mean differences with 95% confidence intervals. Inverse variance weights were used in the pooling of data. For analyses that included cluster-randomized trials, we included outcomes for which cluster-adjusted estimates were reported and used the generic inverse-variance method.19 Heterogeneity across studies within outcomes was explored using visual inspection of the distribution of effect estimates, I2 values, and tested for using chi-square tests for heterogeneity using a p-value of less than 0.10 to denote statistical significance. Data was pooled using a fixed effects model except in cases in which statistically significant heterogeneity was present in which case a random effects (RE) model was used. Recognizing the potential for heterogeneity across studies, we considered baseline VA status, supplement form and dosage, HIV prevalence as a priori sources of heterogeneity. We present disaggregated effect estimates in addition to pooled effect estimates for outcomes in which trials in HIV-positive women were available in order to facilitate policy decisions for populations with high or low HIV prevalence.
A number of trials used multifactorial designs with multiple treatment arms containing VA, β-C, or both. 9, 15, 20–24 For purposes of data extraction and meta analyses we treated these studies as follows: (1) For papers in which results were presented for pooled treatment arms containing VA/β-C vs. those not containing VA/β-C, we also pooled data in this manner (2) For studies using the same factorial design where two separate comparisons isolating the effects were possible (i.e. VA vs. placebo and VA+multivitamins vs. multivitamins alone), data were entered for each comparison and treated as separate studies (3) For studies with multiple arms testing different dosages of VA but only 1 placebo group, data from the two treatment arms was combined when possible.
Results
Literature search and identification of studies
After removing duplicates, our search resulted in 923 articles from which 23 eligible studies were identified with at least one outcome of relevance. Of these, six were ranked “Very Low” on the GRADE criteria and were therefore excluded from meta-analyses (Supplementary Table 2). Our search of the abstracts from key micronutrient conferences led to the identification of several abstracts of relevance although none contained sufficient detail to enable evaluation of study quality or inclusion in pooled analysis.
Study characteristics
Key characteristics of studies included in this review are presented in Table 2. Three large cluster randomized controlled trials (RCT’s) were included: trials from Nepal and Bangladesh assessed the independent and pooled effects of both VA and β-C on maternal and neonatal mortality in addition to other outcomes, while the other, from Ghana, examined the effects of VA alone.15, 25, 26 Of the 17 studies included in the meta-analyses, 10 were conducted in Asian settings and seven in Africa and with the exception of one study from China and one from South Africa all were conducted in low income countries.21, 27 Three RCT’s were undertaken in HIV-positive pregnant women in Africa prior to the availability of anti-retroviral therapy, and had the primary objective of exploring the effect of VA supplementation on mother to child transmission of HIV, but also reported on a number of birth and child health outcomes.9, 28, 29
Table 2.
Description of key studies discussed in the paper
| Study ID | Country | Population | Intervention | Comments |
|---|---|---|---|---|
| Banerjee (2009)31 | India (New Delhi) | Healthy primigravid women with singleton pregnancy gest. wks 12 to 20 | Daily oral dose of (1) 2mg lycopene, n=77 (2) placebo tablet of same appearance, n=82 until delivery | Double blind RCT. Inadequate allocation concealment, lack of intent to treat analysis Grade quality: Low |
| Coutsoudis a (1999)8, 28 | South Africa (KwaZulu-Natal) | HIV-infected women recruited gest. wks 17–39. Baseline: 30.6% ser. retinol <0.7 μmol | Daily oral dose of (1) VA: 5000 IU retinyl palmitate and 30 mg β-C and 200,000 IU VA at delivery, n=368 (2) placebo, n=360. | Double blind RCT. Unclear sequence generation, unclear allocation concealment. Grade quality: High |
| Cox (2005)49 | Ghana | Healthy primigravid <gest. 24 wks | Daily oral dose of (1) VA: 10,000 IU RE, n=48 (2) placebo, n=50. | Double blind RCT. Small sample size, Grade quality: Moderate |
| Dijkhuizen (2004)20, 38 | Indonesia (West Java) | 170 pregnant women. Rural women, gest. age <20 wks | Multifactorial trial of daily (1) 4.5 mg β-C (2) 30 mg zinc sulfate (3) 4.5 mg β-C+ 30 mg zinc sulfate (4) placebo. All women received iron/folate. | Double blind RCT. Grade quality: Moderate |
| Fawzi (1998)9, 10, 33, 35, 36, 40, 71, 78, 81–84 | Tanzania (Dares Salaam) | HIV-1 infected between 12–27 weeks gestation, 34% had ser. retinol <0.7μmol in 2nd trimester. | Multifactorial trial, daily dose of (1) VA: 30 mg β-C plus 5000 IU preformed VA and 1 dose 200,000 IU at delivery, n=272 (2) MV excluding VA+β-C, n=271; (3) MV including VA+β-C and 1 dose 200,000 IU VA at delivery, n=268 (4) Placebo, n=267. All received iron-folate and prophylactic chloroquine phosphate. | Double blind RCT. Adequate sequence generation, allocation concealment. Grade quality: High |
| Kirkwood (2010)25 | Ghana | Rural women aged 15–45. Serum retinol 15.4% <0.70 μmol/L; mean serum retinol 1.18 (0.52) μmol/L. | Weekly oral dose of (1) VA: 25,000 IU RE, n=104,484 women, 39,601 pregnancies (2) placebo, n=103,297 women, 39,234 pregnancies | Double blind RCT. High dropout due to movement but results accounting for movement were similar Grade quality: High |
| Kumwenda (2002)29 | Malawi (Blantyre) | HIV-infected, enrolled at gest. wks 18–28. 51% ser. retinol <0.7μmol during 2nd trimester. | Daily oral dose of (1) VA : 10,000 IU, n=340 (2) Placebo, n=357. Both groups received 200,000 IU VA at delivery | Double blind RCT. Grade quality: High |
| Ma (2008)21 | China (rural) | Anemic (Hb<110g/L) pregnant women, 12–24 wks gestation | Multifactorial trial, women assigned to (1) 60mg iron, n=93 (2) 60 mg iron and 2 mg retinol as retinyl palmitate (≈3636 IU), n=91 (3) 60 mg iron and 1.0 mg riboflavin, n=91 (4) 60 mg iron, 2000 μg retinol, 1.0 mg riboflavin, n=83. | Double blind RCT. Grade quality: High |
| Muslimatun (2001)34, 39, 66, 85, 86 | Indonesia (West Java) | 16–20 wk pregnant at enrollment, aged 17–35 y, mean baseline serum retinol ≈ 1.01+0.03 μmol/L. | 2 arms of relevance (1) VA: Weekly dose of 4800 RE as retinyl acetate (≈14,000 IU) with 60 mg elemental iron and 250 μg folic acid, n=122. (2) Iron and folic acid as above, n=121. | Double blind RCT. Grade quality: High |
| Radhika (2003)87 | India (Hyderabad) | Healthy women gest. age 16–24 weeks. Excluded women with recurrent pregnancy loss, prior preterm delivery. | Randomized to (1) Daily sachet of red palm oil containing 2,173 to 2307 μg β-C per day (≈3621–3845 IU) (2) identical appearing sachet of groundnut oil provided for 8 weeks starting from 26–28 wks gestation. All women received iron-folate for 100 days and prenatal care. | Randomized trial. Seq. generation and allocation conceal. unclear; blinding unclear. Mean hb at baseline higher in red palm oil group than control group. Grade quality: Low |
| Semba (2001)64 | Malawi (Blantyre) | HIV-negative pregnant women, gest. age 18–28 wks | Randomized to daily (1) 30 mg elemental iron, 400 μg folate and 3000 μg VA (≈10,000 IU)or (2) iron and folate, as above. Both groups received prenatal care, treatment for STD’s, malaria prophylaxis (Fansidar). | Double blind RCT. Grade quality: High |
| Sharma (2003)32 | India (New Delhi) | Primigravid women with gest. age 16–20 wks with absence of medical complications | Twice daily oral dose of 1) 2mg lycopene (n=116) (2) placebo tablets of same appearance (n=135) | Double blind RCT. Lack of intent to treat analysis. Grade quality: Low |
| Suharno (1993)22 | Indonesia, West Java | Pregnant women with Hb 8.0–10.9 g/dL, gest. age 16–24 weeks. | Randomized to daily (1) Placebo, n=62 (2) VA 2.4 mg retinol as retinyl palmitate (≈4363 IU) (n=63), (3) 60 mg iron as ferrous sulfate and placebo (n=63), (4) VA+Iron (n=63). | Double blind RCT. Not intent to treat (23 participants excluded for taking supplements <8 weeks). Inadequate allocation concealment. Grade quality: Low |
| Tanumihardjo (2002)23 | Indonesia, West Java | Women in 2nd or 3rd trimester, mean 17 weeks gestation | Women randomized to (1) Placebo, n=7 (2) 8000 IU vitamin A as retinyl palmitate, n=7 (3) 60 mg ferrous sulfate n=5 (4) VA plus iron as above, n=8. | Double blind RCT. Small sample size. Unclear sequence generation/allocation concealment. Grade quality: Moderate |
| Van den Broek (2006)24 | Malawi | Singleton pregnancies with Hb 5–10.9g/dL enrolled at 12–24 weeks, baseline mean ser. retinol 37.1 (13.7) μg/DL, 7.4% <20 μg/dL. | In addition women were randomised to daily doses of (1) 5000 IU VA (2) 10,000 IU VAor (3) placebo. All women received daily iron-folate and antimalarial prophylaxis. | Double blind RCT. Grade quality: High |
| West (1999)3, 5, 15, 30, 37, 50, 56, 88–91 | Nepal (Sarlahi) | Rural married women of reproductive age. 19% with serum retinol <0.70 μmol/l. | Weekly oral dose of (1) 23,300 IU VA (7000 μg RE as retinyl palmitate (n=6,070 pregnancies) (2) 42 mg of all trans β-C 7000 μg RE (n=5650 pregnancies) (3) placebo (n=5653 pregnancies). | Double blind RCT. Grade quality: High |
| West (2011)26 | Bangladesh (Rangpur district) | Rural women with known pregnancy outcome and vital status recorded at 12 weeks. 7.7% with ser. retinol <0.70 μmol/l | Weekly oral dose of (1) VA (7000 μg RE as retinyl palmitate (23,300 IU), (n=19,806 pregnancies) (2) 42 mg of all trans β-C 7000 μg RE, (n=19,998 pregnancies) (3) placebo (n=19,862 pregnancies). | Double blind RCT. in women of reproductive age with 3 arms Grade quality: High |
Additional information for preterm, SGA, and birthweight indicators obtained through personal communication with Drs. Coutsoudis and Kuhn (4/11/2011), Abbreviations: VA=vitamin A, gest.=gestational wks.=weeks, ser.=serum, RE=retinol equivalents, β-C=Beta-carotene, seq.=sequence
Dosage and form of supplements varied across the studies from a weekly dose providing the equivalent of a daily dose of 3333 IU VA to 10,000 IU per day. Two of the studies in HIV+ women included intervention arms with both a supplement of 5000 IU VA per day and 30 mg β-C as well as a 200,000 IU dose of VA at delivery.9, 27 Two studies explored the effects of lycopene supplementation, one of which provided 2 mg daily while the other provided 4 mg per day, although both of these studies were judged to be of low GRADE quality (Supplementary Table 2).
Outcomes
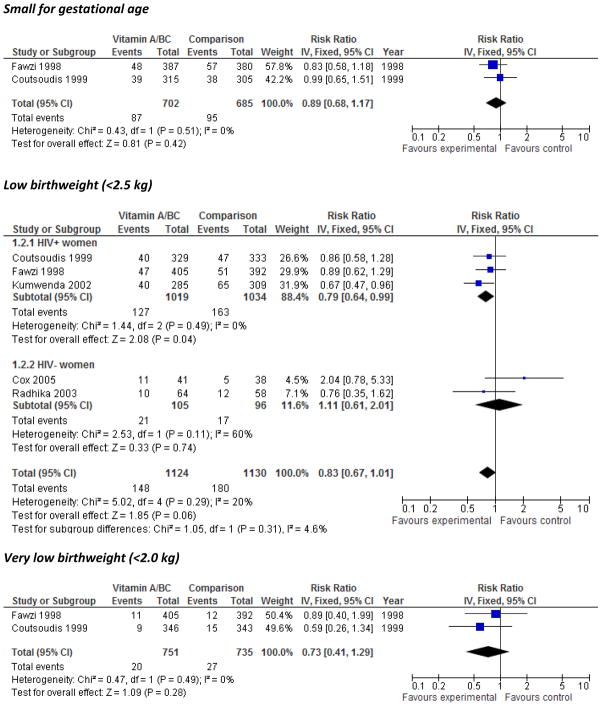
Small-for-gestational age and birthweight
The overall effects of VA on small-for-gestational age (SGA), and very low birthweight (<2.0 kg) were null, and that for low birthweight (<2.5kg) was null but had a trend towards significance, RR=0.83, [0.67, 1.01] (Figure 1). The subgroup of three studies conducted in HIV+ populations showed a significant reduction in risk of LBW, RR=0.79 [0.64, 0.99]. No significant effects of VA on mean birthweight were apparent in meta-analysis (Evaluation of the quality of individual trials for potential inclusion in the meta-analysis using GRADE criteria Supplementary Figure 1) or in a large sub-study from the Nepal trial published only in abstract form,30 and lycopene supplementation did not have a significant effect on mean birthweight in pooled analysis (Supplementary Figure 2).
Figure 1.
Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on small for gestational age, low birthweight (<2.5 kg), and very low birthweight (<2.0 kg)
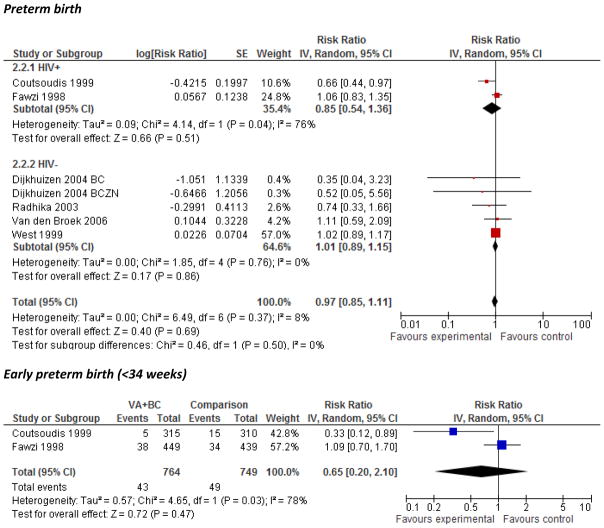
Effect on preterm birth
No significant overall effect was found of VA on preterm birth (<37 weeks) or early preterm birth (<34 weeks) (Figure 2). There was substantial heterogeneity within the HIV+ subgroup for preterm birth (I2=76%, p=0.04), and in the overall effect estimate for early preterm birth (I2=78%, p=0.03). A significant 33% reduction in the prevalence of preterm birth and a 66% reduction in early preterm birth among women was observed in the South African study, but no other individual trials showed statistically significant effects.8 No effect of lycopene supplementation was found on mean gestational age at delivery (Supplementary Figure 3) although substantial heterogeneity was observed (I2=94%, P<0.0001) and one included trial reported an increased risk of preterm birth compared with placebo (10.4% vs. 1.2%, p=0.02).31, 32
Figure 2.
Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on preterm birth (<37 weeks gestational age) and early preterm birth (<34 weeks)
Effect on stillbirth, miscarriage, and fetal death
The overall effect estimates for supplementation with VA or β-C on stillbirth, (Supplementary Figure 4) miscarriage (Supplementary Figure 5), and fetal loss (Supplementary Figure 6) were all null, as were each of the individual studies contributing to the estimates.
Maternal mortality, morbidity, and birth complications
Three large double-blinded, cluster-randomized, placebo-controlled trials assessed the impact of VA supplementation on all-cause pregnancy-related mortality up to 42 days postpartum.15, 25, 26 The combined effect estimate from these studies (Figure 3) indicated a null overall effect (RR=0.86 [0.60, 1.24]), although significant heterogeneity across the two studies was apparent (I2=74%, p=0.02), with the Nepal study exhibiting a 44% decrease in maternal mortality and the Ghana and Bangladesh studies showing no significant effect. The trial in Ghana reported no significant difference in risk of hospital admission with diagnosis of one or more severe pregnancy-related conditions within 6 weeks postpartum between the VA and placebo groups (RR=0.98 [0.89, 1.09]).25 Both the Nepal and Bangladesh trials collected information on cause of death through verbal autopsy but this information was not pooled due to a lack of information about the design effect from the cluster-randomized design. Although one trial with lycopene found a significant protective effect of supplementation on pre-eclampsia risk, the summary estimate from two studies was null (Supplementary Figure 7). Two studies examined risk of delivery using Caesarian section as an outcome, one with VA/βC and the other with lycopene and neither found a significant effect of supplementation8, 31. One study examined the effect of lycopene supplementation in pregnancy and found no effect on incidence of traumatic or atonic hemorrhage, or both.31
Figure 3.
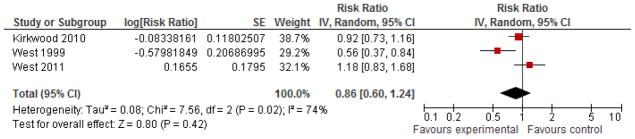
Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on maternal pregnancy-related mortality
Maternal anemia and hemoglobin
Supplementation with any form of VA or β-C was found to significantly (p<0.05) reduce the risk of anemia (Hb<11g/dL) at follow-up during pregnancy by 19% (Figure 4). Three of the individual trials found a significant protective effect, and there was consistency in the direction of effect measures for all of the trials including those with null results. However, there was significant heterogeneity in the effect estimate which completely resolved in a sensitivity analysis that excluded the intervention and comparison arms of the factorial trial by Suharno providing concomitant iron supplementation (RR=0.86 [0.79, 0.93], I2=0, p for heterogeneity=0.42).22 The pooled analysis of studies with severe anemia as an outcome indicated no statistically significant effect of VA supplementation RR=0.93 [0.59, 1.45] (Supplementary Figure 8).
Figure 4.
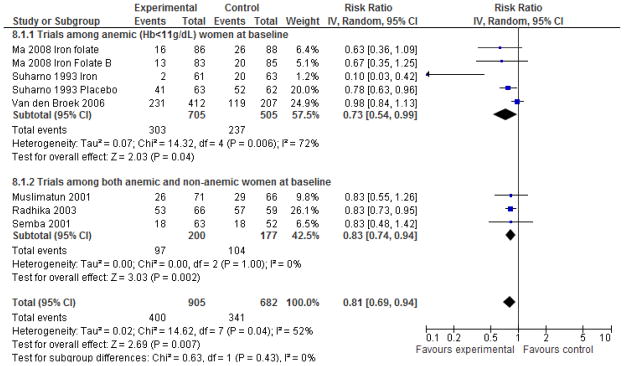
Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on anemia (Hb<110 g/L) during pregnancy
Average follow-up measures of mean hemoglobin assessed after supplementation during pregnancy were found to be significantly higher among women supplemented with VA by 0.35 g/dL [95% CI 0.24, 0.45] (Supplementary Figure 9) and a change in 0.19 g/dL [95% CI 0.06, 0.33] among studies reporting change in hemoglobin levels (Supplementary Figure 10). However, no differences in maternal hemoglobin were observed in group comparisons of women at 4–6 months postpartum (Supplementary Figure 11).
Infant and young child hemoglobin and anemia
Two studies, both in HIV positive populations examined the effect of maternal VA supplementation on risk of anemia (Hb<11.0g/dL) among infants and young children suggested a trend towards a protective effect (RR= 0.71 [0.49, 1.03], p=0.07) Supplementary Figure 12, although considerable heterogeneity was evident (I2=74%, p=0.05) 9, 29 Sensitivity analyses using data on any anemia during an average follow-up of 28 months from the Tanzanian trial rather than time to first episode of anemia led to an attenuation of the effect estimate (RR=0.89 [95% CI 0.74, 1.07], p=0.21).33 No effect of VA supplementation on mean infant hemoglobin was observed at 4–6 months. (Supplementary Figure 13).
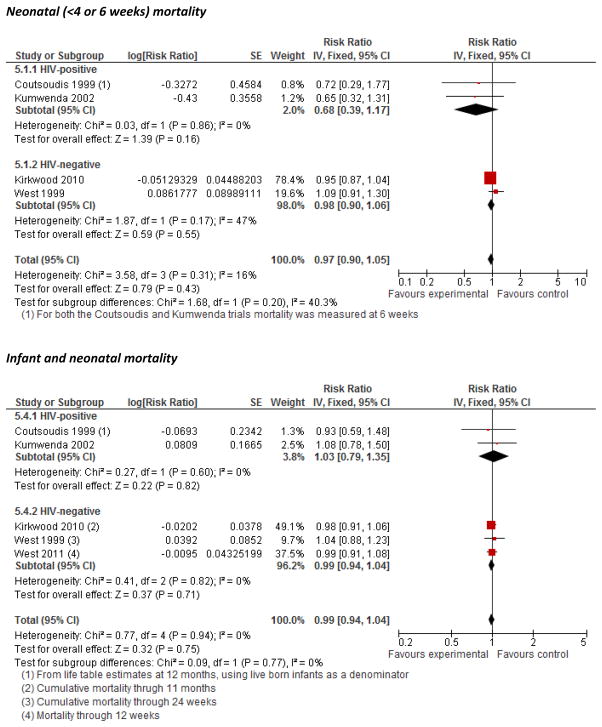
Neonatal and infant mortality, morbidity, and growth
We found strong evidence that maternal VA supplementation during pregnancy does not have an overall effect on mortality during infancy or the neonatal period (Figure 5). The pooled effect estimates were null and weighted strongly by the large cluster randomized trials, none of which suggested a significant effect. An analyses from the Nepal trial stratified by maternal night blindness status during pregnancy suggested that VA supplementation might reduce risk of mortality specifically among infants born to night-blind women although β-C did not appear to be as efficacious.5 The Bangladesh trial was the only one that we identified with cause-specific infant mortality and found no differences across treatment groups.26
Figure 5.
Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on neonatal and infant mortality
Assessment methods for neonatal and infant morbidity differed greatly across trials limiting the utility of pooling. A trial in Indonesia found no significant effect on maternal VA supplementation during pregnancy on incidence of diarrhea, cough, difficulty breathing or other morbidity outcomes over the first year of life.34 In the Tanzania trial of HIV-positive women, VA+ β-C supplementation during pregnancy and in a large dose postpartum was found to significantly reduce risk of cough and rapid respiratory rate among offspring by 31%, (RR=0.69 [0.49–0.96]), but no effect on diarrhea was observed.35 VA+ β-C supplementation alone was also associated with a borderline significant 63% reduction in incidence of clinical malaria in the same study (RR=0.37 [0.13, 1.04], P = 0.06) compared with placebo but no significant effects of supplementation on malarial mortality were observed (RR=0.65 [0.29, 1.46]. 35, 36 In findings from the Nepal trial, published in abstract form, no significant supplementation group differences were observed in 7-day recalls of diarrhea, dysentery, high fever, or cough for infants at 3 or 6 months of life.37 Similarly in Indonesia, no significant effect of β-C was observed on first occurrence of cough or diarrhea.38
A number of studies have reported on anthropometric measures taken during the first year of life although differences in reporting of indicators constrained our ability to incorporate findings into pooled analyses.20, 29, 39, 40 We found little evidence of an effect of VA or β-C supplementation on weight-for-age or height-for-age z score (Supplementary Figure 14, Supplementary Figure 15). In the Malawi trial, infants of HIV-positive women who received VA during pregnancy had greater attained length and weight at 6 weeks, but these differences did not persist at 14 weeks or 6 months of age. 29. In a subset of 833 infants from the Nepal study, published in abstract form only, VA supplementation during pregnancy was found to be associated with significantly greater average daily weight and MUAC gain but not length gain from 0–3 months of age, but did not persist at 3–6 months of age, although there was a trend towards greater attained weight in both intervention groups at 6 months.30
Mother to child HIV transmission
The overall effect of supplementation with VA or VA/βC on HIV transmission was null, although there was significant heterogeneity for this outcome (I2=75%, P=0.02) (Figure 6). Examining the individual effects of these trials, the trial from Tanzania showed a significant 35% increased risk of transmission and the remainder were null. 9 Although not eligible for inclusion in our pooled analysis because it tested the effects of maternal and/or infant VA supplementation during the early postpartum period and not supplementation during pregnancy, we also noted findings from the ZVITAMBO trial suggesting increased risk of HIV infection or death among offspring whose mothers received postpartum VA supplementation but who did not receive direct supplementation themselves.41
Figure 6.
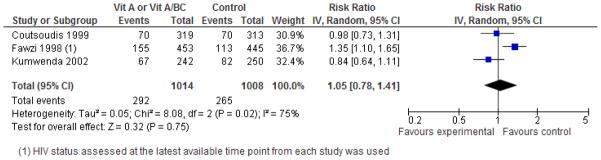
Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on HIV transmission from mother to child
Discussion
IUGR and preterm birth
Some, but not all observational studies have reported that low plasma retinol concentrations are associated with intrauterine growth restriction42–45. We found limited evidence to evaluate the effect of VA supplementation on intrauterine growth restriction, and no effect on mean birthweight was observed overall. Our findings of a significant reduction in the risk of LBW specifically among HIV-positive women are in contrast to previous meta-analyses which reported a null effect. 46, 47 Our analysis differed in two ways: (1) We excluded a trial of multi-micronutrient supplementation which did not isolate the effect of VA and (2) We requested and included additional data from one of the studies in which the published denominators were unclear. 8, 9, 48 We therefore believe our effect estimates provide the most accurate representation of the pooled effects of VA/β-C supplementation during pregnancy on risk of LBW incidence among HIV positive women to date. It remains unclear whether differences in the effect of VA supplementation on LBW incidence among HIV-positive women are the result of a reduction in IUGR, preterm birth, or both, as the pooled estimates of the effects on both of these components of LBW were null.8 The trial from Malawi which showed a protective effect did use a higher dose of retinol (10,000 IU) than other studies and did not include high dose β-C present in the other two trials although there are also potential contextual differences that might explain the strength of the effect 9, 29, 36, 49.
All but one study suggested that VA or β-C supplementation does not influence risk of preterm birth. The one study to show a protective effect of supplementation on preterm birth also noted that this effect disappeared when excluding multiple pregnancies. 8 Interestingly, the large trial in Nepal found a heightened risk of twinning associated with VA/βC supplementation, a phenomenon that might be expected to increase the risk of adverse perinatal outcomes including preterm birth, although no difference in risk of preterm birth was observed overall. 50
Stillbirth, miscarriage, and fetal loss
Our finding of no adverse effect of VA supplementation on outcomes related to fetal loss supports conclusions that VA supplementation appears to be safe throughout pregnancy at levels at or below the amounts tested in the large trials (23,000 IU/week and 10,000 IU per day), although we did not specifically examine birth defects as an outcome.
Maternal all-cause pregnancy-related mortality
The World Health Organization currently does not recommend VA supplementation during pregnancy for the prevention of maternal and infant morbidity and mortality, but does recommend supplementation for the prevention of night blindness in areas where VA deficiency is a serious public health problem.51 Our findings are supportive of this recommendation and are also consistent with those of the recent Cochrane review, though our analysis differed in that we also included the subsequently published trial from Bangladesh and omitted an early trial of cod-liver oil on the grounds that it did not isolate the effects of vitamin A.12, 24, 26
Substantial heterogeneity was observed for the effect estimate on maternal mortality, and reflects the contrast of protective effects observed in Nepal against null findings observed in Bangladesh and Ghana. The heterogeneity observed across these three large and well conducted trials is difficult to explain. Differences in compliance rates are an unlikely explanation, as all studies assessed and reported high compliance.15, 25, 26 It has been argued that findings in the Nepal study may have arisen by chance, based primarily on observations that a large number of deaths were attributable to injury and other causes in the verbal autopsy.25
Other contextual differences related to the prevalence of VA deficiency or pregnancy-related mortality might be responsible in some way for contrasting effects.52 The trial population in Nepal appears to have been worse off than those in Ghana and Bangladesh in many ways including having substantially higher rates of pregnancy related mortality (704, 377, and 231 deaths per 100,000 pregnancies respectively), higher presumed prevalence of wasting malnutrition, and lower intake of eggs and fish compared with Bangladesh.15, 25, 53, 54 Although there was little reported information on availability of health services in the trial reports, it was noted that a greater proportion of deliveries in Bangladesh were attended by traditional or skilled health workers than in Nepal.26
Moderate VA deficiency (plasma retinol <0.7 μmol/L) was also most prevalent in Nepal (19%) followed by Ghana (15%) and Bangladesh (8%).15, 25, 53 While VA, but not βC, supplementation appeared to lead to significant reductions in moderate VA deficiency in Bangladesh and Nepal, findings from Ghana were counterintuitive: A greater proportion of women in the VA arm of the trial had moderate VA deficiency (<0.70 μmol/L) compared with those in the placebo arm (25% vs. 15.4%, p=0.048). Such findings are difficult to explain as investigators reported good compliance with assigned regimens, although serum retinol is known to be an imperfect measure of VA status as it is homeostatically regulated and affected by infection and other factors unrelated to deficiency.55
The prevalence of night blindness during pregnancy in the Nepal and Bangladesh trials was similar (about 9–10%), while night blindness was not measured in the Ghana trial but is reportedly uncommon. 25 Night-blind women in the Nepal trial were observed to have a five-fold greater risk of death due to infection-related causes compared with non-night blind women and much of the mortality risk was found to be attenuated by supplementation with VA or βC.56 In the Bangladesh trial, all women who were identified as night blind when screened at week 28 of gestation were treated with VA according to WHO protocols for ethical reasons.57 If mortality risk was concentrated among such women in Bangladesh, as it appeared to have been in Nepal, it is possible that this may have led to an attenuation of any beneficial effects. In any case, findings from the Nepal study underscore the importance of recommendations that all night blind women be treated with VA.
Maternal and child hemoglobin and anemia
Cross-sectional surveys and observational studies in women and in children often show correlations between anemia and VA deficiency and improving VA status in deficient populations typically results in improvements in anemia.58–60 Our finding that supplementation with VA led to an average increase in mean hemoglobin levels on the order of approximately 0.2 g/dL is consistent with previous estimates that VA supplementation of deficient populations can be expected to raise hemoglobin concentrations by 0.2–1.0 g/dL over a period greater two weeks.59 While not all intervention studies included in our meta-analysis showed a statistically significant reduction in anemia risk, the direction of effect was consistent across all studies. Most of the studies we identified were conducted in trial populations that first screened as anemic and there appeared to be a slightly greater effect of VA supplementation on hemoglobin in those populations. All but two of the studies we identified with outcomes related to maternal hemoglobin or anemia provided iron to all women, so it was not possible to examine potential synergistic effects of VA in the presence or absence of iron supplementation.
The mechanisms through which VA affects anemia remain largely unclear, though a number of hypotheses have been proposed including (1) a potential role of VA in mobilizing iron stores from the liver (2) increasing erythropoiesis (3) decreasing the “anemia of inflammation” by increasing circulating iron through reducing infection and (4) increasing iron absorption.59, 61 Studies of the effects of VA supplementation on concentrations of erythropoietin, a hormone that stimulates production of red blood cells by the bone marrow, have been mixed, with one in pregnant women in Malawi showing no effect, another in preschool aged children in a malarial area of Tanzania a showing an decrease in concentrations, and a third in a non-malarial endemic area showing an increase in erythropoietin concentrations62–64.
Although we did not observe a statistically significant improvement in neonatal/infant anemia associated with supplementation in our pooled analysis of two trials of HIV+ women, a trend toward statistical significance did suggest the possibility of such an effect. However, a previous study in Zimbabwe noted no effect of high dose VA supplementation (400,000 IU) to women postpartum or 50,000 IU to infants on infant hemoglobin or anemia, and another trial from Indonesia noted a similar content of iron in breastmilk in women who were supplemented with β-C vs. those who were not65, 66. It therefore seems likely that any benefits of VA supplementation during pregnancy on infant hemoglobin are more likely to be the result of improving the iron endowment at birth rather than through breastmilk transfer of iron or VA, and that these benefits probably decrease over time. In a sub-study of 728 infants of mothers participating in the large Nepal trial (published in abstract form), a 2 g/L increase in infant hemoglobin at 3 months was observed in the intervention group relative to controls suggesting that it is possible that effects may last at least this long.67
Neonatal and Infant mortality, morbidity, and growth
Pooled estimates for neonatal and infant mortality provide strong evidence that VA/βC supplementation during pregnancy at the doses tested does not appear to reduce neonatal or infant mortality. This is consistent with a meta-analysis showing no effect of maternal postpartum supplementation on mortality, although possible benefits of neonatal supplementation have not been ruled out and several trials are underway to test this hypothesis.68, 69 As with maternal mortality, findings of an effect of supplementation on infant mortality specifically within the subset of infants born to night blind women support recommendations that this condition be treated with VA supplements.5
While VA is known to have an effect on child growth, the precise impact has been difficult to quantify, as VA deficiency often occurs in conjunction with other growth-limiting deficiencies which are often the true “growth-limiting factor.”6 We found little evidence to suggest that VA supplementation during pregnancy affects linear growth or weight gain during infancy, though one limitation of most of the studies we identified through our search was that they examined the effect of supplementation on mean growth rather than indicators more reflective of growth faltering,
HIV transmission
Trials of VA supplementation during pregnancy on risk of HIV transmission from mother to child were initiated based on evidence from observational studies that low maternal serum retinol was associated with increased risk of infant mortality, increased transmission of HIV from mother to child, and greater concentrations of HIV-1 in breast milk and genital tract secretions.8, 10, 16, 29, 41, 43, 70–73 None of these trials showed a reduction in HIV transmission associated with VA supplementation and the trial in Tanzania suggested a greater increase associated with supplementation during pregnancy and lactation, a finding that was supported by a trial in Zimbabwe of high dose postpartum and/or infant supplementation.41, 71
A number of plausible mechanisms have been proposed for potential increased transmission74. Evidence from a prospective cohort study in the United States suggested a U-shaped relationship between dietary intake of VA and HIV disease progression and mortality.75, 76 Some in-vitro studies suggest that VA could increase replication of HIV-1 and increase susceptibility of monocytes and macrophages to HIV infection through increased expression of CCR5 receptors.77 Recent evidence from the Tanzanian trial showed that VA/βC supplementation was associated with increased risk of subclinical mastitis and increased HIV viral load in breast-milk.78, 79 Thus, caution is warranted before initiating VA supplementation programs to pregnant women in HIV endemic areas.
Limitations
We searched multiple sources including conference abstract books for relevant reviews but it is possible that we may have missed relevant studies or that publication bias may have influenced the results of our meta-analysis. Our analysis assumes that pooling of different forms and dosages of vitamin A across different settings and contexts is appropriate—it is possible that different forms of VA may have different effects depending on the extent of deficiency and other contextual factors. While there are undoubtedly many different plausible explanations for apparent heterogeneity in results for some outcomes, our ability to identify sources of heterogeneity was limited by the small number of published studies. The recent Cochrane review on maternal vitamin A supplementation during pregnancy provides complementary disaggregated analysis to ours that may be useful in examining sources of heterogeneity including by concomitant micronutrient supplementation, dosage, and duration of intervention.80
Conclusions and future research directions
Our review examined the effects of VA/βC supplementation on a broad range of adverse perinatal, maternal, and neonatal outcomes prevalent in low income countries. In pooled analysis, we found little evidence of an overall effect of VA supplementation on either maternal or neonatal/infant mortality although we found significant heterogeneity in estimates of the effect on maternal mortality. VA/βC supplementation appears to also improve hematologic status of women during pregnancy in the presence of concomitant supplementation with iron and folate. Supplementation may also reduce risk of LBW among HIV-positive women, although in areas of high HIV prevalence these benefits should be weighed against the potential risk of increased risk of transmission from mother to child. Findings of no adverse effect on outcomes including stillbirth, fetal loss, miscarriage, support the safety of supplements at levels tested. The evidence level for some outcomes remains low or moderate, and more studies are needed to be able to better understand potential sources of heterogeneity potentially attributable to dosage, HIV status, VA status, interaction with other nutrients, and other contextual factors.
Supplementary Material
Supplementary Figure 1. Forest plot of the effects of vitamin A and/or β-C on mean birthweight
Supplementary Figure 2. Forest plot of the effects of lycopene supplementation on mean birthweight (kg)
Supplementary Figure 3. Forest plot of the effects of lycopene supplementation on mean gestational age at delivery (weeks)
Supplementary Figure 4. Forest plot of the effects of vitamin A and/or β-C on stillbirth
Supplementary Figure 5. Forest plot of the effects of vitamin A and/or β-C on miscarriage
Supplementary Figure 6. Forest plot of the effects of vitamin A and/or β-C on fetal loss
Supplementary Figure 7. Forest plot of the effect of lycopene supplementation on pre-eclampsia
Supplementary Figure 8. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on severe anemia during pregnancy (<8 or 8.5 g/dL)
Supplementary Figure 9. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on follow-up hemoglobin during pregnancy (g/dL)
Supplementary Figure 10. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on change in hemoglobin during pregnancy (g/dL)
Supplementary Figure 11. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on mean maternal postpartum hemoglobin (4–6 months postpartum)
Supplementary Figure 12. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on neonatal/infant anemia (Hb<11.0 g/dL)
Supplementary Figure 13. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on mean infant hemoglobin at 4–6 months
Supplementary Figure 14. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on mean weight-for-age z score at 6 months
Supplementary Figure 15. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on mean height-for-age z score at 6 months
Supplementary Table 1. Search strategy and keywords
Supplementary Table 2. Evaluation of the quality of individual trials for potential inclusion in the meta-analysis using GRADE criteria
Table 3.
Summary of findings and overall assessment of quality of evidence, by outcome
| Quality Assessment | Summary of Findings | ||||||
|---|---|---|---|---|---|---|---|
| No. studies and study design | Heterogeneity of results? | Consistent size of effect? | Generalizable to intervention of interest? | Other sources of bias (e.g., major limitations in study design) | N | Statistical method | Effect estimate [95% CI] |
| Small for gestational age, Overall quality of evidence grade=Low | |||||||
| 2 RCTs27,9 (both in HIV positive women) | Low (I2=0%, p=0.51); Neither study showed a significant effect | Both studies null | Both studies provided the same supplement (5,000 IU Vit. A+30 mg β-C+postpartum 200,000 IU. | None | 1387 | RR (IV, Fixed) | 0.89 [0.68, 1.17] |
| Low birthweight <2.5 kg, Overall quality of evidence grade=Low | |||||||
| 5 RCTs9, 27, 29, 49, 87 (3 in HIV+ women) | Moderate (I2=20%, p=0.29); 1 study showed significant effect | All but 1 study had protective direction of effect | Different supplements: VA+ βC, VA, Palm oil. Iron-folate, malaria prophylaxis varied across trials. | All but one study were of high or moderate grade quality | 2254 | Risk Ratio (IV, Fixed, 95% CI) | 0.83 [0.67, 1.01] HIV+: 0.79 [0.64, 0.99] HIV−: 1.11 [0.61, 2.01] |
| Very low birthweight <2.0 kg, HIV-positive: Overall quality of evidence grade=Low | |||||||
| 2 RCTs27,9 (both in HIV-positive women) | Low (I2=0%, p=0.49); Neither study showed a significant effect | Both studies null, effect estimates towards protective | Both used the same supplement (5,000 IU Vit. A+30 mg β-C). | None, all were high grade quality | 1486 | Risk Ratio (IV, Fixed, 95% CI) | 0.73 [0.41, 1.29] |
| Mean birthweight (kg): Overall quality of evidence grade=Moderate | |||||||
| 7 RCT’sb, 9, 27, 29, 49, 87, 92 (3 in HIV positive women) | High (I2=53%, p=0.05); 2 studies showed significant effects in opposite directions | 5 were null, 2 showed opposite effects. | Supplements used included VA, VA+βC, βC, and Palm oil as a source of βC. | All but one study were of high or moderate grade quality | 2417 | MD IV, Random | 0.03 [−0.04, 0.10]kg HIV+: 0.04 [−0.01, 0.08] HIV−: 0.02 [−0.17, 0.21] |
| Lycopene supplementation on mean birthweight (kg): Overall quality of evidence grade= Very low | |||||||
| 2 RCTs31, 32 | High (I2=65%, p=0.09); | Both studies null, RR’s in opposite directions | One study supplemented with 2 mg/day lycopene, the other with 4 mg/day | Both studies had low grade quality, neither used intent-to treat analysis | 410 | MD IV Random | 0.03 kg [−0.12, 0.18] |
| Preterm birth <37 weeks: Overall quality of evidence grade=High | |||||||
| 7 RCT’sb, 9, 24, 27, 87, 92, 93 | Low, (I2=8%, p=0.37) | One study protective, 6 null | Two studies in HIV+ women; 5 in HIV-negative; Differences in supplement type/dosage | All but one study were of high or moderate quality | 19,799a | Risk Ratio (IV, Fixed, 95% CI) | Overall 0.99 [0.88, 1.10] HIV+: 0.93 [0.75, 1.14] HIV−: 1.01 [0.89, 1.15] |
| Early preterm birth <34 weeks: Overall quality of evidence grade=Low | |||||||
| 2 RCT’s27,9 (both in HIV-positive women) | High, (I2=78%, p=0.03); | No, one study protective, one null | Both studies used the same supplement (VA+βC) | Both studies of high GRADE quality | 1513 | RR IV, Random | HIV+ 0.65 [0.20, 2.11] |
| Lycopene, mean gestational age at delivery: Overall quality of evidence grade=Very low | |||||||
| 2 RCTs31, 32 | (I2=94%, P<0.0001); | 1 null; one positive | 1 with 2 mg/day lycopene, the other with 4 mg/day | Both low GRADE quality, neither used intent-to treat analysis | 410 | MD IV Random | 0.40 weeks [−1.1, 1.9] |
| Stillbirth: Overall quality of evidence grade=High | |||||||
| 5 RCT’s (2 HIV+) 9, 25, 29, 88, 94 | Low (I2=0%, p=0.98); All studies null | Yes, all studies null | Variation in supplements used; VA, VA+ βC, | All studies of high GRADE quality | 106,894a | RR IV Fixed | 1.03 [0.97, 1.10] HIV+ 1.07 [0.66, 1.74] HIV− 1.03 [0.97, 1.10] |
| Fetal loss: Overall quality of evidence grade=High | |||||||
| 7 RCT’s (3 HIV+) 9, 25, 27, 29, 54, 88, 94 | Low (I2=0%, p=0.78); All studies null | Yes, all studies null | Variation in supplements used; | All studies of high GRADE quality | 113,207a | RR IV Fixed | 0.99 [0.95, 1.04] HIV+ 0.92 [0.50, 1.67] HIV− 0.99 [0.95, 1.04] |
| Miscarriage: Overall quality of evidence grade=Moderate | |||||||
| 4 RCT’s (2 HIV+) 9, 24, 29, 88 | Low (I2=0%, p=0.58); All studies null | Yes, all studies null | Variation in supplements used; VA, βC, VA+ βC | All studies of high GRADE quality | 62,138a | RR IV Fixed | 0.99 [0.95, 1.04] HIV+ 0.92 [0.51, 1.67] HIV− 0.99 [0.95, 1.04] |
| Maternal pregnancy related mortality: Overall quality of evidence grade=High | |||||||
| 3 RCT’s25, 54, 93 | High, (I2=74%, p=0.02); 1 study showed protective effect, 2 were null | 1 large study protective; others null | Variation in supplements used; VA, VA+ βC, | All studies of high GRADE quality | 160,690a | RR IV Random | 0.86 [0.60, 1.24] |
| Maternal mortality due to sepsis: Overall quality of evidence grade=Low | |||||||
| 2 RCT’s54, 93 | Low: (I2=0%, P=0.72) | All studies null | Both were pooled estimates of VA and βC arms | All studies of high GRADE quality | 81,885a | RR IV Fixed | 0.67 [0.31, 1.46] |
| Maternal mortality due to hemorrhage: Overall quality of evidence grade=Low | |||||||
| 2 RCT’s54, 93 | Moderate: (I2=44%, p=0.18) | All studies null | Both were pooled estimates of VA and βC arms | All studies of high GRADE quality | 81,885a | RR IV Fixed | 1.33 [0.61, 2.91] |
| Maternal mortality due to eclampsia/pre-eclampsia: Overall quality of evidence grade=Low | |||||||
| 2 RCT’s54, 93 | High: (I2=77%, p=0.04); | All studies null | Both were pooled estimates of VA and βC arms | All studies of high GRADE quality | 81,885a | RR IV Random | 1.09 [0.24, 4.88] |
| Maternal mortality due to infection related causes: Overall quality of evidence grade=Low | |||||||
| 2 RCT’s54, 93 | Low: (I2=9%, p=0.29) | All studies null | Both were pooled estimates of VA and βC arms | All studies of high GRADE quality | 81,885a | RR IV Fixed | 0.93 [0.53, 1.61] |
| Lycopene supplementation on pre-eclampsia: Overall quality of evidence grade=Very low | |||||||
| 2 RCTs31, 32 | (I2=54%, p=0.14); | 1 protective, 1 null | 2 mg/day lycopene vs. 4 mg/day | Both low GRADE quality, neither intent-to treat | 410 | RR IV Fixed | 0.71 [0.44, 1.14] |
| Neonatal and early infant mortality: Overall quality of evidence grade= High | |||||||
| 4 RCT’s25, 27, 29, 88 (2 in HIV-positive women) | Moderate: overall (I2=40.3%. p=0.31); in HIV-negative I2=47%, p=0.17), in HIV− I2=0%, p=0.86). | All studies null. | Variation in supplements used; VA, βC, VA+ βC, | All studies of high GRADE quality | 91,022a | RR IV Fixed | 0.97 [0.90, 1.05] HIV+: 0.68 [0.39, 1.17] HIV−: 0.98 [0.90, 1.06] |
| Infant and neonatal mortality: Overall quality of evidence grade=High | |||||||
| 5 RCT’s25, 27, 29, 47, 88 | Low; (I2=0%); | All studies null | Variation in supplements used; VA, βC, VA+ βC; variation in postpartum supplements. | All studies of high GRADE quality | 132,903a | RR IV Fixed | 0.99 [0.94, 1.04] HIV+: 1.03 [0.79, 1.35] HIV−: 0.99 [0.94, 1.04] |
| HIV transmission from mother to child: Overall quality of evidence grade=Moderate | |||||||
| 3 RCT’s9, 27, 29 | High; (I2=75%, p=0.02); | 2 studies null, 1 study showed significant increase in HIV transmission | Variation in supplements used; two had VA+ βC, 1 had VA | All studies of high GRADE quality | 2022 | RR IV Random | HIV+: 1.05 [0.78, 1.41] |
| Maternal anemia (Hb<11 g/dL) during follow-up in pregnancy: Overall quality of evidence grade=High | |||||||
| 8 RCT’sb, 21, 22, 24, 64, 86, 87 | High: overall (I2=54%, p=0.03%), (Anemic women I2=74%, p=0.004); (Non anemic women I2=0%) | All studies had effect estimates in a prot. direction 3/8 sig. | Variation in supplements used; VA, Differences in provision of iron-folate supplements | All studies but 2 of high GRADE quality | 1587 | RR IV Random | 0.81 [0.69, 0.94] Anemic: 0.72 [0.53, 0.99] Unscreened for anemia: 0.83 [0.69, 0.95] |
| Maternal severe anemia <8.0 or 8.5 g/dL) during pregnancy: Overall quality of evidence grade=Low | |||||||
| 3 RCT’sb, 21, 24 | Low; (I2=0%, p=0.03) | All studies null | Both trials used vitamin A | Both studies of high GRADE quality | 961 | RR IV Fixed | 0.93 [0.60, 1.46] |
| Mean maternal hemoglobin during follow-up in pregnancy: Overall quality of evidence grade=Moderate | |||||||
| 8 RCT’sb22–24, 49, 86, 87 | Some; (I2=17%, p=0.29) | All studies null but one factorial trial null where both comparisons showed significantly positive effects | Five studies used vitamin A; one used palm oil. All women except those in placebo arms of two factorial trials received iron folate | All but one study of high grade quality | 1034 | MD IV Fixed | 0.35 [0.24, 0.45] |
| Change in maternal hemoglobin during pregnancy: Overall quality of evidence grade=Moderate | |||||||
| 8 RCT’s21, 24, 64, 86, 87 | Some; (I2=15%) | All studies but the factorial trial null | Variation in supplements used; all had iron-folate | All but one study of high grade quality | 1131 | MD IV Random | 0.19 [0.06, 0.33] |
| Mean postpartum maternal hemoglobin (4–6 weeks postpartum): Overall quality of evidence grade=Moderate | |||||||
| 4 RCT’s49, 86, 92 | Low; (I2=0%, p=0.31) | All studies null, | 1 study with 2 arms used βC, 2 studies used VA | All but one study of high grade quality | 365 | MD IV FIXED | 0.01 [−0.24, 0.25] |
| Infant anemia: Overall quality of evidence grade=Low | |||||||
| 2 RCT’s29, 33 (Both in HIV+ women) | Yes; (I2=74%, p=0.05); | 1 study protective, 1 null | Variation in supplements used | All studies of high GRADE quality | 894 | RR IV Random | HIV+ 0.71 [0.49, 1.03], p=0.07 |
| Infant hemoglobin at 4–6 months (g/dL): Overall quality of evidence grade=Low | |||||||
| 2 RCT’s92, 95 (1 factorial) | No; (I2=0%, p=0.96) | All three studies null | Variation in supplements used; | All studies of high GRADE | 284 | MD IV Fixed | −0.13 [−2.29, 2.03] |
| Infant weight-for-age z score at 6 months: Overall quality of evidence grade=Low | |||||||
| 2 RCT’s40, 92 (2 factorial) | Some; (I2=22%) | Yes, all studies null | Variation in supplements used | All studies of high GRADE | 1022 | MD IV Fixed | 0.07 [−0.07, 0.22] |
| Infant height-for-age z score at 6 months: Overall quality of evidence grade=Low | |||||||
| 2 RCT’s40, 92 (2 factorial) | No; (I2=0%) | Yes, all studies null | Variation in supplements used | All studies of high GRADE | 1022 | MD IV Fixed | 0.01 [−0.12, 0.13] |
Effective sample size for analysis is actually smaller due to inclusion of cluster randomized trials in the estimation of variance
Different arms of factorial trials counted as separate trials, prot=protective
Acknowledgments
Acknowledgements and support: We gratefully acknowledge the additional data provided by Dr. Anna Coutsoudis and Dr. Louise Kuhn for inclusion in this analysis and the feedback received from Dr. Reynaldo Martorell and Dr. Beth Imhoff-Kunsch on an earlier draft of this manuscript. Andrew Thorne-Lyman is supported by NIH training grant T32 #DK 007703 and a Julius B. Richmond Fellowship from the Center on the Developing Child at Harvard University.
References
- 1.WHO. WHO Global Database on Vitamin A deficiency. Geneva: World Health Organization; 2009. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. [cited. Available from: http://whqlibdoc.who.int/publications/2009/9789241598019_eng.pdf. [Google Scholar]
- 2.West KP., Jr Extent of vitamin A deficiency among preschool children and women of reproductive age. Journal of Nutrition. 2002;132:2857S–2866S. doi: 10.1093/jn/132.9.2857S. [DOI] [PubMed] [Google Scholar]
- 3.Christian P, West KP, Jr, Khatry SK, Katz J, LeClerq S, Pradhan EK, et al. Vitamin A or beta-carotene supplementation reduces but does not eliminate maternal night blindness in Nepal. Journal of Nutrition. 1998;128:1458–1463. doi: 10.1093/jn/128.9.1458. [DOI] [PubMed] [Google Scholar]
- 4.Christian P, West KP, Jr, Khatry SK, Katz J, Shrestha SR, Pradhan EK, et al. Night blindness of pregnancy in rural Nepal--nutritional and health risks. International Journal of Epidemiology. 1998;27:231–237. doi: 10.1093/ije/27.2.231. [DOI] [PubMed] [Google Scholar]
- 5.Christian P, West KP, Jr, Khatry SK, LeClerq SC, Kimbrough-Pradhan E, Katz J, et al. Maternal night blindness increases risk of mortality in the first 6 months of life among infants in Nepal. Journal of Nutrition. 2001;131:1510–1512. doi: 10.1093/jn/131.5.1510. [DOI] [PubMed] [Google Scholar]
- 6.Sommer A, KW . Vitamin A deficiency: Health, Survival, and Vision. New York: Oxford University Press; 1996. [Google Scholar]
- 7.World Health Organization and Food and Agriculture Organization. Vitamin and mineral requirements in human nutrition: report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21–30 September 1998. 2. Geneva: World Health Organization; 2004. [Google Scholar]
- 8.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Randomized trial testing the effect of vitamin A supplementation on pregnancy outcomes and early mother-to-child HIV-1 transmission in Durban, South Africa. South African Vitamin A Study Group. AIDS. 1999;13:1517–1524. doi: 10.1097/00002030-199908200-00012. [DOI] [PubMed] [Google Scholar]
- 9.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. The Lancet. 1998;351:1477–1482. doi: 10.1016/s0140-6736(98)04197-x. [DOI] [PubMed] [Google Scholar]
- 10.Villamor E, Fawzi WW. Vitamin A supplementation: implications for morbidity and mortality in children. Journal of Infectious Diseases. 2000;182 (Suppl 1):S122–133. doi: 10.1086/315921. [DOI] [PubMed] [Google Scholar]
- 11.Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011;343:d5094. doi: 10.1136/bmj.d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green H, Pindar D, Davis G, Melanby E. Diet as a prophylactic agent against puerperal sepsis-with special reference to vitamin A as an anti-infective agent. BMJ. 1931:595–598. doi: 10.1136/bmj.2.3691.595. ii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semba RD. Vitamin A as “anti-infective” therapy, 1920–1940. Journal of Nutrition. 1999;129:783–791. doi: 10.1093/jn/129.4.783. [DOI] [PubMed] [Google Scholar]
- 14.Mellanby E, Green H. Vitamin A as an anti-infective agent. Its use in the treatment of puerperal septicaemia. The British Medical Journal. 1929;1:984–986. doi: 10.1136/bmj.1.3569.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West KP, Jr, Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, et al. Double blind, cluster randomised trial of low dose supplementation with vitamin A or beta carotene on mortality related to pregnancy in Nepal. The NNIPS-2 Study Group. BMJ. 1999;318:570–575. doi: 10.1136/bmj.318.7183.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semba RD, Miotti PG, Chiphangwi JD, Saah AJ, Canner JK, Dallabetta GA, et al. Maternal vitamin A deficiency and mother-to-child transmission of HIV-1. The Lancet. 1994;343:1593–1597. doi: 10.1016/s0140-6736(94)93056-2. [DOI] [PubMed] [Google Scholar]
- 17.Suharno D, West CE, Muhilal, Logman MH, de Waart FG, Karyadi D, et al. Cross-sectional study on the iron and vitamin A status of pregnant women in West Java, Indonesia. American Journal of Clinical Nutrition. 1992;56:988–993. doi: 10.1093/ajcn/56.6.988. [DOI] [PubMed] [Google Scholar]
- 18.Walker N, Fischer-Walker C, Bryce J, Bahl R, Cousens S. Standards for CHERG reviews of intervention effects on child survival. International Journal of Epidemiology. 2010;39 (Suppl 1):i21–31. doi: 10.1093/ije/dyq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Nordic Cochrane Centre TCC. Review Manager (RevMan) Version 5.1. Copenhagen: 2011. [Google Scholar]
- 20.Dijkhuizen MA, Wieringa FT, West CE, Muhilal Zinc plus beta-carotene supplementation of pregnant women is superior to beta-carotene supplementation alone in improving vitamin A status in both mothers and infants. American Journal of Clinical Nutrition. 2004;80:1299–1307. doi: 10.1093/ajcn/80.5.1299. [DOI] [PubMed] [Google Scholar]
- 21.Ma AG, Schouten EG, Zhang FZ, Kok FJ, Yang F, Jiang DC, et al. Retinol and riboflavin supplementation decreases the prevalence of anemia in Chinese pregnant women taking iron and folic Acid supplements. Journal of Nutrition. 2008;138:1946–1950. doi: 10.1093/jn/138.10.1946. [DOI] [PubMed] [Google Scholar]
- 22.Suharno D, West CE, Muhilal, Karyadi D, Hautvast JG. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. The Lancet. 1993;342:1325–1328. doi: 10.1016/0140-6736(93)92246-p. [DOI] [PubMed] [Google Scholar]
- 23.Tanumihardjo SA. Vitamin A and iron status are improved by vitamin A and iron supplementation in pregnant Indonesian women. Journal of Nutrition. 2002;132:1909–1912. doi: 10.1093/jn/132.7.1909. [DOI] [PubMed] [Google Scholar]
- 24.van den Broek NR, White SA, Flowers C, Cook JD, Letsky EA, Tanumihardjo SA, et al. Randomised trial of vitamin A supplementation in pregnant women in rural Malawi found to be anaemic on screening by HemoCue. British Journal of Obstetrics and Gynaecology. 2006;113:569–576. doi: 10.1111/j.1471-0528.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 25.Kirkwood BR, Hurt L, Amenga-Etego S, Tawiah C, Zandoh C, Danso S, et al. Effect of vitamin A supplementation in women of reproductive age on maternal survival in Ghana (ObaapaVitA): a cluster-randomised, placebo-controlled trial. The Lancet. 2010;375:1640–1649. doi: 10.1016/S0140-6736(10)60311-X. [DOI] [PubMed] [Google Scholar]
- 26.West K, Christian P, Labrique A, Rashid M, Shamim A, Klemm R, et al. Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: A cluster randomized trial. Journal of the American Medical Association. 2011;305:1986–1995. doi: 10.1001/jama.2011.656. [DOI] [PubMed] [Google Scholar]
- 27.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Randomized trial testing the effect of vitamin A supplementation on pregnancy outcomes and early mother-to-child HIV-1 transmission in Durban, South Africa. AIDS. 1999;13:1517–24. doi: 10.1097/00002030-199908200-00012. [DOI] [PubMed] [Google Scholar]
- 28.Coutsoudis A, Moodley D, Pillay K, Harrigan R, Stone C, Moodley J, et al. Effects of vitamin A supplementation on viral load in HIV-1-infected pregnant women. Journal of acquired immune deficiency syndromes and human retrovirology. 1997;15:86–87. doi: 10.1097/00042560-199705010-00015. [DOI] [PubMed] [Google Scholar]
- 29.Kumwenda N, Miotti PG, Taha TE, Broadhead R, Biggar RJ, Jackson JB, et al. Antenatal vitamin A supplementation increases birth weight and decreases anemia among infants born to human immunodeficiency virus-infected women in Malawi. Clinical Infectious Diseases. 2002;35:618–624. doi: 10.1086/342297. [DOI] [PubMed] [Google Scholar]
- 30.Dreyfuss M, KW, Katz J, LeClerq S, Pradhan E, Adhikari R, et al. Effects of maternal vitamin A or beta-carotene supplementation on intrauterine/neonatal and early infant growth in Nepal. Report of the XVIII International Vitamin A Consultative Group Meeting; 1997; Cairo, Egypt. IVACG Secretariat, ILSI Research Foundation; 1997. p. 139. [Google Scholar]
- 31.Banerjee S, Jeyaseelan S, Guleria R. Trial of lycopene to prevent pre-eclampsia in healthy primigravidas: results show some adverse effects. Journal of Obstetrics and Gynaecology Research. 2009;35:477–482. doi: 10.1111/j.1447-0756.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- 32.Sharma JB, Kumar A, Malhotra M, Arora R, Prasad S, Batra S. Effect of lycopene on pre-eclampsia and intra-uterine growth retardation in primigravidas. International Journal of Gynecology & Obstetrics. 2003;81:257–262. doi: 10.1016/s0020-7292(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 33.Fawzi WW, Msamanga GI, Kupka R, Spiegelman D, Villamor E, Mugusi F, et al. Multivitamin supplementation improves hematologic status in HIV-infected women and their children in Tanzania. American Journal of Clinical Nutrition. 2007;85:1335–1343. doi: 10.1093/ajcn/85.5.1335. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt MK, Muslimatun S, Schultink W, West CE, Hautvast JG. Randomised double-blind trial of the effect of vitamin A supplementation of Indonesian pregnant women on morbidity and growth of their infants during the first year of life. European Journal of Clinical Nutrition. 2002;56:338–346. doi: 10.1038/sj.ejcn.1601318. [DOI] [PubMed] [Google Scholar]
- 35.Fawzi WW, Msamanga GI, Wei R, Spiegelman D, Antelman G, Villamor E, et al. Effect of providing vitamin supplements to human immunodeficiency virus-infected, lactating mothers on the child’s morbidity and CD4+ cell counts. Clinical Infectious Diseases. 2003;36:1053–1062. doi: 10.1086/374223. [DOI] [PubMed] [Google Scholar]
- 36.Villamor E, Msamanga G, Saathoff E, Fataki M, Manji K, Fawzi WW. Effects of maternal vitamin supplements on malaria in children born to HIV-infected women. American Journal of Tropical Medicine and Hygiene. 2007;76:1066–1071. [PubMed] [Google Scholar]
- 37.Adhikari R, Katz J, Pradhan EK, LeClerq S, Connor PB, Khatry S, et al. Impact of maternal vitamin A or beta-carotene supplementation on infectious morbidity during early infancy in Nepal. Report of the XVII International Vitamin A Consultative Group Meeting; 1997 22–26 September 1997; Cairo, Egypt. ILSI Research Foundation; 1997. [Google Scholar]
- 38.Wieringa FT, Dijkhuizen MA, Muhilal, Van der Meer JW. Maternal micronutrient supplementation with zinc and beta-carotene affects morbidity and immune function of infants during the first 6 months of life. European Journal of Clinical Nutrition. 2010 doi: 10.1038/ejcn.2010.115. [DOI] [PubMed] [Google Scholar]
- 39.Muslimatun S, Schmidt MK, West CE, Schultink W, Gross R, Hautvast JG. Determinants of weight and length of Indonesian neonates. European Journal of Clinical Nutrition. 2002;56:947–951. doi: 10.1038/sj.ejcn.1601439. [DOI] [PubMed] [Google Scholar]
- 40.Villamor E, Saathoff E, Bosch RJ, Hertzmark E, Baylin A, Manji K, et al. Vitamin supplementation of HIV-infected women improves postnatal child growth. American Journal of Clinical Nutrition. 2005;81:880–888. doi: 10.1093/ajcn/81.4.880. [DOI] [PubMed] [Google Scholar]
- 41.Humphrey JH, Iliff PJ, Marinda ET, Mutasa K, Moulton LH, Chidawanyika H, et al. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. Journal of Infectious Diseases. 2006;193:860–871. doi: 10.1086/500366. [DOI] [PubMed] [Google Scholar]
- 42.Tammela O, Aitola M, Ikonen S. Cord blood concentrations of vitamin A in preterm infants. Early Human Development. 1999;56:39–47. doi: 10.1016/s0378-3782(99)00032-8. [DOI] [PubMed] [Google Scholar]
- 43.Semba RD, Miotti PG, Chiphangwi JD, Liomba G, Yang LP, Saah AJ, et al. Infant mortality and maternal vitamin A deficiency during human immunodeficiency virus infection. Clinical Infectious Diseases. 1995;21:966–972. doi: 10.1093/clinids/21.4.966. [DOI] [PubMed] [Google Scholar]
- 44.Semba RD, Miotti P, Chiphangwi JD, Henderson R, Dallabetta G, Yang LP, et al. Maternal vitamin A deficiency and child growth failure during human immunodeficiency virus infection. Journal of acquired immune deficiency syndromes and human retrovirology. 1997;14:219–222. doi: 10.1097/00042560-199703010-00004. [DOI] [PubMed] [Google Scholar]
- 45.Burns DN, FitzGerald G, Semba R, Hershow R, Zorrilla C, Pitt J, et al. Vitamin A deficiency and other nutritional indices during pregnancy in human immunodeficiency virus infection: prevalence, clinical correlates, and outcome. Women and Infants Transmission Study Group. Clinical Infectious Diseases. 1999;29:328–334. doi: 10.1086/520210. [DOI] [PubMed] [Google Scholar]
- 46.Kongnyuy EJ, Wiysonge CS, Shey MS. A systematic review of randomized controlled trials of prenatal and postnatal vitamin A supplementation of HIV-infected women. International Journal of Gynecology & Obstetrics. 2009;104:5–8. doi: 10.1016/j.ijgo.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 47.Wiysonge CS, Shey M, Kongnyuy EJ, Sterne JA, Brocklehurst P. Vitamin A supplementation for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database of Systematic Reviews. 2011:CD003648. doi: 10.1002/14651858.CD003648.pub3. [DOI] [PubMed] [Google Scholar]
- 48.Friis H, Gomo E, Nyazema N, Ndhlovu P, Krarup H, Kaestel P, et al. Effect of multimicronutrient supplementation on gestational length and birth size: a randomized, placebo-controlled, double-blind effectiveness trial in Zimbabwe. American Journal of Clinical Nutrition. 2004;80:178–184. doi: 10.1093/ajcn/80.1.178. [DOI] [PubMed] [Google Scholar]
- 49.Cox SE, Staalsoe T, Arthur P, Bulmer JN, Tagbor H, Hviid L, et al. Maternal vitamin A supplementation and immunity to malaria in pregnancy in Ghanaian primigravids. Tropical Medicine & International Health. 2005;10:1286–1297. doi: 10.1111/j.1365-3156.2005.01515.x. [DOI] [PubMed] [Google Scholar]
- 50.Katz J, West KP, Jr, Khatry SK, LeClerq SC, Christian P, Pradhan EK, et al. Twinning rates and survival of twins in rural Nepal. International Journal of Epidemiology. 2001;30:802–807. doi: 10.1093/ije/30.4.802. [DOI] [PubMed] [Google Scholar]
- 51.WHO. Guideline: Vitamin A supplementation in pregnant women. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 52.West KP, Jr, Christian P, Katz J, Labrique A, Klemm R, Sommer A. Effect of vitamin A supplementation on maternal survival. The Lancet. 2010;376:873–874. doi: 10.1016/S0140-6736(10)61411-0. author reply 874. [DOI] [PubMed] [Google Scholar]
- 53.Christian P, West KP, Labrique A, Klemm R, et al. Effects of maternal vitamin A or beta-carotene supplementation on maternal and infant mortality in rural Bangladesh: the JIVITA-1 trial. Micronutrient Forum; Istambul, Turkey. 2007. [Google Scholar]
- 54.West KP, Jr, Christian P, Labrique AB, Rashid M, Shamim AA, Klemm RD, et al. Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: a cluster randomized trial. Journal of the American Medical Association. 2011;305:1986–1995. doi: 10.1001/jama.2011.656. [DOI] [PubMed] [Google Scholar]
- 55.de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. Journal of Nutrition. 2002;132:2895S–2901S. doi: 10.1093/jn/132.9.2895S. [DOI] [PubMed] [Google Scholar]
- 56.Christian P, West KP, Jr, Khatry SK, Kimbrough-Pradhan E, LeClerq SC, Katz J, et al. Night blindness during pregnancy and subsequent mortality among women in Nepal: effects of vitamin A and beta-carotene supplementation. American Journal of Epidemiology. 2000;152:542–547. doi: 10.1093/aje/152.6.542. [DOI] [PubMed] [Google Scholar]
- 57.Labrique AB, Christian P, Klemm RD, Rashid M, Shamim AA, Massie A, et al. A cluster-randomized, placebo-controlled, maternal vitamin A or beta-carotene supplementation trial in Bangladesh: design and methods. Trials. 2011;12:102. doi: 10.1186/1745-6215-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semba RD, Bloem MW. The anemia of vitamin A deficiency: epidemiology and pathogenesis. European Journal of Clinical Nutrition. 2002;56:271–281. doi: 10.1038/sj.ejcn.1601320. [DOI] [PubMed] [Google Scholar]
- 59.West K, Gernand A, Sommer A. Vitamin A in nutritional anemia. In: Kraemer K, Zimmermann M, editors. Nutritional Anemia. Basel, Switzerland: Sight and Life Press; 2007. pp. 133–153. [Google Scholar]
- 60.Radhika MS, Bhaskaram P, Balakrishna N, Ramalakshmi BA, Devi S, Kumar BS. Effects of vitamin A deficiency during pregnancy on maternal and child health. BJOG. 2002;109:689–693. doi: 10.1111/j.1471-0528.2002.01010.x. [DOI] [PubMed] [Google Scholar]
- 61.Bloem MW. Interdependence of vitamin A and iron: an important association for programmes of anaemia control. Proc Nutr Soc. 1995;54:501–508. doi: 10.1079/pns19950018. [DOI] [PubMed] [Google Scholar]
- 62.Zimmermann MB, Biebinger R, Rohner F, Dib A, Zeder C, Hurrell RF, et al. Vitamin A supplementation in children with poor vitamin A and iron status increases erythropoietin and hemoglobin concentrations without changing total body iron. American Journal of Clinical Nutrition. 2006;84:580–586. doi: 10.1093/ajcn/84.3.580. [DOI] [PubMed] [Google Scholar]
- 63.Cusick SE, Tielsch JM, Ramsan M, Jape JK, Sazawal S, Black RE, et al. Short-term effects of vitamin A and antimalarial treatment on erythropoiesis in severely anemic Zanzibari preschool children. American Journal of Clinical Nutrition. 2005;82:406–412. doi: 10.1093/ajcn.82.2.406. [DOI] [PubMed] [Google Scholar]
- 64.Semba RD, Kumwenda N, Taha TE, Mtimavalye L, Broadhead R, Garrett E, et al. Impact of vitamin A supplementation on anaemia and plasma erythropoietin concentrations in pregnant women: a controlled clinical trial. European Journal of Haematology. 2001;66:389–395. doi: 10.1034/j.1600-0609.2001.066006389.x. [DOI] [PubMed] [Google Scholar]
- 65.Miller MF, Stoltzfus RJ, Iliff PJ, Malaba LC, Mbuya NV, Humphrey JH. Effect of maternal and neonatal vitamin A supplementation and other postnatal factors on anemia in Zimbabwean infants: a prospective, randomized study. American Journal of Clinical Nutrition. 2006;84:212–222. doi: 10.1093/ajcn/84.1.212. [DOI] [PubMed] [Google Scholar]
- 66.Muslimatun S, Schmidt MK, West CE, Schultink W, Hautvast JG, Karyadi D. Weekly vitamin A and iron supplementation during pregnancy increases vitamin A concentration of breast milk but not iron status in Indonesian lactating women. Journal of Nutrition. 2001;131:2664–2669. doi: 10.1093/jn/131.10.2664. [DOI] [PubMed] [Google Scholar]
- 67.Stoltzfus R, Dreyfuss M, Shrestha J, Khatry S, Schulze K, KW Effect of Maternal vitamin A or beta-carotene supplementation on iron-deficiency anemia in nepalese pregnant women, post-partum mothers, and infants. Report of the XVIII International Vitamin A Consultative Group Meeting: Sustainable control of vitamin A deficiency; 1997 22–26 September; Cairo, Egypt. [Google Scholar]
- 68.Gogia S, Sachdev HS. Maternal postpartum vitamin A supplementation for the prevention of mortality and morbidity in infancy: a systematic review of randomized controlled trials. International Journal of Epidemiology. 2010;39:1217–1226. doi: 10.1093/ije/dyq080. [DOI] [PubMed] [Google Scholar]
- 69.Kirkwood B, Humphrey J, Moulton L, Martines J. Neonatal vitamin A supplementation and infant survival. The Lancet. 2010;376:1643–1644. doi: 10.1016/S0140-6736(10)61895-8. [DOI] [PubMed] [Google Scholar]
- 70.Greenberg BL, Semba RD, Vink PE, Farley JJ, Sivapalasingam M, Steketee RW, et al. Vitamin A deficiency and maternal-infant transmissions of HIV in two metropolitan areas in the United States. AIDS. 1997;11:325–332. doi: 10.1097/00002030-199703110-00010. [DOI] [PubMed] [Google Scholar]
- 71.Fawzi WW, Msamanga GI, Hunter D, Renjifo B, Antelman G, Bang H, et al. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS. 2002;16:1935–1944. doi: 10.1097/00002030-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 72.Nduati RW, John GC, Richardson BA, Overbaugh J, Welch M, Ndinya-Achola J, et al. Human immunodeficiency virus type 1-infected cells in breast milk: association with immunosuppression and vitamin A deficiency. Journal of Infectious Diseases. 1995;172:1461–1468. doi: 10.1093/infdis/172.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.John GC, Nduati RW, Mbori-Ngacha D, Overbaugh J, Welch M, Richardson BA, et al. Genital shedding of human immunodeficiency virus type 1 DNA during pregnancy: association with immunosuppression, abnormal cervical or vaginal discharge, and severe vitamin A deficiency. Journal of Infectious Diseases. 1997;175:57–62. doi: 10.1093/infdis/175.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fawzi WW. The benefits and concerns related to vitamin a supplementation. Journal of Infectious Diseases. 2006;193:756–759. doi: 10.1086/500369. [DOI] [PubMed] [Google Scholar]
- 75.Tang AM, Graham NM, Saah AJ. Effects of micronutrient intake on survival in human immunodeficiency virus type 1 infection. American Journal of Epidemiology. 1996;143:1244–1256. doi: 10.1093/oxfordjournals.aje.a008712. [DOI] [PubMed] [Google Scholar]
- 76.Tang AM, Graham NM, Kirby AJ, McCall LD, Willett WC, Saah AJ. Dietary micronutrient intake and risk of progression to acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus type 1 (HIV-1)-infected homosexual men. American Journal of Epidemiology. 1993;138:937–951. doi: 10.1093/oxfordjournals.aje.a116814. [DOI] [PubMed] [Google Scholar]
- 77.MacDonald KS, Malonza I, Chen DK, Nagelkerke NJ, Nasio JM, Ndinya-Achola J, et al. Vitamin A and risk of HIV-1 seroconversion among Kenyan men with genital ulcers. AIDS. 2001;15:635–639. doi: 10.1097/00002030-200103300-00014. [DOI] [PubMed] [Google Scholar]
- 78.Villamor E, Koulinska IN, Aboud S, Murrin C, Bosch RJ, Manji KP, et al. Effect of vitamin supplements on HIV shedding in breast milk. American Journal of Clinical Nutrition. 2010;92:881–886. doi: 10.3945/ajcn.2010.29339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arsenault JE, Aboud S, Manji KP, Fawzi WW, Villamor E. Vitamin supplementation increases risk of subclinical mastitis in HIV-infected women. Journal of Nutrition. 2010;140:1788–1792. doi: 10.3945/jn.110.122713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van den Broek N, Dou L, Othman M, Neilson JP, Gates S, Gulmezoglu AM. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database of Systematic Reviews. 2010:CD008666. doi: 10.1002/14651858.CD008666.pub2. [DOI] [PubMed] [Google Scholar]
- 81.Kawai K, Msamanga G, Manji K, Villamor E, Bosch RJ, Hertzmark E, et al. Sex differences in the effects of maternal vitamin supplements on mortality and morbidity among children born to HIV-infected women in Tanzania. British Journal of Nutrition. 2010;103:1784–1791. doi: 10.1017/S0007114509993862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. New England Journal of Medicine. 2004;351:23–32. doi: 10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- 83.Villamor E, Msamanga G, Spiegelman D, Antelman G, Peterson KE, Hunter DJ, et al. Effect of multivitamin and vitamin A supplements on weight gain during pregnancy among HIV-1-infected women. American Journal of Clinical Nutrition. 2002;76:1082–1090. doi: 10.1093/ajcn/76.5.1082. [DOI] [PubMed] [Google Scholar]
- 84.Merchant AT, Msamanga G, Villamor E, Saathoff E, O’Brien M, Hertzmark E, et al. Multivitamin supplementation of HIV-positive women during pregnancy reduces hypertension. Journal of Nutrition. 2005;135:1776–1781. doi: 10.1093/jn/135.7.1776. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt MK, Muslimatun S, West CE, Schultink W, Hautvast JG. Vitamin A and iron supplementation of Indonesian pregnant women benefits vitamin A status of their infants. British Journal of Nutrition. 2001;86:607–615. doi: 10.1079/bjn2001444. [DOI] [PubMed] [Google Scholar]
- 86.Muslimatun S, Schmidt MK, Schultink W, West CE, Hautvast JA, Gross R, et al. Weekly supplementation with iron and vitamin A during pregnancy increases hemoglobin concentration but decreases serum ferritin concentration in Indonesian pregnant women. Journal of Nutrition. 2001;131:85–90. doi: 10.1093/jn/131.1.85. [DOI] [PubMed] [Google Scholar]
- 87.Radhika MS, Bhaskaram P, Balakrishna N, Ramalakshmi BA. Red palm oil supplementation: a feasible diet-based approach to improve the vitamin A status of pregnant women and their infants. Food and Nutrition Bulletin. 2003;24:208–217. doi: 10.1177/156482650302400207. [DOI] [PubMed] [Google Scholar]
- 88.Katz J, West KP, Jr, Khatry SK, Pradhan EK, LeClerq SC, Christian P, et al. Maternal low-dose vitamin A or beta-carotene supplementation has no effect on fetal loss and early infant mortality: a randomized cluster trial in Nepal. American Journal of Clinical Nutrition. 2000;71:1570–1576. doi: 10.1093/ajcn/71.6.1570. [DOI] [PubMed] [Google Scholar]
- 89.Christian P, West KP, Jr, Khatry SK, Katz J, LeClerq SC, Kimbrough-Pradhan E, et al. Vitamin A or beta-carotene supplementation reduces symptoms of illness in pregnant and lactating Nepali women. Journal of Nutrition. 2000;130:2675–2682. doi: 10.1093/jn/130.11.2675. [DOI] [PubMed] [Google Scholar]
- 90.Christian P, West KP, Jr, Katz J, Kimbrough-Pradhan E, LeClerq SC, Khatry SK, et al. Cigarette smoking during pregnancy in rural Nepal. Risk factors and effects of beta-carotene and vitamin A supplementation. European Journal of Clinical Nutrition. 2004;58:204–211. doi: 10.1038/sj.ejcn.1601767. [DOI] [PubMed] [Google Scholar]
- 91.Christian P, Katz J, Wu L, Kimbrough-Pradhan E, Khatry SK, LeClerq SC, et al. Risk factors for pregnancy-related mortality: a prospective study in rural Nepal. Public Health. 2008;122:161–172. doi: 10.1016/j.puhe.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dijkhuizen MA, Wieringa FT, West CE, Muhilal Zinc plus beta-carotene supplementation of pregnant women is superior to beta-carotene supplementation alone in improving vitamin A status in both mothers and infants. American Journal of Clinical Nutrition. 2004;80:1299–107. doi: 10.1093/ajcn/80.5.1299. [DOI] [PubMed] [Google Scholar]
- 93.West KP, Jr, Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, et al. Double blind, cluster randomised trial of low dose supplementation with vitamin A or beta carotene on mortality related to pregnancy in Nepal. BMJ. 1999;318:570–75. doi: 10.1136/bmj.318.7183.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt MK, Muslimatun S, West CE, Schultink W, Hautvast JG. Vitamin A and iron supplementation of Indonesian pregnant women benefits vitamin A status of their infants. British Journal of Nutrition. 2001;86:607–15. doi: 10.1079/bjn2001444. [DOI] [PubMed] [Google Scholar]
- 95.Muslimatun S, Schmidt MK, West CE, Schultink W, Hautvast JG, Karyadi D. Weekly vitamin A and iron supplementation during pregnancy increases vitamin A concentration of breast milk but not iron status in Indonesian lactating women. Journal of Nutrition. 2001;131:2664–9. doi: 10.1093/jn/131.10.2664. [DOI] [PubMed] [Google Scholar]
- 96.Aggarwal A. Effect of vitamin A and iron supplementation on hemoglobin and serum vitamin A level in pregnancy. Indian Pediatrics. 1998;35:1148–1150. [PubMed] [Google Scholar]
- 97.Antartani R, Ashok K. Effect of lycopene in prevention of preeclampsia in high risk pregnant women. Journal of the Turkish German Gynecology Association. 2011;12:35–38. doi: 10.5152/jtgga.2011.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chawla PK, Puri R. Impact of nutritional supplements on hematological profile of pregnant women. Indian Pediatrics. 1995;32:876–880. [PubMed] [Google Scholar]
- 99.Wieringa FT, Dijkhuizen MA, Muhilal, Van der Meer JW. Maternal micronutrient supplementation with zinc and beta-carotene affects morbidity and immune function of infants during the first 6 months of life. European Journal of Clinical Nutrition. 2010;64:1072–1079. doi: 10.1038/ejcn.2010.115. [DOI] [PubMed] [Google Scholar]
- 100.Lietz G, Henry CJ, Mulokozi G, Mugyabuso JK, Ballart A, Ndossi GD, et al. Comparison of the effects of supplemental red palm oil and sunflower oil on maternal vitamin A status. American Journal of Clinical Nutrition. 2001;74:501–9. doi: 10.1093/ajcn/74.4.501. [DOI] [PubMed] [Google Scholar]
- 101.Panth M, Shatrugna V, Yasodhara P, Sivakumar B. Effect of vitamin A supplementation on haemoglobin and vitamin A levels during pregnancy. British Journal of Nutrition. 1990;64:351–8. doi: 10.1079/bjn19900037. [DOI] [PubMed] [Google Scholar]
- 102.Suprapto B, Widardo, Suhanantyo Effect of low-dosage vitamin A and riboflavin on iron-folate supplementation in anaemic pregnant women. Asia Pacific Journal of Clinical Nutrition. 2002;11:263–267. doi: 10.1046/j.1440-6047.2002.00310.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Forest plot of the effects of vitamin A and/or β-C on mean birthweight
Supplementary Figure 2. Forest plot of the effects of lycopene supplementation on mean birthweight (kg)
Supplementary Figure 3. Forest plot of the effects of lycopene supplementation on mean gestational age at delivery (weeks)
Supplementary Figure 4. Forest plot of the effects of vitamin A and/or β-C on stillbirth
Supplementary Figure 5. Forest plot of the effects of vitamin A and/or β-C on miscarriage
Supplementary Figure 6. Forest plot of the effects of vitamin A and/or β-C on fetal loss
Supplementary Figure 7. Forest plot of the effect of lycopene supplementation on pre-eclampsia
Supplementary Figure 8. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on severe anemia during pregnancy (<8 or 8.5 g/dL)
Supplementary Figure 9. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on follow-up hemoglobin during pregnancy (g/dL)
Supplementary Figure 10. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on change in hemoglobin during pregnancy (g/dL)
Supplementary Figure 11. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on mean maternal postpartum hemoglobin (4–6 months postpartum)
Supplementary Figure 12. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on neonatal/infant anemia (Hb<11.0 g/dL)
Supplementary Figure 13. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on mean infant hemoglobin at 4–6 months
Supplementary Figure 14. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on mean weight-for-age z score at 6 months
Supplementary Figure 15. Forest plot of the effect of vitamin A/β-C supplementation during pregnancy on mean height-for-age z score at 6 months
Supplementary Table 1. Search strategy and keywords
Supplementary Table 2. Evaluation of the quality of individual trials for potential inclusion in the meta-analysis using GRADE criteria