Abstract
Chronic cocaine use in humans and animal models is known to lead to pronounced alterations in glutamatergic function in brain regions associated with reinforcement. Previous studies have examined ionotropic glutamate receptor (iGluR) subunit protein level changes following acute and chronic experimenter-administered cocaine or after withdrawal periods from experimenter-administered cocaine. To evaluate whether alterations in expression of iGluRs are associated with cocaine reinforcement, protein levels were assessed after binge (8 h/day, 15 days; 24-h access, days 16–21) cocaine self-administration and following 2 weeks of abstinence from this binge. Western blotting was used to compare levels of iGluR protein expression (NR1–3B, GluR1–7, KA2) in the ventral tegmental area (VTA), substantia nigra (SN), nucleus accumbens (NAc), striatum and prefrontal cortex (PFC) of rats. iGluR subunits were altered in a time-dependent manner in all brain regions studied; however, selective alterations in certain iGluR subtypes appeared to be associated with binge cocaine self-administration and withdrawal in a region-specific manner. In the SN and VTA, alterations in iGluR protein levels compared with controls occurred only following binge access, whereas in the striatum and PFC, iGluR alterations occurred with binge access and following withdrawal. In the NAc, GluR2/3 levels were increased following withdrawal compared with binge access, and were the only changes observed in this region. Because subunit composition determines the functional properties of iGluRs, the observed changes may indicate alterations in the excitability of dopamine transmission underlying long-term biochemical and behavioral effects of cocaine.
Keywords: cocaine, glutamate, mesolimbic, nigrostriatal, reinforcement, self-administration
Neuropharmacological studies in animal models and imaging studies in humans indicate that the mesocorticolimbic dopamine pathway is regulated by cocaine administration, and that the functional integrity of this pathway is essential for the reinforcing and euphoric effects of cocaine (Ritz et al. 1987; Wise and Bozarth 1987; Di Chiara and Imperato 1988; Koob and Bloom 1988; Volkow et al. 1999). Chronic cocaine administration induces neuroadaptive changes that lead to transient and perhaps persistent alterations in regional brain function that may underlie persistent-drug taking behaviors, ‘craving’ and relapse (Nestler 1993, 1997; White et al. 1995; Pierce and Kalivas 1997; White and Kalivas 1998). Alterations in the expression of genes associated with dopaminergic neurotransmission have been an area of investigation. Previously described neuroadaptations induced by chronic cocaine administration include a generalized up-regulation of the cyclic AMP pathway (Nestler et al. 1990; Terwilliger et al. 1991; Striplin and Kalivas 1992; Miserendino and Nestler 1995; Carlezon et al. 1998; Pliakas et al. 2001), activation of members of the activator protein 1 family (Hope et al. 1992; Nye et al. 1995; Hiroi et al. 1997; Pich et al. 1997; Haile et al. 2001), and other potential transcriptional regulators (Freeman et al. 2001).
More recent evidence indicates involvement of glutamate transmission in the ventral tegmental area (VTA) and nucleus accumbens (NAc) in the mediation of the behavioral and neurochemical effects of cocaine, as well as the neuroadaptations induced by chronic cocaine administration. Previous studies have shown that repeated cocaine administration increases extracellular glutamate concentrations in the NAc and VTA (Pierce et al. 1996; Reid et al. 1997; Kalivas and Duffy 1998) and produces behavioral sensitization (Pierce et al. 1996). This results in an increased responsivity of glutamate receptors to stimulation in the NAc and VTA (White et al. 1995; Zhang et al. 1997) with as little as one exposure to cocaine (Ungless et al. 2001). Increased α-amino-3-hydroxy-5-methylisoxazole-4-propionate(AMPA) and NMDA receptor expression in the VTA has been proposed as a possible mechanism for increased excitability of mesocorticolimbic dopamine neurons and behavioral sensitization to cocaine (White et al. 1995; Zhang et al. 1997). Indeed, glutamine receptor (GluR)1, GluR2/3 and NMDA receptor (NR)1 protein levels are increased in the VTA following chronic cocaine administration (Fitzgerald et al. 1996; Churchill et al. 1999; Loftis and Janowsky 2000b; Lu et al. 2002), which return to control levels following withdrawal (Churchill et al. 1999; Lu et al. 2002). Further evidence for regulation of glutamatergic transmission is provided by the recent observation of significant increases in the mRNA and protein levels of NR1, GluR2, GluR5 and kainate receptor (KA)2 in the VTA, but not substantia nigra (SN), of cocaine overdose victims (Tang et al. 2003).
The ionotropic glutamate receptors (iGluRs) are classified as NMDA (NR1, NR2A–D, NR3), AMPA (GluR1–4) and kainate (GluR5–7, KA1–2) receptor subunits, based on their pharmacological characteristics and sequence information (Hollmann and Heinemann 1994; Borges and Dingledine 2002). Whereas AMPA and kainate subunits contribute to fast neurotransmission, all three ionotropic subtypes are thought to play roles in long-term potentiation (Bortolotto et al. 1999; Nestler 2001; Ungless et al. 2001). As subunit composition determines the functional properties of iGluRs (Borges and Dingledine 2002), alterations in these receptors in the VTA may indicate alterations in the excitability of dopamine transmission underlying long-term biochemical and behavioral effects of cocaine which, in turn, may affect subsequent drug intake.
With regard to glutamatergic input to the regions under study, the VTA and SN receive inputs from the amygdala, hypothalamus and frontal and orbitofrontal cortical regions (Sesack and Pickel 1992; Vankova et al. 1992; Wallace et al. 1992), whereas the SN also receives projections from the subthalamic nucleus (Berendse and Groenewegen 1990). Glutamatergic afferents to the NAc and dorsal striatum are markedly different. Regions projecting to the NAc include the amygdala (McDonald 1991; Zahm and Brog 1992), intermediodorsal, paraventricular and parataenial thalamic nuclei (Berendse et al. 1988; Berendse and Groenewegen 1990), and mesocortical and allocortical regions (Gerfen 1984). In the dorsal striatum, glutamatergic afferents arise from midline and intralaminar thalamic nuclei (Berendse et al. 1988; Berendse and Groenewegen 1990) and the sensorimotor, visual and auditory cortices (Gerfen 1984; Donoghue and Herkenham 1986; McGeorge and Faull 1989). The prefrontal cortex (PFC) receives glutamatergic input from the intralaminar and mediodorsal thalamic nuclei (Krettek and Price 1977b; Divac et al. 1978a, 1978b) and the amygdala (Krettek and Price 1977a; Divac et al. 1978b).
The role of iGluR alterations in cocaine-induced behavioral sensitization is well documented; however, the effect of cocaine self-administration on alterations in the abundance of glutamate receptor subunits is less clear. The majority of studies evaluating the effects of cocaine on mRNA and protein expression have employed experimenter-administered cocaine and evaluated the effects following acute or chronic withdrawal. These studies have advanced understanding of the neurobiological basis of sensitization, learning and memory, but do not address the role of iGluR subunits in cocaine reinforcement inasmuch as several studies indicate pronounced biochemical differences between the contingent and non-contingent administration of drugs (Hemby et al. 1995, 1996, 1997a; Mark et al. 1999; McFarland et al. 2003). The present study was undertaken to evaluate changes in the abundance of iGluR subunit expression in mesocorticolimbic structures involved in cocaine reinforcement (VTA, NAc, PFC) following binge cocaine self-administration and withdrawal from cocaine. Similar changes were evaluated in the nigrostriatal pathway (SN and dorsal striatum) as a measure of regional and pathway specificity.
Materials and methods
Animals
Male Sprague–Dawley rats (aged 60–90 days, 225–275 g; Charles River, Wilmington, MA, USA) were housed individually in operant conditioning cages in a temperature-controlled vivarium on a 12-h reversed light–dark cycle (lights on at 20.00 hours) with food and water available ad libitum throughout the experiment.
Intravenous catheterization
Rats were anesthetized with halothane and implanted with chronic indwelling venous catheters, as described previously (Hemby et al. 1995, 1996, 1997a, 1999). Catheters were inserted into the right jugular vein, terminating just outside the right atrium and anchored to muscle near the point of entry into the vein. The distal end of the catheter was guided subcutaneously to exit above the scapulae through a Teflon shoulder harness. The harness provided a point of attachment for a spring leash connected to a single-channel swivel at the opposing end. The catheter was threaded through the leash for attachment to the swivel where the fixed end of the swivel was connected to a syringe by polyethylene tubing. Infusions were administered by a motor-driven syringe pump controlled by a computer. Intravenous infusions of methohexital (100 μL; 10 mg/kg) were administered to assess catheter patency, as needed. Health of the rats was monitored three times daily by the experimenter and biweekly by institutional veterinarians according to the guidelines issued by the Emory University Institutional Animal Care and Use Committee and the National Institutes of Health.
Self-administration procedures
Subjects were housed in standard operant conditioning chambers (24.5 × 23.5 × 21cm) containing a retractable lever and a stimulus light mounted directly above the lever. The chambers were enclosed in sound-attenuating boxes containing an exhaust fan, a house light, a tone source and a water bottle. A motor-driven syringe pump was located on the side of this external chamber. Extraneous noise was masked by the exhaust fan. Immediately after surgery, rats were placed in their respective chambers where they received infusions of heparinized 0.9% bacteriostatic saline (1.7 U/mL; 200 μL per 30 min) for 48 h. On the following day, the self-administration procedure was initiated.
Rats were randomly divided into two groups: (binge access and withdrawal) and allowed to self-administer cocaine (0.5 mg/infusion; 200 μL/infusion; 6.2 s/infusion) during an 8-h self-administration session (dark phase of the light cycle) under a fixed ratio (FR) 5, time-out 20 s schedule of reinforcement. Upon completion of the response requirement, a cocaine infusion was delivered and a 20-s time-out was in effect. Responding was initially maintained under an FR1 that was gradually increased to FR5. During the timeout, the lever light was extinguished, the house light illuminated, and a tone was generated. The end of the time-out was signaled by illumination of the lever light, and the house light and tone were extinguished. During the time-out, lever responses were recorded but had no scheduled consequence. IBM-compatible computers were used for session programming and data collection. Once attaining the terminal ratio of FR5, rats were given limited access (8 h/day, 7 days/week) for 14 consecutive days. On the 15th day, the self-administration session was changed to multiple 3-h access components separated by 1-h time outs as depicted in Table 1.
Table 1.
Self-administration protocol
| Dark cycle (8.31 to 20.30 hours)
|
Light cycle (20.31 to 8.30 hours)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-h access self-admin | TO | 3 h access self-admin | TO | 3 h access self-admin | TO | 3 h access self-admin | TO | 3 h access self-admin | TO | 3 h access self-admin | TO |
TO, time-out; self-admin, self-administration.
Twenty-four sessions were looped such that the program began each day at the beginning of the dark cycle. After completion of the self-administration session on the 20th day, rats in the binge access group were killed. On days 21–34, rats in the withdrawal group remained in the self-administration chambers but did not have access to cocaine or related stimuli; they were killed on day 35.
Tissue preparation and western blot analysis
Rats were anesthetized with halothane and killed by intracardial perfusion with phosphate-buffered saline (pH 7.2). Brains were removed and sectioned on ice in the coronal plane using a brain matrix. Areas of interest were dissected immediately on ice-cooled aluminum plates from 1-mm slices (approximately + 3.2 to + 2.2, PFC; + 1.7–0.7, NAc; 0.48 to − 0.4, striatum; − 5.2 to − 6.2, SN/VTA; all measures relative to bregma) (Paxinos and Watson 1998) and immediately frozen at − 80°C in Eppendorf tubes.
The sample size for the binge, withdrawal and control groups for the NAc, PFC and striatum was n = 8 per region. Because of the size of the VTA and SN, two samples were pooled such that n = 4 for the withdrawal, binge and control groups for these regions. Tissue samples were homogenized in 10 mM HEPES, 10 mM NaCl, 1 mM KH2PO4, 5 mM NaHCO3, 1 mM CaCl2, 0.5 mM MgCl2, 5 mM EDTA and protease inhibitors (1 mM phenylmethylsulfonylfluoride, 10 mM benzamidine, 10 μg/mL aprotinin, 10 μg/mL leupeptin and 1 μg/mL pepstatin) and centrifuged using a Beckman Coulter SW55Ti swinging bucket rotor at 5333 g for 5 min. Supernatant (cytosol and crude membrane) was removed and centrifuged at 59 255 g for 30 min at 4°C; the pure cytosolic supernatant was removed and stored at − 80°C. The pellet containing the crude plasma membrane was resuspended in 20 mM Tris-HCl, 1 mM EDTA (pH 8.0) and 300 mM sucrose with protease inhibitors and centrifuged at 5333 g for 5 min. This procedure was repeated twice and the pellet was resuspended in phosphate-buffered saline and stored at − 80°C (crude plasma membrane fraction). The pellet from the intial centrifugation was resuspended in 10 mM Tris (pH 7.5), 300 mM sucrose, 1 mM EDTA (pH 8.0), 0.1% NP40 and protease inhibitors, and centrifuged at 2370 g for 5 min at 4°C. The supernatant was discarded and the pellet was resuspended in the buffer and washed three times before resuspension in the buffer containing protease inhibitors. Samples were stored at − 80°C (nuclear fraction), as described previously (Tang et al. 2003; http://www.pmci.unimelb.edu.au/core_facilities/manual/b080.asp).
Protein concentrations were calculated using the bicinochoninic acid protein assay kit (Pierce, Rockford, IL, USA) and diluted in Laemmli sample buffer to achieve the equivalent final protein concentrations. Five micrograms of protein was loaded on to 10% sodium dodecyl sulfate–polyacrylamide gels, electrophoresed and transferred to nitrocellulose by electroblotting (30 V, overnight at 4°C) in 1 × transfer buffer (Bio-Rad, Richmond, CA, USA). Nitrocellulose membranes were blocked in 0.5% w/v non-fat dry milk and 0.1% v/v Tween 20 in phosphate-buffered saline (pH 7.4, 0.12 M) for 1 h at 25°C before being incubated overnight at 4°C with primary antibodies in blocking buffer (Bio-Rad). Membranes were then incubated with secondary antibody for 1 h at room temperature. Protein bands were visualized on a Kodak (Rochester, NY, USA) XAR-5 film with enhanced chemiluminescence (ECL plus, Amersham Pharmacia Biotech, Piscataway, NJ, USA). Primary antibodies were as follows: mouse monoclonal antibodies directed against NR1 (Chemicon International, Temecula, CA, USA) and rabbit polyclonal antibodies directed against NR2A, NR2B, NR3A, NR3B, GluR1, GluR2/3, GluR4, GluR5, GluR6/7 and KA2 (Upstate Biotechnology Cell Signaling Systems, Waltham, MA, USA). Equal protein loading was confirmed by stripping the blots and re-probing them with a monoclonal β-tubulin antibody (1 : 5000 v/v; Upstate Biotechnology Cell Signaling Systems) followed by incubation with secondary antibody and visualization as described above. No significant differences were detected in β-tubulin abundance between the groups for any of the blots, indicating that any differences in ionotropic glutamate receptor abundance between the groups was not due in unequal loading of protein in the gels. Protein abundances were calculated by optical densitometry with a Scan Jet 2200C and imported into NIH Image 1.61 software for analysis. Film background was subtracted from the optical density values to give the optical density value for each subject. All assays were conducted under conditions in which densitometric signal intensity was linear with protein concentration as determined by preliminary experiments. Data were expressed as a percentage of control levels (mean ± SEM).
Data analysis
Self-administration data were analyzed by two-way ANOVA with repeated measures (time) with number of infusions as the dependent measure. Data for each iGluR subunit/region were normalized based on control levels and were analyzed using one-way ANOVA. Null hypotheses were rejected when p < 0.05.
Results
Behavioral data
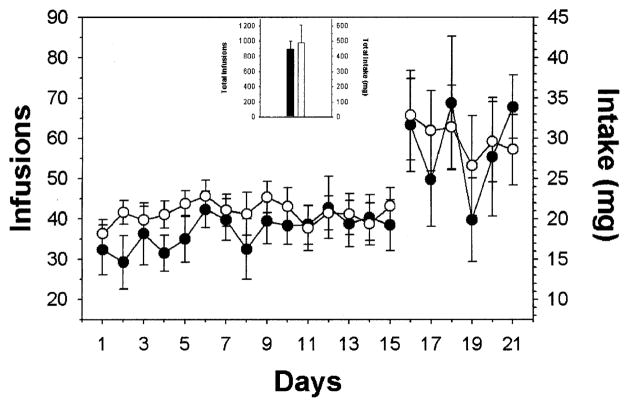
Cocaine engendered and maintained rates of self-administration observed previously under limited access, FR schedules of reinforcement in rats (Hemby et al. 1996, 1997a; 1999). There was no statistically significant difference in the number of infusions, and likewise cocaine intake, between the two groups over the course of the experiment (F1,377 = 0.401, p = 0.544) (Fig. 1). The total number of infusions was 895.8 ± 101.7 for the binge group and 981.3 ± 232.9 for the withdrawal group. During the 15 days of limited access, rats in the binge group had 551.3 ± 60.9 infusions (275.7 ± 30.5 mg cocaine), whereas the withdrawal group had 621.7 ± 42.7 infusions (310.9 ± 21.4 mg cocaine). Similarly, during the 6 days of unlimited access, the binge group self-administered approximately 344.4 ± 48.3 infusions (172.2 ± 24.2 mg cocaine) and the withdrawal group self-administered 359.5 ± 48.0 infusions (179.8 ± 24.0 mg cocaine).
Fig. 1.
Mean ± SEM number of cocaine infusions and intake self-administered during limited and binge access periods for the two groups. Responding was engendered and maintained by intravenous cocaine infusions by both the binge (●) and withdrawal (○) groups. There was no significant difference in the number of infusions or intake between the two groups. The inset depicts mean ± SEM total number of infusions and intake for the experiments.
Regional comparison of iGluR subunit protein levels in controls
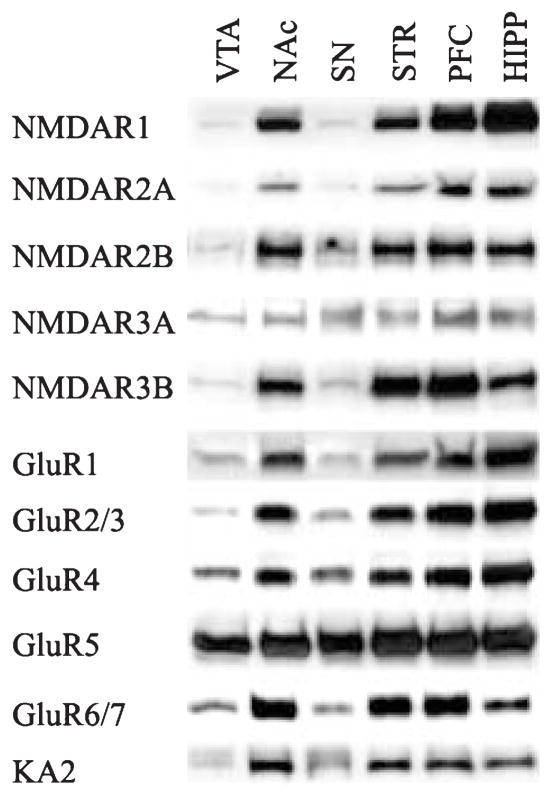
Analysis of the relative abundance of iGluR subunits across various brain regions was performed. Western blots of the iGluR subunits in each of the brain regions studied revealed single bands at the appropriate molecular weight (Fig. 2). For the NMDA subunits, NR1 was most abundant in the hippocampus, followed by the PFC, the NAc and striatum (hippocampus > PFC > NAc, striatum > VTA, SN). There were no apparent differences in abundances of NR2A, NR2B, or NR3A between the hippocampus, PFC, NAc and striatum, whereas these subunits were in low abundance in the VTA and SN. Interestingly, the NR3B subunit appeared to be most abundant in the PFC and striatum followed by the hippocampus and NAc. Owing to the paucity of protein from the VTA and SN, NR3A and NR3B levels were not assessed. The abundances of GluR1, GluR2/3 and GluR4 were greater in the hippocampus and PFC than the NAc and striatum (hippocampus, PFC > NAc, striatum > VTA, SN). GluR5 protein levels appeared to be equally abundant in all regions tested. For the kainate receptor subunits, GluR6/7 was most abundant in the NAc, striatum and PFC followed by the hippocampus, then the SN and VTA. In contrast, KA2 was most abundant in the NAc, moderately abundant in the striatum, PFC and hippocampus, and least abundant in the VTA and SN.
Fig. 2.
Regional distribution of iGluR subunits in rat brain. Aliquots of 10 μg membrane fraction combined from four control rats were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting for NMDR1 (176 kDa), NMDR2A (170 kDa), NMDAR2B (180 kDa), NMDAR3A (130 kDa), NMDAR3B (98 kDa), GluR1 (110 kDa), GluR2/3 (110 kDaA), GluR4 (110 kDa), GluR5 (105 kDa), GluR6/7 (115 kDa), KA2 (123 kDa). STR, striatum; HIPP, hippocampus.
Effects of cocaine self-administration history on iGluR subunit levels
NR1
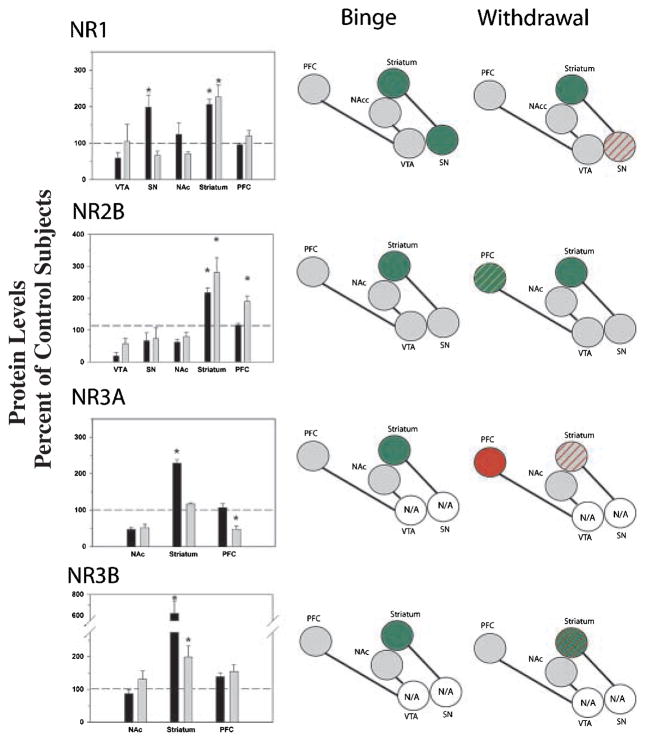
ANOVA revealed a significant effect of cocaine history on protein levels in the SN (F2,16 = 9.785; p = 0.002) and striatum (F2,22 = 11.217; p < 0.001). No significant differences were observed in the VTA, NAc or PFC. Post-hoc analyses revealed increased levels in the SN following binge access which returned to control levels following withdrawal, whereas levels in the striatum following binge access and withdrawal were both significantly greater than control levels (Fig. 3).
Fig. 3.
Comparisons of NMDA receptor subunit protein levels in VTA, SN, NAc, striatum and PFC following binge cocaine access (black bars) and withdrawal (grey bars). Data are mean ± SEM percentage of values for control rats in each region. Data for each subunit were statistically evaluated using one-way ANOVA. See Table 2 for significant comparisons between groups. *p < 0.05 by post-hoc analysis. Schematics of mesolimbic and nigrostriatal pathways represent graphical depiction of changes for each subunit. Green filled, increased compared with control; red filled, decreased compared with control; green diagonal bands, increased relative to binge; red diagonal bands, decreased relative to binge. N/A, not available; grey filled, no change.
NR2B
There was a significant effect of cocaine history on NR2B levels in the striatum (F2,22 = 12.996; p < 0.001) and PFC (F2,18 = 11.881; p < 0.001). Similar to NR1, NR2B levels in the striatum were increased following binge access and withdrawal, both at levels significantly greater than control levels. In the PFC, levels were significantly greater following withdrawal than either control or binge access (Fig. 3).
NR3A
ANOVA revealed a significant effect of cocaine history on protein levels in the striatum (F2,22 = 12.169; p < 0.001) and PFC (F3,26 = 3.928; p = 0.011). Owing to the relatively small amounts of protein available in the VTA and SN, NR3A levels were not assessed in these regions. In the striatum, binge access produced significantly greater levels than control conditions and withdrawal. In the PFC, protein levels were significantly reduced following withdrawal below levels observed in controls and binge access (Fig. 3).
NR3B
Protein levels were significantly altered by cocaine in the striatum (F2,23 = 101.821; p < 0.001). No changes were observed in the other brain regions. As noted for NR3A, levels were not assessed in the VTA or SN. Levels following binge access were significantly greater than levels in the withdrawal group which, in turn, were greater than control levels (Fig. 3).
GluR1
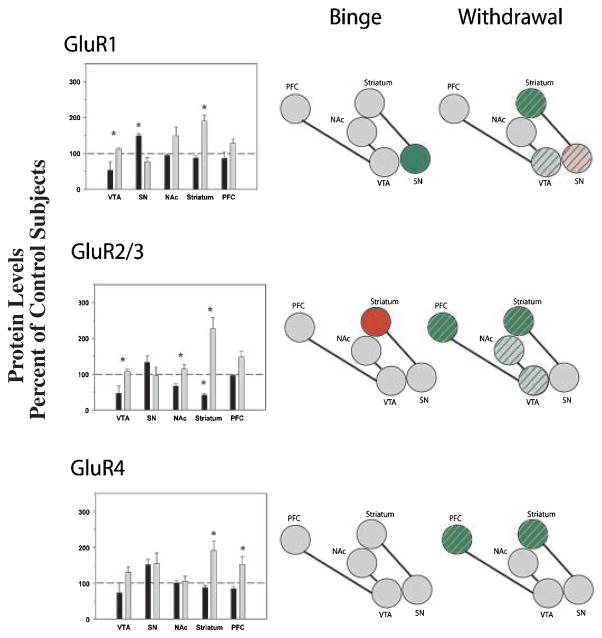
ANOVA revealed a significant effect of cocaine history on protein levels in the VTA (F2,16 = 5.924; p = 0.014), SN (F2,19 = 8.890; p = 0.002), striatum (F2,22 = 42.004; p < 0.001) and PFC (F2,20 = 4.431; p = 0.0270), but there was no significant difference in the NAc. In the VTA, GluR1 levels were significantly greater during withdrawal compared with binge access. In contrast, GluR1 levels in the SN were increased during binge access but returned to control levels during withdrawal. In the striatum, levels were significantly raised during withdrawal only (Fig. 4).
Fig. 4.
Comparisons of AMPA receptor subunit protein levels in VTA, SN, NAc, striatum and PFC following binge cocaine access (black bars) and withdrawal (grey bars). Data are mean ± SEM percentage of values for control rats for each antibody. Data for each subunit were statistically evaluated using one-way ANOVA. See Table 2 for significant comparisons between groups. *p < 0.05 by post-hoc analysis. Schematics of mesolimbic and nigrostriatal pathways represent graphical depiction of changes for each subunit. Green filled, increased compared with control; red filled, decreased compared with control; green diagonal bands, increased relative to binge; red diagonal bands, decreased relative to binge.
GluR2/3
Protein levels were significantly altered in the VTA (F2,11 = 5.494; p = 0.028), NAc (F2,22 = 5.105; p = 0.016), striatum (F2,22 = 38.918; p < 0.001) and pre-frontal cortex (F2,20 = 6.953; p = 0.006), although no significant differences were observed in the SN. In the VTA, NAc and PFC, withdrawal produced significantly greater levels than binge access. In the striatum and PFC, GluR2/3 levels following withdrawal were significantly more abundant than control levels (Figs 4 and 5).
Fig. 5.

Representative immunoblot of GluR2/3 protein levels in the striatum following binge cocaine access and withdrawal. Note the decrease in protein levels following binge access and the increase in levels following withdrawal. Data were analyzed by quantitative densiotometry and were found to be statistically different from control levels. Refer to Fig. 4(b) for graphic representation.
GluR4
ANOVA revealed significant effects of cocaine on GluR4 protein levels in the striatum (F2,33 = 23.629; p = 0.007) and PFC (F2,19 = 8.211; p = 0.003). There was no significant difference in the VTA, SN or NAc. Similar patterns of changes were observed in the striatum and PFC, with levels following withdrawal significantly increased above both control and binge levels (Fig. 4).
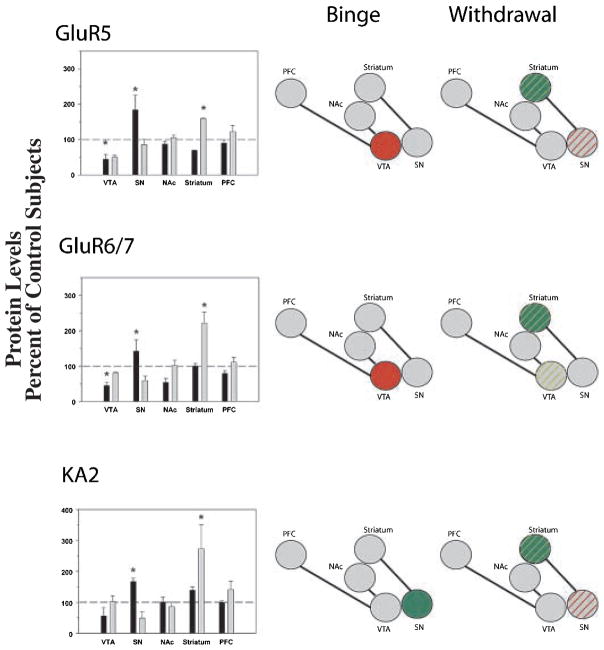
GluR5
There was a significant effect of cocaine history on GluR5 levels in the VTA (F2,11 = 5.583; p = 0.027), SN (F2,16 = 4.032; p = 0.041) and striatum (F2,16 = 10.706; p = 0.002). No significant differences between the groups were observed in the NAc or PFC. In the VTA, levels were significantly decreased during binge access compared with control levels. In contrast, GluR5 levels in the SN increased during binge cocaine access and returned to control levels during withdrawal. In the PFC, levels were significantly increased during withdrawal above both control and binge levels (Fig. 6).
Fig. 6.
Comparisons of kainate receptor subunit protein levels in VTA, SN, NAc, striatum and PFC following binge cocaine access (black bars) and withdrawal (grey bars). Data are mean ± SEM percentage of values for control rats for each antibody. Data for each subunit were statistically evaluated using one-way ANOVA. See Table 2 for significant comparisons between groups. *p < 0.05 by post-hoc analysis. Schematics of mesolimbic and nigrostriatal pathways represent graphical depiction of changes for each subunit. Green filled, increased compared with control; red filled, decreased compared with control; green diagonal bands, increased relative to binge; red diagonal bands, decreased relative to binge.
GluR6/7
ANOVA revealed significant effects of cocaine on GluR6/7 protein levels in the VTA (F2,11 = 20.731; p < 0.001), SN (F2,20 = 4.232; p = 0.031) and striatum (F2,22 = 23.934; p < 0.001). No significant differences were observed in the PFC or NAc, although there was a trend towards significance in the NAc (F2,22 = 3.339; p = 0.056). In the VTA, protein levels were significantly decreased during binge access but returned to control levels following 2 weeks of withdrawal. In contrast, SN GluR6/7 levels increased following binge cocaine access and returned to control levels during withdrawal. In the striatum, GluR6/7 protein levels were increased above control and binge access levels following 2 weeks of withdrawal (Fig. 6).
KA2
ANOVA revealed significant effects of cocaine on protein levels in the SN (F2,20 = 11.357; p < 0.001) and striatum (F2,22 = 42.004; p < 0.001). There were no significant differences in KA2 levels between the groups in the VTA, NAc or PFC. Levels were significantly increased in the SN during binge access and returned to control levels following withdrawal. In contrast, striatal KA2 protein levels were significantly increased during withdrawal but were not different from control levels following binge access (Fig. 6).
Discussion
In the present study, western blot analysis was used to examine the expression of iGluR protein subunits following binge cocaine self-administration and 2 weeks of withdrawal in the brain mesocorticolimbic and nigrostriatal dopamine pathways. Binge cocaine self-administration and withdrawal induced changes in protein levels of iGluR subunits in a region-specific manner and were dependent upon the history of cocaine exposure. The present results provide the first composite assessment of iGluR subunit protein alterations in mesocorticolimbic brain regions associated with cocaine reinforcement (VTA, NAc, and PFC) compared with regions in the nigrostriatal pathway (SN and striatum).
Previous studies demonstrated that chronic cocaine administration induced biochemical and molecular changes that altered the functional integrity of neural pathways related to the reinforcing effects of cocaine. Alterations in glutamatergic function have been proposed as a potential mechanism of cocaine-related neuroadaptations. Glutamate transmission in the mesocorticolimbic circuit is involved in the development and expression of behavioral sensitization to cocaine (White et al. 1995; Bell and Kalivas 1996; Pierce et al. 1996; Li et al. 1997; Wolf 1998), and the maintenance (Pulvirenti et al. 1992; Jackson et al. 1998) and reinstatement of cocaine self-administration in rats (Cornish et al. 1999; Cornish and Kalivas 2000; Park et al. 2002; McFarland et al. 2003). Previous studies demonstrated that cocaine administration selectively increased extracellular glutamate concentrations in the VTA, NAc and PFC in rats (Pierce et al. 1996; Reid et al. 1997; Kalivas and Duffy 1998), and resulted in increased responsivity of AMPA and NMDA receptor stimulation in the VTA (White et al. 1995; Zhang et al. 1997; Ungless et al. 2001). Transient increases in AMPA and NMDA receptor expression have been proposed as one mechanism underlying hyperglutamatergia induced by cocaine (White et al. 1995; Zhang et al. 1997). For example, protein levels of NR1, GluR1and GluR2/3 subunits in the VTA were increased by chronic cocaine administration (Fitzgerald et al. 1996; Churchill et al. 1999; Loftis and Janowsky 2000b; Lu et al. 2002) and returned to control levels during withdrawal (Churchill et al. 1999; Lu et al. 2002). Previously, we demonstrated significant up-regulation of NR1, GluR2/3, GluR5 and KA2 protein levels in the VTA, but not SN, of cocaine overdose victims (Tang et al. 2003), confirming that such a mechanism may exist in cocaine overdose in humans. The present study was undertaken in an attempt to recapitulate these changes and explore additional changes in an animal model of cocaine reinforcement.
NMDA receptor subunits
The present results confirm previous studies in which the NAc, striatum and PFC exhibited moderated to dense immunoreactivity for NR1 and NR2A/2B subunits (Petralia et al. 1994b, 1994c; Fitzgerald et al. 1995, 1996), and moderate and low immunoreactivity in the VTA and SN respectively (Fitzgerald et al. 1996). Previous studies have demonstrated the regulation of cocaine self-administration by NMDA receptor subunits. For example, intra-accumbens administration of the NMDA antagonist 2-amino-5-phos-phonovaleric acid (AP5) increased cocaine self-administration (Pulvirenti et al. 1992) whereas intra-accumbens administration of 1-aminocyclobutane-cis-1,3-dicarboxylic acid (cis-ACDA) decreased self-administration (Cornish et al. 1999) suggesting that NMDA receptor stimulation in the NAc may increase the reinforcing effects of cocaine. Previous studies have demonstrated increased NR1 protein levels in the VTA (Fitzgerald et al. 1996; Churchill et al. 1999) and NAc (Churchill et al. 1999) following chronic cocaine administration (experimenter administered) and withdrawal (Loftis and Janowsky 2000a; Lu et al. 2003). In the present study, no significant differences were observed in the VTA, NAc or PFC NR1 levels following binge cocaine self-administration, suggesting that differences between the studies may be due to the contingency of drug administration and/or the drug regimen. However, NR1 protein levels were increased following 6 days of binge cocaine access in the SN compared with control and withdrawal levels, and in the striatum following binge and withdrawal compared with control levels. Interestingly, increased levels returned to baseline following 2 weeks of withdrawal in the SN whereas levels remained raised following withdrawal in the striatum.
The involvement of other NMDA receptor subunits in cocaine-induced neuroadaptations has received less attention than that of NR1. In the present study, NR2B and NR3B levels were increased in the striatum following binge cocaine self-administration and remained raised above control levels after 2 weeks of withdrawal suggesting that these changes may represent long-term alterations in NMDA receptor function following cocaine exposure. In contrast, NR3A levels in the striatum were increased following binge cocaine access but returned to control levels following 2 weeks of withdrawal. In the PFC, withdrawal from cocaine increased NR2B above control and binge access levels, whereas withdrawal levels of NR3A were decreased compared with control and binge access levels. The presence of NR3A subunits, which must co-express with NR1 for membrane expression, leads to decreased Ca2+ permeability through the NMDA receptor complex (Perez-Otano et al. 2001). As increased Ca2+ permeability is necessary for long-term synaptic changes, the present data suggest an increased opportunity for long term potentiation (LTP) to occur in PFC neurons. From a functional perspective, increased NR2B levels indicate a slow deactivation time (~400 ms) for the receptor complex which, when paired with decreased expression of NR3A, may yield an increase in Ca2+ permeability and hyperexcitability of prefrontal function. NR1 subunits are required for the normal function of the NMDA ionophore. The subunit is phosphorylated by protein kinase A, protein kinase C and possibly by calcium/calmodulin dependent protein Kinase II (CamKII), which causes increased Ca2+ influx through the activated receptor leading to the slow onset of the excitatory post-synaptic potential (EPSP). NR1 subunits may lead to enhanced Ca2+ influx in dopamine neurons resulting in hyperexcitability of these cells. With no apparent change in NR1 protein levels, NR3A alterations may provide an alternative means by which to increase intracellular Ca2+ levels without altering the number of receptors.
AMPA receptor subunits
GluR1, GluR2/3 and GluR4 subunits exhibited moderate to strong immunoreactivity in the NAc, striatum and PFC (Petralia and Wenthold 1992; Martin et al. 1993; Fitzgerald et al. 1995; Bernard et al. 1996; Bernard et al. 1997), and low to moderate levels in the SN and VTA (Petralia and Wenthold 1992; Martin et al. 1993; Chen et al. 2001). The effects of acute and chronic cocaine administration (experimenter administered) and withdrawal on GluR1 and GluR2/3 subunit levels in several brain regions have been examined previously. Chronic cocaine (7 or 14 days) increased GluR1 levels in the VTA, but not in the SN, NAc, striatum or PFC (Fitzgerald et al. 1996) and following 1 day but not 3 weeks of withdrawal (Churchill et al. 1999); however, others reported no significant alterations in GluR1 protein levels in the VTA following experimenter-administered cocaine (Lu et al. 2002) or withdrawal from self-administered cocaine (Lu et al. 2003). In contrast to the aforementioned studies, GluR1 and GluR2/3 levels in the VTA were significantly increased during withdrawal compared with binge access. Similarly in the NAc, GluR2/3 levels were increased following withdrawal from cocaine compared with binge access, an effect also observed in human cocaine overdose victims (W. Tang, W. Freeman, D. C. Nash and S. E. Hemby, unpublished observations). Significant up-regulation of GluR2/3 and GluR4 in the PFC was observed following withdrawal compared with control and binge access levels. As noted in a previous study, the increases in NAc GluR2/3 levels may be transient as up-regulation was observed 1 and 30 days after withdrawal from cocaine self-administration, but returned to control levels after 90 days (Lu et al. 2003). Further studies are warranted to provide a detailed temporal profile of such changes. In contrast, no significant alterations in NAc GluR1 or GluR2/3 levels were observed following chronic experimenter-administered cocaine (Fitzgerald et al. 1996), or 1 or 21 days of withdrawal from cocaine (Churchill et al. 1999). The most probable explanation for the differences between the present results and those of previous studies is the contingency of cocaine administration. Such differences are not surprising inasmuch as several studies have demonstrated significant differences in various biochemical measures between self-administered (contingent) and experimenter-administered (non-contingent) cocaine delivery (Wilson et al. 1994; Hemby et al. 1995, 1997a, 1997b; Hemby 1999). The present results confirm previous findings of no change in GluR1 and GluR2 levels in the NAc during withdrawal from cocaine (Sutton et al. 2003) and increased GluR1 and GluR2/3 levels in the NAc following extinction training withdrawal (Sutton et al. 2003).
In the nigrostriatal pathway, GluR1 levels in the SN were increased during binge access compared with control and withdrawal levels, whereas AMPA subunit levels in the striatum were increased during 2 weeks of withdrawal from cocaine compared with control and binge access levels. In addition, binge GluR2 levels in the striatum were significantly lower than control and withdrawal levels. Previous studies using experimenter-administered cocaine did not observe significant alterations in AMPA subunits in the nigrostriatal pathway following chronic cocaine or withdrawal (Fitzgerald et al. 1996; Churchill et al. 1999), again suggesting the potential relative importance of contingent drug administration.
Kainate receptor subunits
In agreement with previous studies, GluR6 and KA2 exhibited similar moderate to strong immunoreactivity in the NAc, striatum and PFC (Petralia et al. 1994a; Fitzgerald et al. 1996) and light immunoreactivity in the SN and VTA (Fitzgerald et al. 1996). GluR5 levels were moderate to strong in the PFC, as described previously (Toda et al. 2002), and were similarly expressed in the NAc, striatum, VTA and SN. Interestingly, GluR5 levels were expressed at considerably higher levels in the rat compared with human VTA (Tang et al. 2003). Unlike NMDA and AMPA receptor subunits, the physiological function of kainate receptors remains unclear; however, they appear to be important for controlling Ca2+ influx through the kainate ionophore in different pathological states, such as cocaine addiction (Paschen and Djuricic 1994).
In the present study, GluR5, GluR6/7 and KA2 receptor subunit levels were regulated in a region-specific manner. In the VTA, GluR5 and GluR6/7 immunoreactivities were decreased during binge access, whereas binge access increased GluR5, GluR6/7 and KA2 levels in the SN compared with withdrawal levels. Conversely, the kainate receptor subunits in the striatum were significantly up-regulated during withdrawal compared with control and binge access levels. Presently, there is a paucity of information on the regulation and function of kainate receptor subunits and even less on the role of these subunits in cocaine abuse and addiction. GluR5 mRNA and protein levels were found to be up-regulated in the PFC of rats following 3 weeks of withdrawal from chronic cocaine (Toda et al. 2002) and in the VTA of human cocaine overdose victims (Tang et al. 2003). However, no changes in VTA or PFC GluR5 protein levels were identified in the present study. Probable reasons for such differences include the regimen and chronicity of administration, contingency of administration, and differences in glutamatergic innervation between species.
Summary
The present study demonstrated the regional and subunit-specific changes in iGluR protein expression following binge cocaine self-administration and withdrawal. Differences between the present data and previous rat studies require further investigation, including the evaluation of longer-duration cocaine self-administration histories, alternate binge–withdrawal conditions and further evaluation of potential differences in iGluR subunit distribution. Although additional studies are needed to establish causal relationships between changes in iGluR protein expression and function, these results provide a significant addition to the knowledge of altered glutamatergic function induced by chronic cocaine self-administration and withdrawal. The study is unique in that it is the first to assess alterations in multiple iGluR subtypes in various brain regions following binge cocaine self-administration and withdrawal. Such changes may be related to behaviors associated with withdrawal such as decreased locomotion, increased anxiety and behavioral sensitization and other enduring effects, and may be an important mechanism by which cocaine exerts long-term effects on the mesolimbic dopamine system.
Table 2.
Comparisons of ionotropic glutamate receptor immunoreactivity in brain regions following limited and binge cocaine self-administration and withdrawal
| VTA
|
SN
|
NAc
|
Striatum
|
PFC
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA | Post hoc | ANOVA | Post hoc | ANOVA | Post hoc | ANOVA | Post hoc | ANOVA | Post hoc | |
| NR1 | 0.002 | B > C,W | < 0.001 | W,B > C | ||||||
| NR2B | < 0.001 | W,B > C | < 0.001 | W > C,B | ||||||
| NR3A | < 0.001 | B > C,W | 0.011 | C,B > W | ||||||
| NR3B | < 0.001 | B > W > C | ||||||||
| GluR1 | 0.014 | W > B | 0.002 | B > C,W | < 0.001 | W > C,B | 0.027 | |||
| GluR2/3 | 0.028 | W > B | 0.016 | W > B | < 0.001 | W > C > B | 0.006 | W > C,B | ||
| GluR4 | < 0.001 | W > C,B | 0.003 | W > C,B | ||||||
| GluR5 | 0.027 | C > B | 0.041 | B > W | 0.002 | W > C,B | ||||
| GluR6/7 | < 0.001 | C,W > B | 0.031 | B > W | < 0.001 | W > C,B | ||||
| KA2 | < 0.001 | B > C,W | < 0.001 | W > C,B | ||||||
C, control; B, binge; W, withdrawal. Post-hoc comparisons revealed statistically significant differences at p < 0.05.
Acknowledgments
This work was supported in part by the National Institute on Drug Abuse (DA13772, S.E.H.).
Abbreviations used
- AMPA (+/−)
α-amino-3-hydroxy-5-methylis-oxazole-4-propionate
- FR
fixed ratio
- GluR
glutamate receptor
- iGluR
ionotropic glutamate receptor
- NAc
nucleus accumbens
- NR
NMDA receptor
- KA
kainate receptor
- PFC
prefrontal cortex
- SN
substantia nigra
- VTA
ventral tegmental area
References
- Bell K, Kalivas PW. Context-specific cross-sensitization between systemic cocaine and intra-accumbens AMPA infusion in the rat. Psychopharmacologia. 1996;127:377–383. doi: 10.1007/s002130050101. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol. 1990;299:187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Voorn P, te Kortschot A, Groenewegen HJ. Nuclear origin of thalamic afferents of the ventral striatum determines their relation to patch/matrix configurations in enkephalin-immunoreactivity in the rat. J Chem Neuroanat. 1988;1:3–10. [PubMed] [Google Scholar]
- Bernard V, Gardiol A, Faucheux B, Bloch B, Agid Y, Hirsch EC. Expression of glutamate receptors in the human and rat basal ganglia: effect of the dopaminergic denervation on AMPA receptor gene expression in the striatopallidal complex in Parkinson’s disease and rat with 6-OHDA lesion. J Comp Neurol. 1996;368:553–568. doi: 10.1002/(SICI)1096-9861(19960513)368:4<553::AID-CNE7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges K, Dingledine R. Molecular pharmacology and physiology of glutamate receptors. In: Herman BH, Frankenheim J, Litten RZ, Sheridan PH, Weight FF, Zukin SR, editors. Glutamate and Addiction. Humana Press; Totawa: 2002. pp. 3–22. [Google Scholar]
- Bortolotto ZA, Clarke VR, Delany CM, et al. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Chen LW, Wei LC, Lang B, Ju G, Chan YS. Differential expression of AMPA receptor subunits in dopamine neurons of the rat brain: a double immunocytochemical study. Neuroscience. 2001;106:149–160. doi: 10.1016/s0306-4522(01)00255-x. [DOI] [PubMed] [Google Scholar]
- Churchill L, Swanson CJ, Urbina M, Kalivas PW. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem. 1999;72:2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divac I, Bjorklund A, Lindvall O, Passingham RE. Converging projections from the mediodorsal thalamic nucleus and mesencephalic dopaminergic neurons to the neocortex in three species. J Comp Neurol. 1978a;180:59–71. doi: 10.1002/cne.901800105. [DOI] [PubMed] [Google Scholar]
- Divac I, Kosmal A, Bjorklund A, Lindvall O. Subcortical projections to the prefrontal cortex in the rat as revealed by the horseradish peroxidase technique. Neuroscience. 1978b;3:785–796. doi: 10.1016/0306-4522(78)90031-3. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Herkenham M. Neostriatal projections from individual cortical fields conform to histochemically distinct striatal compartments in the rat. Brain Res. 1986;365:397–403. doi: 10.1016/0006-8993(86)91658-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Deutch AY, Gasic G, Heinemann SF, Nestler EJ. Regulation of cortical and subcortical glutamate receptor subunit expression by antipsychotic drugs. J Neurosci. 1995;15:2453–2461. doi: 10.1523/JNEUROSCI.15-03-02453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Nader MA, Nader SH, Robertson DJ, Gioia L, Mitchell SM, Daunais JB, Porrino LJ, Friedman DP, Vrana KE. Chronic cocaine-mediated changes in non-human primate nucleus accumbens gene expression. J Neurochem. 2001;77:542–549. doi: 10.1046/j.1471-4159.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Haile CN, Hiroi N, Nestler EJ, Kosten TA. Differential behavioral responses to cocaine are associated with dynamics of mesolimbic dopamine proteins in Lewis and Fischer 344 rats. Synapse. 2001;41:179–190. doi: 10.1002/syn.1073. [DOI] [PubMed] [Google Scholar]
- Hemby SE. Recent advances in the biology of addiction. Curr Psychiatry Rep. 1999;1:159–165. doi: 10.1007/s11920-999-0026-9. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE. The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis. J Pharmacol Exp Ther. 1995;273:591–598. [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. J Pharmacol Exp Ther. 1996;277:1247–1258. [erratum appears in J. Pharmacol. Exp. Ther. 1996; 279, 442] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997a;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Johnson BA, Dworkin SI. Neurobiological basis of drug reinforcement. In: Johnson BA, Roache JD, editors. Drug Addiction and its Treatment: Nexus of Neuroscience and Behavior. Lippincott-Raven Publishers; Philadelphia: 1997b. pp. 137–169. [Google Scholar]
- Hemby SE, Co C, Dworkin SI, Smith JE. Synergistic elevations in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine/heroin combinations (Speedball) in rats. J Pharmacol Exp Ther. 1999;288:274–280. [PubMed] [Google Scholar]
- Hiroi N, Brown JR, Haile CNYeH, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine’s psychomotor and rewarding effects. Proc Natl Acad Sci USA. 1997;94:10397–10402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci USA. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Mead AN, Rocha BA, Stephens DN. AMPA receptors and motivation for drug: effect of the selective antagonist NBQX on behavioural sensitization and on self-administration in mice. Behav Pharmacol. 1998;9:457–467. doi: 10.1097/00008877-199809000-00009. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977a;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977b;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Li Y, Vartanian AJ, White FJ, Xue CJ, Wolf ME. Effects of the AMPA receptor antagonist NBQX on the development and expression of behavioral sensitization to cocaine and amphetamine. Psychopharmacologia. 1997;134:266–276. doi: 10.1007/s002130050449. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. Regulation of NMDA receptor subunits and nitric oxide synthase expression during cocaine withdrawal. J Neurochem. 2000a;75:2040–2050. doi: 10.1046/j.1471-4159.2000.0752040.x. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. Regulation of NMDA receptor subunits and nitric oxide synthase expression during cocaine withdrawal. J Neurochem. 2000b;75:2040–2050. doi: 10.1046/j.1471-4159.2000.0752040.x. [DOI] [PubMed] [Google Scholar]
- Lu W, Monteggia LM, Wolf ME. Repeated administration of amphetamine or cocaine does not alter AMPA receptor subunit expression in the rat midbrain. Neuropsychopharmacology. 2002;26:1–13. doi: 10.1016/S0893-133X(01)00272-X. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Levey AI, Huganir RL, Price DL. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience. 1993;53:327–358. doi: 10.1016/0306-4522(93)90199-p. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Nestler EJ. Behavioral sensitization to cocaine: modulation by the cyclic AMP system in the nucleus accumbens. Brain Res. 1995;674:299–306. doi: 10.1016/0006-8993(95)00030-t. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Cellular responses to chronic treatment with drugs of abuse. Crit Rev Neurobiol. 1993;7:23–39. [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of opiate and cocaine addiction. Curr Opin Neurobiol. 1997;7:713–719. doi: 10.1016/s0959-4388(97)80094-3. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Neurobiology. Total recall − the memory of addiction. Science. 2001;292:2266–2267. doi: 10.1126/science.1063024. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Terwilliger RZ, Walker JR, Sevarino KA, Duman RS. Chronic cocaine treatment decreases levels of the G protein subunits Gi alpha and Go alpha in discrete regions of rat brain. J Neurochem. 1990;55:1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Nye HE, Hope BT, Kelz MB, Iadarola M, Nestler EJ. Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. J Pharmacol Exp Ther. 1995;275:1671–1680. [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W, Djuricic B. Extent of RNA editing of glutamate receptor subunit GluR5 in different brain regions of the rat. Cell Mol Neurobiol. 1994;14:259–270. doi: 10.1007/BF02088324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Perez-Otano I, Schulteis CT, Contractor A, Lipton SA, Trimmer JS, Sucher NJ, Heinemann SF. Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J Neurosci. 2001;21:1228–1237. doi: 10.1523/JNEUROSCI.21-04-01228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol. 1994a;349:85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. The NMDA receptor subunits NR2A and NR2B show histological and ultra-structural localization patterns similar to those of NR1. J Neurosci. 1994b;14:6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994c;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvirenti L, Maldonado-Lopez R, Koob GF. NMDA receptors in the nucleus accumbens modulate intravenous cocaine but not heroin self-administration in the rat. Brain Res. 1992;594:327–330. doi: 10.1016/0006-8993(92)91145-5. [DOI] [PubMed] [Google Scholar]
- Reid MS, Hsu K, Jr, Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Striplin CD, Kalivas PW. Correlation between behavioral sensitization to cocaine and G protein ADP-ribosylation in the ventral tegmental area. Brain Res. 1992;579:181–186. doi: 10.1016/0006-8993(92)90049-f. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Tang WX, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911–924. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Toda S, McGinty JF, Kalivas PW. Repeated cocaine administration alters the expression of genes in corticolimbic circuitry after a 3-week withdrawal: a DNA macroarray study. J Neurochem. 2002;82:1290–1299. doi: 10.1046/j.1471-4159.2002.01083.x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vankova M, Arluison M, Leviel V, Tramu G. Afferent connections of the rat substantia nigra pars lateralis with special reference to peptide-containing neurons of the amygdalo–nigral pathway. J Chem Neuroanat. 1992;5:39–50. doi: 10.1016/0891-0618(92)90032-l. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol (Oxf) 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS. Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic, and adrenergic cell groups in the rat. Brain Res Bull. 1992;28:447–454. doi: 10.1016/0361-9230(92)90046-z. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- White FJ, Xiu Y-H, Henry DJ, Zhang X-F. Neurophysiological alterations in the mesocorticolimbic doapmine system during cocaine administration. In: Hammer RP Jr, editor. The Neurobiology of Cocaine Addiction. CRC Press; Boca Raton: 1995. pp. 99–120. [Google Scholar]
- Wilson JM, Nobrega JN, Corrigall WA, Coen KM, Shannak K, Kish SJ. Amygdala dopamine levels are markedly elevated after self- but not passive-administration of cocaine. Brain Res. 1994;668:39–45. doi: 10.1016/0006-8993(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the ‘accumbens’ part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]