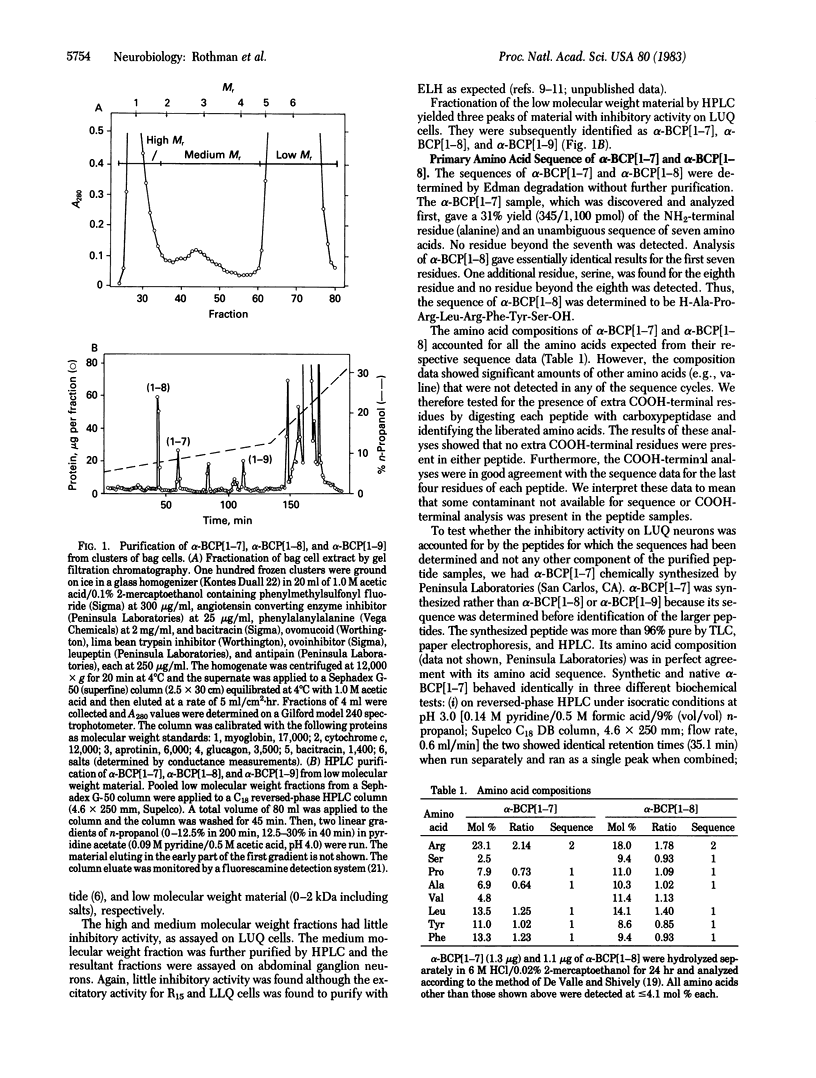
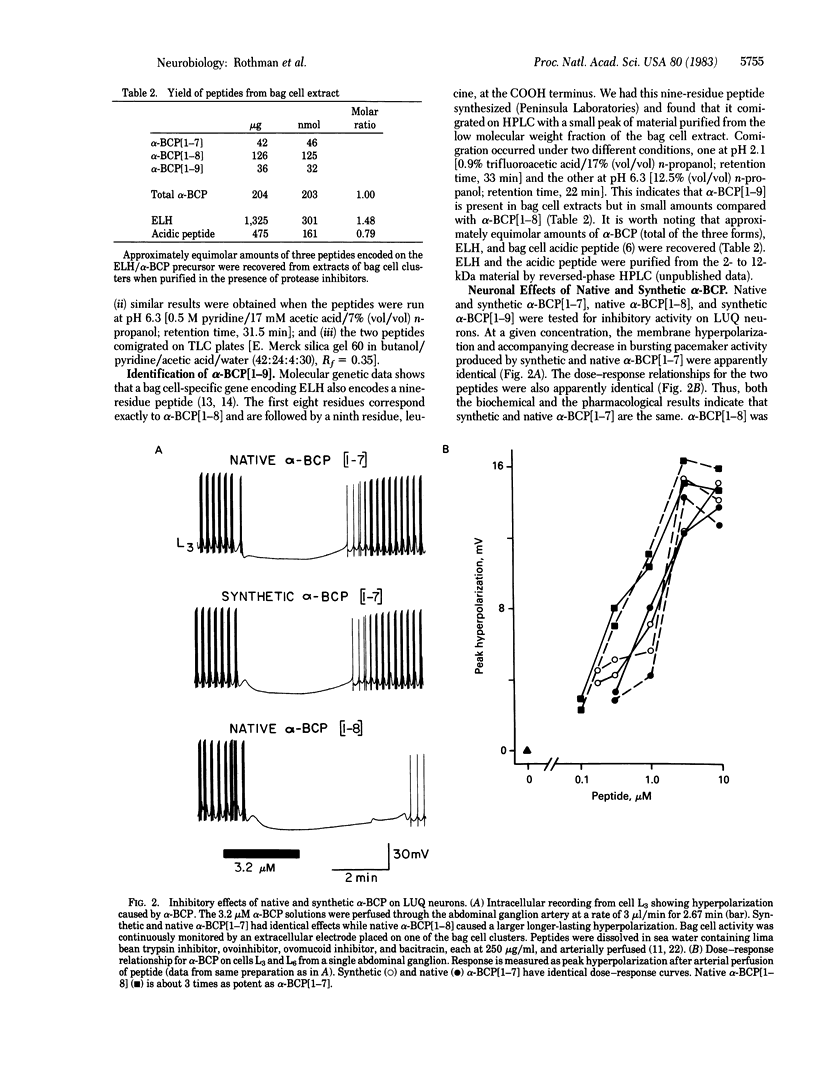
Abstract
A discharge of impulse activity in a group of neuroendocrine cells, the bag cells, produces several types of prolonged responses in various identified neurons of the abdominal ganglion of Aplysia. Two excitatory responses are almost certainly mediated by egg-laying hormone, but this peptide cannot account for other responses, such as inhibition of left upper quadrant neurons. We report here the isolation from bag cell clusters of three structurally similar peptides, seven, eight, and nine residues long, that are candidate transmitters for mediating bag cell-induced inhibition. They may also serve as autoexcitatory transmitters since the seven-residue peptide produces a slow depolarization of the bag cells similar to that which occurs during bag cell discharge. The amino acid sequence of the largest peptide, termed α-bag cell peptide[1-9], is H-Ala-Pro-Arg-Leu-Arg-Phe-Tyr-Ser-Leu-OH. The other two peptides are identical to α-BCP[1-9] except that they lack the COOH-terminal Ser-Leu or leucine residues. The three peptides inhibit left upper quadrant neurons at relative potencies of 10:30:1 (seven-, eight-, and nine-residue peptides, respectively). Recent molecular genetic analysis shows that both α-BCP[1-9] and egg-laying hormone are encoded by the same bag cell-specific gene. The multiple neuronal effects of bag cells are therefore likely to be mediated by at least two transmitters that are cleaved from a common precursor molecule.
Keywords: high pressure liquid chromatography, autotransmitter, egg-laying behavior, co-transmitter, long-term inhibition
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch S. Biosynthesis of the egg-laying hormone (ELH) in the bag cell neurons of Aplysia californica. J Gen Physiol. 1972 Jul;60(1):102–119. doi: 10.1085/jgp.60.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch S., Earley P., Smock T. Biochemical isolation and physiological identification of the egg-laying hormone in Aplysia californica. J Gen Physiol. 1976 Aug;68(2):197–210. doi: 10.1085/jgp.68.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton W. D., Arch S., Smock T., Mayeri E. Evidence for mediation of a neuronal interaction by a behaviorally active peptide. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5732–5736. doi: 10.1073/pnas.75.11.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton W. D., Mayeri E., Brownell P., Simon S. B. Evidence for local hormonal communication between neurones in Aplysia. Nature. 1978 Jul 6;274(5666):70–72. doi: 10.1038/274070a0. [DOI] [PubMed] [Google Scholar]
- Brownell P., Mayeri E. Prolonged inhibition of neurons by neuroendocrine cells in Aplysia. Science. 1979 Apr 27;204(4391):417–420. doi: 10.1126/science.35827. [DOI] [PubMed] [Google Scholar]
- Chiu A. Y., Hunkapiller M. W., Heller E., Stuart D. K., Hood L. E., Strumwasser F. Purification and primary structure of the neuropeptide egg-laying hormone of Aplysia californica. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6656–6660. doi: 10.1073/pnas.76.12.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle U., Shively J. E. Two-column system for determination of glucosamine, galactosamine, and amino acids on a Beckman 121MB amino acid analyzer: separation of the anomers of glucosamine and galactosamine. Anal Biochem. 1979 Jul 1;96(1):77–83. doi: 10.1016/0003-2697(79)90556-6. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Stimulation of egg laying by extracts of neuroendocrine cells (bag cells) of abdominal ganglion of Aplysia. J Neurophysiol. 1970 Nov;33(6):877–881. doi: 10.1152/jn.1970.33.6.877. [DOI] [PubMed] [Google Scholar]
- Mayeri E., Brownell P., Branton W. D. Multiple, prolonged actions of neuroendocrine bag cells on neurons in Aplysia. II. Effects on beating pacemaker and silent neurons. J Neurophysiol. 1979 Jul;42(4):1185–1197. doi: 10.1152/jn.1979.42.4.1185. [DOI] [PubMed] [Google Scholar]
- Mayeri E., Brownell P., Branton W. D., Simon S. B. Multiple, prolonged actions of neuroendocrine bag cells on neurons in Aplysia. I. Effects on bursting pacemaker neurons. J Neurophysiol. 1979 Jul;42(4):1165–1184. doi: 10.1152/jn.1979.42.4.1165. [DOI] [PubMed] [Google Scholar]
- Pinsker H. M., Dudek F. E. Bag cell control of egg laying in freely behaving aplysia. Science. 1977 Jul 29;197(4302):490–493. doi: 10.1126/science.197.4302.490. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Four basic characteristics of the gastrin-cholecystokinin system. Am J Physiol. 1981 Apr;240(4):G255–G266. doi: 10.1152/ajpgi.1981.240.4.G255. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Jackson J. F., McAllister L. B., Rothman B. S., Mayeri E., Axel R. A single gene encodes multiple neuropeptides mediating a stereotyped behavior. Cell. 1983 Jan;32(1):7–22. doi: 10.1016/0092-8674(83)90492-0. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Jackson J. F., McAllister L. B., Schwartz J. H., Kandel E. R., Axel R. A family of genes that codes for ELH, a neuropeptide eliciting a stereotyped pattern of behavior in Aplysia. Cell. 1982 Apr;28(4):707–719. doi: 10.1016/0092-8674(82)90050-2. [DOI] [PubMed] [Google Scholar]
- Shively J. E. Sequence determinations of proteins and peptides at the nanomole and subnanomole level with a modified spinning cup sequenator. Methods Enzymol. 1981;79(Pt B):31–48. doi: 10.1016/s0076-6879(81)79011-6. [DOI] [PubMed] [Google Scholar]
- Stuart D. K., Chiu A. Y., Strumwasser F. Neurosecretion of egg-laying hormone and other peptides from electrically active bag cell neurons of Aplysia. J Neurophysiol. 1980 Feb;43(2):488–498. doi: 10.1152/jn.1980.43.2.488. [DOI] [PubMed] [Google Scholar]
- Stuart D. K., Strumwasser F. Neuronal sites of action of a neurosecretory peptide, egg-laying hormone, in Aplysia californica. J Neurophysiol. 1980 Feb;43(2):499–519. doi: 10.1152/jn.1980.43.2.499. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B. Amino acid sequence studies on ten ribosomal proteins of Escherichia coli with an improved sequenator equipped with an automatic conversion device. Hoppe Seylers Z Physiol Chem. 1973 Oct-Nov;354(10-11):1415–1431. doi: 10.1515/bchm2.1973.354.2.1415. [DOI] [PubMed] [Google Scholar]


