Summary
Caspase-8 or cFLIP deficiency leads to embryonic lethality in mice due to defects in endothelial tissues. Caspase-8−/−, RIPK3−/−, but not cFLIP−/−, RIPK3−/−, double-knockout animals develop normally, indicating that caspase-8 antagonizes the lethal effects of RIPK3 during development. Here we show that the acute deletion of caspase-8 in the gut of adult mice induces enterocyte death, disruption of tissue homeostasis and inflammation, resulting in sepsis and mortality. Likewise, acute deletion of caspase-8 in a focal region of the skin induces local keratinocyte death, tissue disruption and inflammation. Strikingly, RIPK3 ablation rescues both phenotypes. Acute loss of cFLIP in the skin produces a similar phenotype, which, however, is not rescued by RIPK3 ablation. TNF neutralization protects from either acute loss of caspase-8 or cFLIP. These results demonstrate that caspase-8-mediated suppression of RIPK3-induced death is required not only during development, but also for adult homeostasis. Furthermore, RIPK3-dependent inflammation is dispensable for the skin phenotype.
Introduction
Upon death receptor ligation, caspase-8 is recruited to initiator complexes through the adapter molecule FADD, and forms active homodimers by induced proximity, propagating the apoptotic signal (Fuentes-Prior and Salvesen, 2004). cFLIPL (FLIP), a caspase-8 homolog lacking the catalytic cysteine, is recruited to the same complexes, forms heterodimers with caspase-8 and blocks the formation of the pro-apoptotic caspase-8 homodimers (Krueger et al., 2001). Caspase-8-, FADD-, or FLIP-deficient embryos die around embryonic day 10.5 associated with a failure to remodel the yolk sac vasculature (Varfolomeev et al., 1998) (Yeh et al., 2000; Yeh et al., 1998), an effect unrelated to the ability of caspase-8 to promote apoptosis. Further, animals with conditional deletion of caspase-8 with endothelium-specific Tie-1 promoter die with the same gross pathology and at the same developmental stage as do caspase-8-deficient embryos (Kang et al., 2004). No embryonic lethality was observed when caspase-8 was deleted elsewhere, including the heart (Dillon et al., 2012), liver (Kang et al., 2004), myeloid cells (Kang et al., 2004), and B or T lymphocytes (Beisner et al., 2005; Salmena et al., 2003). In skin (Kovalenko et al., 2009; Lee et al., 2009), or gut (Gunther et al., 2011), deletion of caspase-8 results in post-natal lethality due to loss of barrier function and inflammation.
Receptor Interacting Protein Kinase-3 (RIPK3) promotes an alternative mode of cell death with characteristics of necrosis, often called “necroptosis” (Zhang et al., 2009). Ablation of RIPK3 fully rescues the development of mice lacking caspase-8, FADD, or both FADD and FLIP (Dillon et al., 2012; Kaiser et al., 2011; Oberst et al., 2011). These and other approaches showed that the FADD-induced heterodimer of caspase-8 and FLIP suppresses RIPK3-mediated lethality (Dillon et al., 2012; Oberst et al., 2011). In this paper, we address whether the developmental roles of caspase-8 and FLIP in suppressing the lethal effects of RIPK3 persist into adult life.
Results and Discussion
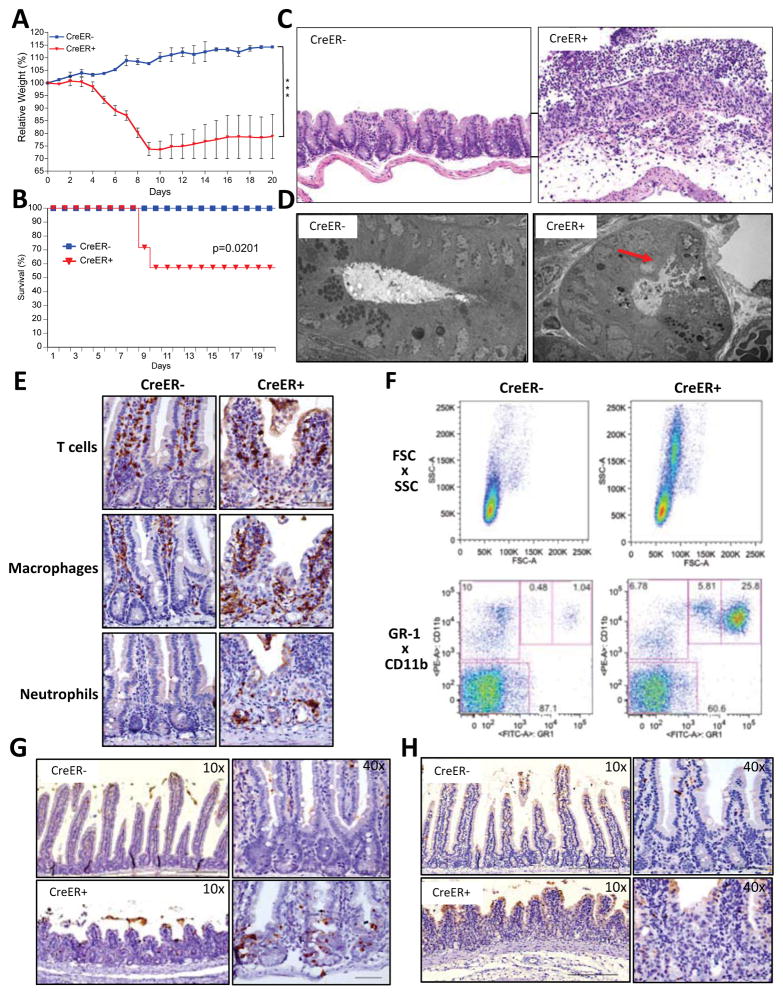
In order to achieve acute, systemic deletion of caspase-8 in adult animals, Rosa26.CreER+ (CreER+), Caspase-8flox/flox (casp8f/f) animals were gavaged with tamoxifen. Acute caspase-8 deletion through tamoxifen gavage induced severe weight loss and lethality in Cre+ animals, but not in Cre− littermates (Fig. 1A–B). Cre+ animals that survived the treatment recovered weight over time (Fig. S1A). Neither the activation of Cre in WT animals (Fig. S1B) nor the deletion of an unrelated gene (Fig. S1C) induced weight loss. Therefore, the effects were specifically caused by acute caspase-8 ablation.
Figure 1. Acute deletion of caspase-8 induces cell death, tissue damage, loss of weight and lethality.
(A–H) Rosa26.CreER−, casp8f/f (CreER−) and Rosa26.CreER+, casp8f/f (CreER+) animals were gavaged with 1mg tamoxifen per 25g animal body weight for 6 consecutive days. Animals were observed over 20 days for weight loss (A) and lethality (B). (C) Day +9 cecum sections from tamoxifen-treated animals were stained with hematoxylin and eosin. (D) Transmission electron microscopy of proximal small intestine crypts at day +6. Arrow points to necrotic cell. (E) Proximal small intestine sections at day +9 were immunostained for T cells (anti-CD3), macrophages (anti-F4/80) and neutrophils (anti-LY-6B.2). (F) Peripheral blood leukocytes at day +9 were stained with anti-CD11b and anti-GR-1 and then analyzed by FACS. Upper panels show cell size (FSC-A) versus granulosity (SSC-A) while the lower panels show anti-GR-1 versus anti-CD11b. Proximal small intestine sections from day +6 were stained for TUNEL (G) or cleaved caspase-3 (H). (***)p<0.001.
To assess the efficiency of gene recombination, CreER+ animals were crossed to lox/stop/lox-YFP (LSL-YFP) animals. Upon gavage, these animals showed widespread YFP expression throughout the gut (Fig. S1D), lymph nodes, spleen, thymus (Fig. S1E) and peripheral blood leukocytes (Fig. S1F). Direct assessment of caspase-8 deletion was further performed by PCR in tamoxifen-treated CreER+, casp8f/f animals. Gene deletion was detected in all tissues and was nearly complete throughout the gut (Fig. S1G), consistent with the YFP expression pattern in the LSL-YFP reporter animal (Fig. S1D–F).
Histology during the period of acute weight loss showed major disruption of tissue homeostasis and organization, with vacuolation of the villi, enterocyte death, marked inflammation and infiltration of immune cells (Fig. 1C–E, S1H). The disruption of tissue homeostasis occurred throughout the intestines, including the small intestine (Fig. S1H, top row), cecum (Fig. 1C) and colon (Fig. S1H, bottom row). Infiltration was by macrophages (F4/80+) and neutrophils (Ly6B.2+), with a minor infiltration of T cells (CD3+) (Fig. 1E). Analysis of the peripheral blood leukocytes showed a marked increase in granulocytes (Fig. 1F).
No significant alterations were observed in other organs, including liver, brain, heart, kidney, lungs and lymphoid organs during the course of these experiments (data not shown). Indeed, blood cultures from casp8-deleted animals detected gut-resident microorganisms (Fig. S2A), and oral-gavaged FITC-Dextran was detected in the peripheral blood of CreER+, casp8f/f animals, indicating abnormal gut permeability (Fig. S2B). Therefore, the weight loss and lethality in CreER+, casp8f/f animals were most likely related to disruption of the gut epithelial barrier.
Histology of the gut in the pre-weight loss phase upon casp8 deletion revealed early enterocyte cell death, by H&E and TUNEL staining, especially in the crypts and in the base of the villi, before any signs of inflammation (Fig. 1G and Fig. S1I). Most TUNEL-positive cells did not stain for cleaved caspase-3 (Fig. 1H), indicating that apoptosis was not the prevalent mode of cell death. In addition to apoptosis, TUNEL positivity in vivo can result from DNAse-II-mediated digestion of engulfed non-apoptotic cell corpses (Mcllroy et al., 2000). Indeed, ultrastructure of these dying cells resembled that of necrotic cells (Fig. 1D). Although there is no specific marker for necroptosis, the combination of H&E, TUNEL, cleaved caspase-3 staining and ultrastructure is evidence for non-apoptotic, necrosis-like cell death (Bonnet et al., 2011; Gunther et al., 2011; Welz et al., 2011).
The liver, a major site for the conversion of tamoxifen into its active form 4-hydroxytamoxifen (4OHT), was not affected by acute casp8 deletion (Fig. S1G and data not shown). Liver is sensitive to concavalin A (ConA)-induced cell death that can be blocked by the RIPK1 inhibitor, necrostatin-1 (Jouan-Lanhouet et al., 2012). However, ConA-injected RIPK3 deficient animals displayed the same levels of liver damage as their control littermates (Fig. S3A–C). Damaged areas in the liver were cleaved caspase-3+, indicating apoptosis (Fig. S3C–D). This is in agreement with the finding that liver-specific ablation of caspase-8 has no pathological effect (Kang et al., 2004). Moreover, neither TNF nor anti-CD95, in combination with the pan-caspase inhibitor zVAD-fmk, induces liver necrosis (Chandler et al., 1998; Kunstle et al., 1997) and liver-specific ablation of caspase-8 is protective against anti-CD95-induced liver damage (Kang et al., 2004). Hence, there is no evidence to date that liver cells can undergo RIPK3-dependent necrosis; rather, they seem to be refractory to this type of cell death.
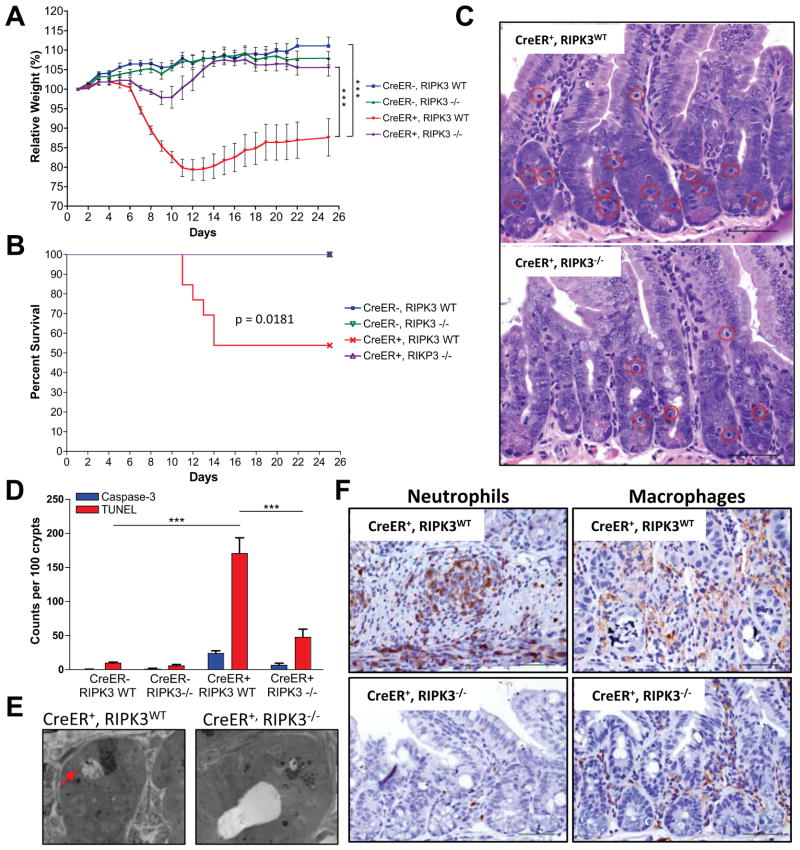
Embryonic lethality in caspase-8−/− mice can be fully rescued by ablation of RIPK3 (Kaiser et al., 2011; Oberst et al., 2011); therefore four genotypes were examined: CreER−, casp8f/f, ripk3+/+; CreER−, casp8f/f, ripk3−/−; CreER+, casp8f/f, ripk3+/+ and CreER+, casp8f/f, ripk3−/−. Strikingly, RIPK3 deficiency protected animals from the weight loss induced by acute casp8 deletion (Fig. 2A, Fig. S2C). Moreover, no lethality was observed in the CreER+, casp8f/f, ripk3−/− animals (Fig. 2B).
Figure 2. RIPK3 deficiency protects from acute deletion of caspase-8.
(A–F) Rosa26.CreER−, casp8f/f, ripk3+/+ (CreER−, RIPK3WT), Rosa26.CreER−, casp8f/f ripk3−/− (CreER−, RIPK3−/−), Rosa26.CreER+, caspase-8flox/flox, RIPK3+/+ (CreER+, RIPK3WT) and Rosa26.CreER+, casp8f/f, ripk3−/− (CreER+, RIPK3−/−) animals were gavaged with 1mg tamoxifen per 25g animal body weight for 6 consecutive s. Animals were observed over 25 days for weight loss (A) and lethality (B). (C) Day +6 proximal small intestine sections from tamoxifen-treated CreER+, RIPK3WT and CreER+, RIPK3−/− animals were stained with hematoxylin and eosin. Red circles highlight dead cells. (D) Proximal small intestine sections were stained for TUNEL and cleaved caspase-3 and the number of TUNEL-positive or cleaved caspase-3 positive cells per 100 crypts was determined by manual counts. (E) Transmission electron microscopy of proximal small intestine crypts at day +6. Arrow points to necrotic cell. (F) Day +9 proximal small intestine sections from tamoxifen-treated CreER+, RIPK3WT and CreER+, RIPK3−/− animals were immunostained for neutrophils (anti-LY-6B.2) (left panel) or macrophages (anti-F4/80) (right panel). (***)p<0.001.
A marked reduction in the number of dying enterocytes was observed during the pre-weight loss phase in CreER+, casp8f/f, ripk3−/− animals as compared to their CreER+, casp8f/f, ripk3WT littermates (Fig. 2C), as was confirmed by TUNEL and cleaved caspase-3 staining (Fig. 2D, S2D–E) and ultrastructure (Fig. 2E). RIPK3-dependent death of enterocytes during the early phases of tamoxifen treatment was associated with more intact gut tissue architecture at later time points (Fig. S2F). At the peak of weight loss and disruption of gut homeostasis in CreER+, casp8f/f, ripk3WT animals, CreER+, casp8f/f, ripk3−/− mice showed dramatically lower levels of edema and infiltration of the gut (Fig. S2F), including the small intestine (Fig. 2F) and the cecum (Fig. S2G). No FITC-Dextran or bacteria were found in the peripheral blood in the these animals, indicating that there was no gut epithelial barrier breakdown or subsequent infection (Fig. S2A–B).
Collectively, these findings demonstrate that caspase-8 is required for normal gut homeostasis during adulthood, and upon its acute deletion, RIPK3-mediated effects, including necrosis, can induce rapid and extensive gut damage, leading to epithelial barrier disruption and sepsis.
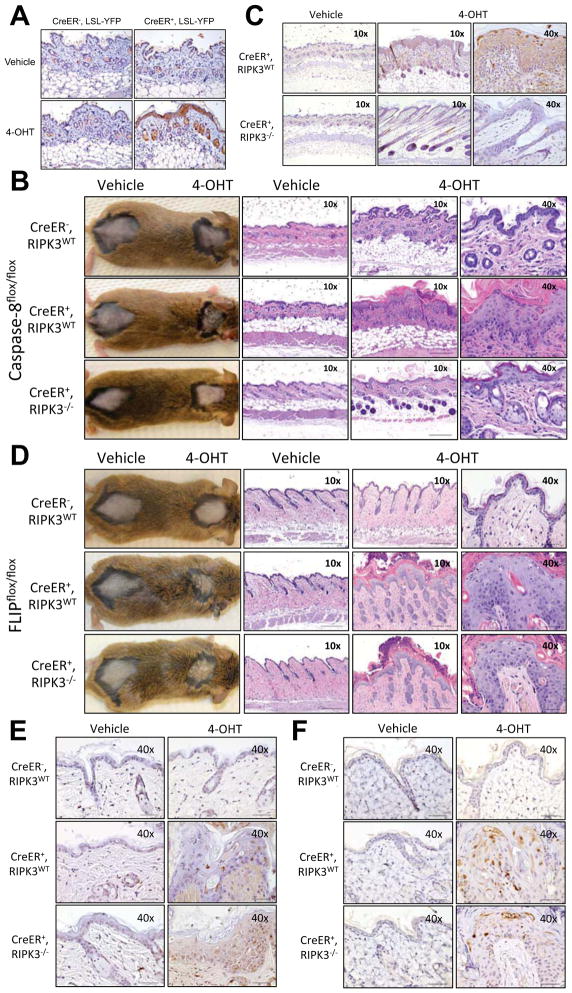
Skin homeostasis can also be severely affected by tissue-specific loss of caspase-8 during development (Kovalenko et al., 2009; Lee et al., 2009). Therefore, we painted skin with 4OHT to assess the effects of a localized, acute loss of caspase-8 in adult animals. YFP expression in CreER+, LSL- YFP animals was analyzed 24h after the last day of painting showed YFP expression, while no YFP+ cells were detected in the vehicle-only painted area of the same animal (Fig. 3A).
Figure 3. Acute deletion of caspase-8 or FLIP in the skin by 4-hydroxytamoxifen painting produces local inflammation and tissue damage.
Animals were shaved in two distinct dorsal areas. The shaved area around the neck was painted with 4-hydroxytamoxifen (40HT) while the shaved area near the tailbase was painted only with vehicle. (A) Histological sections from the painted skin areas from Rosa26.CreER−, LSL-YFP (CreER−, LSL-YFP) and Rosa26.CreER+, LSL-YFP (CreER+, LSL-YFP) were taken the day after the last painting and stained with anti-GFP antibodies to detect YFP expression. (B–C) Rosa26.CreER−, casp8f/f ripk3+/+ (CreER−, RIPK3WT), Rosa26.CreER+, casp8f/f, ripk3+/+ (CreER+, RIPK3WT) and Rosa26.CreER+, casp8f/f ripk3−/− (CreER+, RIPK3−/−) were painted four times and at day +12 (B) photos and H&E-stained sections or (C) TUNEL-stained sections were produced. (D–F) Rosa26.CreER−, cflarf/f ripk3+/+ (CreER+, RIPK3WT), Rosa26.CreER+, cflarf/f, ripk3+/+ (CreER+, RIPK3WT) and Rosa26.CreER+, cflarf/f, ripk3−/− (CreER+, RIPK3−/−) were painted two times and at day +10 (D) photos and H&E-stained sections, (E) TUNEL-stained sections or (F) cleaved caspase-3 were produced.
Local deletion of casp8 in the skin induced a large ulcerative area, with epidermal hyperplasia and marked superficial hyperkeratosis, forming a thick crust (Fig. 3B, left panel), with dyskeratotic foci and central pustule formation, edema, and immune cell infiltration (Fig. 3B, right panel). Enhanced keratinocyte death was evidenced by TUNEL staining (Fig. 3C). In contrast, 4OHT-painted areas from CreER+, casp8f/f, ripk3−/− mice showed minimal epidermal hyperplasia, rare dead keratinocytes and minimal dermal inflammation (Fig. 3B–C). Similarly, embryonic skin-specific deletion of FADD induced chronic skin inflammation, which was also blocked by concurrent ablation of RIPK3 (Bonnet et al, 2011).
All previous models of skin-specific Cre-induced deletion of caspase-8 or FADD used either the K5 or the K14 promoters, which are first expressed during embryonic development (Bonnet et al., 2011; Kovalenko et al., 2009; Lee et al., 2009). It is, therefore, possible that the effects seen in young animals were the result of loss during development. This same argument can be made for models of gut-specific deletion of caspase-8, FADD or FLIP employing the Villin-Cre promoter, which is also expressed during embryogenesis (Gunther et al., 2011; Piao et al., 2012; Welz et al., 2011). Our results demonstrate that caspase-8 is required not only during development but also are essential to maintain skin and gut homeostasis in adult animals.
Recently, RIPK3 activation was suggested to be involved not only in necroptosis induction but also in inflammatory responses (Kang et al., 2012; Vince et al., 2012). Ripk3−/− bone marrow-derived macrophages (BMDM), however, presented similar levels of pro-inflammatory cytokine production compared to RIPK3-sufficient BMDM both after stimulation with LPS or infection with E. coli (Fig. S4A). Likewise, RIPK3 deficiency did not influenced LPS-induced cytokine production in vivo (Fig. S4B). The detection of RIPK3-dependent necrosis at early time points, when no signs of inflammation were observed (Fig. S1I), together with the similar pro-inflammatory response to LPS between the RIPK3-sufficient and -deficient mice (Figure S4A–B), suggests that cell death may be the major driving force behind the damaging outcomes of caspase-8 loss in adult animals. To directly test this, a model in which cflar (encoding FLIP), rather than casp8, is acutely deleted in the skin was developed. In the absence of FLIP, caspase-8 fails to block RIPK3-mediated necroptosis (Oberst et al., 2011), and can also sensitize to FADD-caspase-8-dependent apoptosis (Dillon et al., 2012; Irmler et al., 1997; Yeh et al., 2000). 4OHT-painted skin areas from CreER+, cflarf/f, ripk3+/+ presented a similar phenotype to that observed in acute casp8 deletion, with epidermal hyperplasia, superficial hyperkeratosis, and the formation of a thick crust (Fig. 3D, left panel), with enhanced edema and infiltration (Fig. 3D, right panels) as well as higher levels of cell death, evidenced either by TUNEL (Fig. 3E) or cleaved caspase-3 staining (Fig. 3F). In contrast to acute casp8 deletion, apoptosis rather than necrosis was responsible for the cell death. Further, RIPK3 ablation was not able to prevent or lessen the skin disease, as CreER+, cflarf/f, ripk3−/− presented similar levels of skin damage when painted with 4OHT (Fig. 3D–F and S4C).
Therefore, regardless of the mode of cell death, the consequences of acute deletion of either caspase-8 or FLIP appear to produce similar pathologies, yet with different time courses (Fig. S4C–D), perhaps due to the relative stabilities of the proteins (Fulda et al., 2000). Since, in the latter case, cells can only undergo apoptosis and the phenotype developed independently of RIPK3-mediated pathways, it is reasonable to conclude that RIPK3-induced inflammation is not required in this model. Further, this implies that apoptosis and RIPK3-mediated necrosis must be actively suppressed to maintain skin tissue homeostasis, which is in agreement with the observation that FLIP embryonic lethality is only rescued by ablation of both RIPK3 and FADD (Dillon et al., 2012).
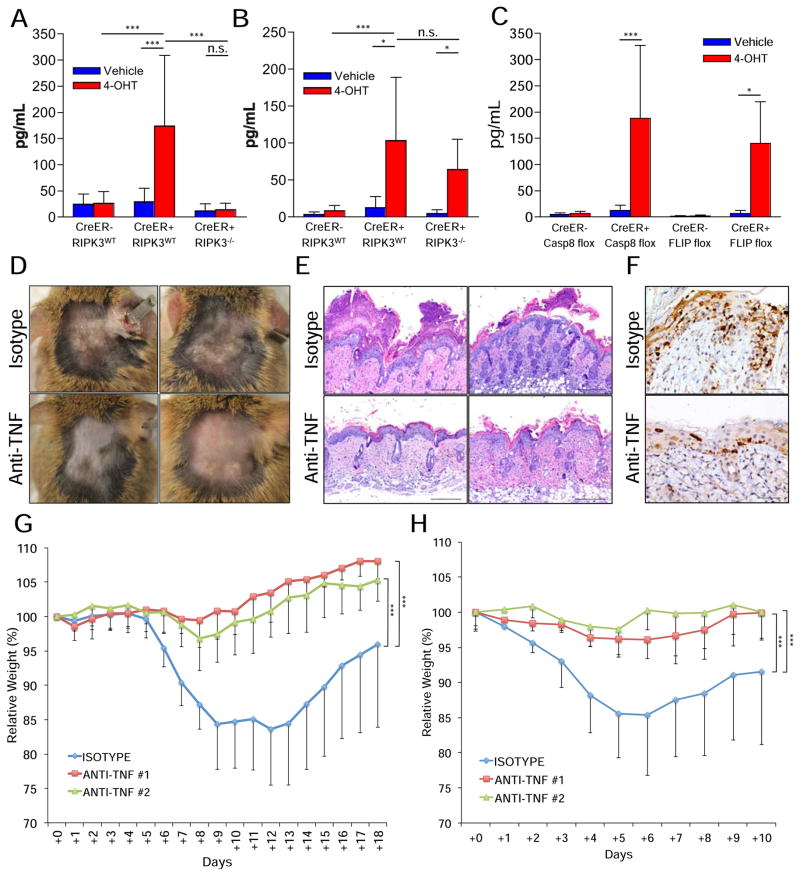
We suggest that either necroptotic or apoptotic cell death is responsible for the inflammatory effects. However, while necrosis is known to be pro-inflammatory, apoptosis is not (Green, 2010). Thus, it is possible that the inflammatory response is due to cell death-mediated loss of barrier function, rather than an effect of dying cells on the inflammatory response. Indeed, increased production of IL-1β in the skin correlates with the skin damage rather than with RIPK3-induced inflammation (Fig. 4A–B). Moreover, loss of gut tissue homeostasis by embryonic gut-specific ablation of FADD is attenuated by treatment with antibiotics as well as by germ-free conditions (Gunther et al., 2011).
Figure 4. Neutralization of TNF protects from the deleterious effects of caspase-8 or FLIP acute deletion.
(A–E) Animals were shaved in two distinct dorsal areas. The shaved area around the neck was painted with 4-hydroxytamoxifen (4OHT) while the shaved area near the tailbase was painted only with vehicle. (A–B) IL-1b levels in the skins of (A) Casp8f/f animals at day +10 or (B) cflarf/f animals at day +5. (C) TNF levels in the skins of casp8f/f (Casp8 flox) animals at day +10 or cflarf/f (FLIP flox) animals at day +5. (D–F) CreER+, cflarf/f animals were i.p. injected at days −1, +1 and +3 with 0.5mg of anti-TNF antibodies (clone XT3.11) or isotype control antibodies (clone HRPN) and examples of (D) photos, (E) sections stained with hematoxylin and eosin (40X) and (F) cleaved caspase-3 staining sections (10X) were produced at day +8. (G) CreER+, casp8f/f animals were gavaged with 1mg tamoxifen per 25g animal body weight for 6 consecutive days and i.p. injected at days +0, +2 and +4 with 0.5mg of two different neutralizing anti-TNF antibodies (#1 = XT3.11, #2 = HB10649) or isotype control (clone HRPN). Weight loss was followed for 18 days. (H) CreER+, cflarf/f animals were treated as in (G), but gavaged only twice, at days +0 and +2, and their weights were tracked for 10 days. (*) p<0.05. (***) p<0.001. (n.s.) not statistically significant.
Caspase-8 was suggested to suppress the exacerbated RIPK3-mediated inflammatory response to normal skin cornification via RIG-I and its adaptor protein MAVS (Rajput et al., 2011; Wallach et al., 2010). To assess the contribution of this pathway to the observed skin phenotype, CreER+, casp8f/f, mavs−/− animals were generated. We found, however, that the pathological effects of acute deletion of casp8 in the skin were not rescued by ablation of MAVS (Fig. S4E), demonstrating that caspase-8 suppression of the RIG-I/MAVS-pathway is not essential for skin homeostasis.
TNF, via its receptor TNF-R1, is the best-described trigger of necroptosis, both in vitro and in vivo, and it is also a well known-inducer of apoptosis (Weinlich et al., 2011). Upon acute deletion of either casp8 or cflar, TNF is produced at detectable levels in the skin at early stages of disease (Fig. 4C). Therefore, CreER+, cflarf/f, animals were injected with neutralizing anti-TNF or control isotype antibodies and painted with 40HT (Fig. 4D–E). While all isotype-injected animals fully developed the skin disease, anti-TNF treatment resulted in a marked protection: either they did not show any signs of the disease or presented much milder lesions (Figure 4D–F).
To further analyze the role of TNF, CreER+, casp8f/f or CreER+, cflarf/f animals were gavaged with tamoxifen simultaneously with injections of two different neutralizing anti-TNF antibodies or isotype control. Both anti-TNF antibodies protected from the effects of acute deletion of casp8 (Fig. 4G) or cflar (Fig. 4H). These data indicate that, independent of the cell death type induced, TNF plays a central role in the onset of the disease.
The therapeutic use of pan-caspase inhibitors has been proposed and, to date, no severe toxic effects of these inhibitors have been reported in mice (Callus and Vaux, 2007) or humans (Pockros et al., 2007; Ratziu et al., 2012). Genetic studies that rescued caspase-8-deficiency in vivo (Kaiser et al., 2011; Oberst et al., 2011), as well as in vitro studies on inhibition of RIPK3-dependent necrosis (Oberst et al., 2011), have shown that the protease activity of caspase-8 is required for its protective effects. Therefore, in light of our results, a closer evaluation of how these inhibitors affect caspase-8 activity in vivo, as well as their pharmacokinetics, bioavailability and tissue distribution is warranted. This might be particularly important in patients with elevated levels of circulating TNF, as the addition of exogenous TNF to animals lacking intestinal caspase-8 strikingly exacerbated damage to gut tissue (Gunther et al., 2011). Furthermore, TNF-induced shock as well as hyperacute shock induced by TNF in combination with caspase inhibitors severely impact the gut and are mainly mediated by RIPK3 (Duprez et al., 2011; Linkermann et al., 2012). Attempts to generate pan-caspase inhibitors that may be more active in vivo may therefore reveal toxicities associated with the effects we describe.
Experimental Procedures
Tamoxifen Treatments
To induce systemic deletion of caspase-8, animals were gavaged for six consecutive days with tamoxifen (cat#T5648, Sigma) dissolved in sunflower seed oil (cat#S5007, Sigma) at a concentration of 1mg tamoxifen per 25g of animal body weight per day. Weight loss, morbidity, and mortality were assessed everyday. Acute deletion of FLIP was induced by two rounds of gavage with a 48h interval between them.
To induce localized deletion of caspase-8 or cFLIP in the skin, animals were shaved in two different dorsal areas (one around the neck and one near the tailbase) using a hair trimmer. The dorsal area around the neck was painted four times, once every other day, for casp8f/f animals and twice, once every other day, for cflarf/f animals with 100ul of 10mg/mL 4-hydroxytamoxifen (cat#H6278, Sigma) dissolved in ethanol. The dorsal area near the tailbase was painted only with vehicle and served as an internal control area.
For further Experimental Procedures, please see supplemental info online.
Supplementary Material
Highlights.
Acute deletion of caspase-8 or cFLIP in the gut or skin disrupts tissue homeostasis
Ablation of RIPK3 rescues the damaging effects of acute caspase-8 but not cFLIP loss
RIPK3-mediated inflammation is dispensable for the skin damage by acute cFLIP loss
Neutralization of TNF rescues from the effects of acute loss of caspase-8 or cFLIP
Acknowledgments
The authors thank Patrick Fitzgerald and Laura L. McCormick for providing essential logistical and administrative support, and maintenance of the mouse colony, respectively. We thank Sharon Frase and Fara Sudlow, from the St. Jude Cellular Image Shared Resource, for providing technical support for the electron microscopy analysis. We thank Dr. Peter Murray and Dr. Scott Brown from St. Jude Children’s Research Hospital, for kindly providing key reagents for this project. We also thank the St. Jude Animal Resource Center and the Veterinary Pathology Core Histology Lab. Supported by CA169291, AI44828, and AI47891 from the U.S. National Institutes of Health, and by the American Lebanese Syrian Associated Charities. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beisner DR, Ch’en IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175:3469–3473. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35:572–582. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Callus BA, Vaux DL. Caspase inhibitors: viral, cellular and chemical. Cell Death Differ. 2007;14:73–78. doi: 10.1038/sj.cdd.4402034. [DOI] [PubMed] [Google Scholar]
- Chandler JM, Cohen GM, MacFarlane M. Different subcellular distribution of caspase-3 and caspase-7 following Fas-induced apoptosis in mouse liver. J Biol Chem. 1998;273:10815–10818. doi: 10.1074/jbc.273.18.10815. [DOI] [PubMed] [Google Scholar]
- Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, Declercq W, Libert C, Cauwels A, Vandenabeele P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384:201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Meyer E, Debatin KM. Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by down-regulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression. Cancer Res. 2000;60:3947–3956. [PubMed] [Google Scholar]
- Green DR. Means to and End: Apoptosis and Other Cell Death Mechanisms. 1. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2010. [Google Scholar]
- Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F, Takahashi N, Sergent O, Lagadic-Gossmann D, Vandenabeele P, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19:2003–2014. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 Blocks Kinase RIPK3-Mediated Activation of the NLRP3 Inflammasome. Immunity. 2012;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Kovalenko A, Kim JC, Kang TB, Rajput A, Bogdanov K, Dittrich-Breiholz O, Kracht M, Brenner O, Wallach D. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633–20640. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- Kunstle G, Leist M, Uhlig S, Revesz L, Feifel R, MacKenzie A, Wendel A. ICE-protease inhibitors block murine liver injury and apoptosis caused by CD95 or by TNF-alpha. Immunol Lett. 1997;55:5–10. doi: 10.1016/s0165-2478(96)02642-9. [DOI] [PubMed] [Google Scholar]
- Lee P, Lee DJ, Chan C, Chen SW, Ch’en I, Jamora C. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature. 2009;458:519–523. doi: 10.1038/nature07687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A, Brasen JH, De Zen F, Weinlich R, Schwendener RA, Green DR, Kunzendorf U, Krautwald S. Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-alpha-induced shock. Mol Med. 2012;18:577–586. doi: 10.2119/molmed.2011.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy D, Tanaka M, Sakahira H, Fukuyama H, Suzuki M, Yamamura K, Ohsawa Y, Uchiyama Y, Nagata S. An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev. 2000;14:549–558. [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao X, Komazawa-Sakon S, Nishina T, Koike M, Piao JH, Ehlken H, Kurihara H, Hara M, Van Rooijen N, Schutz G, et al. c-FLIP Maintains Tissue Homeostasis by Preventing Apoptosis and Programmed Necrosis. Sci Signal. 2012;5:ra93. doi: 10.1126/scisignal.2003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros PJ, Schiff ER, Shiftman ML, McHutchison JG, Gish RG, Afdhal NH, Makhviladze M, Huyghe M, Hecht D, Oltersdorf T, et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46:324–329. doi: 10.1002/hep.21664. [DOI] [PubMed] [Google Scholar]
- Rajput A, Kovalenko A, Bogdanov K, Yang SH, Kang TB, Kim JC, Du J, Wallach D. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–351. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Sheikh MY, Sanyal AJ, Lim JK, Conjeevaram H, Chalasani N, Abdelmalek M, Bakken A, Renou C, Palmer M, et al. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55:419–428. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, Tamblyn L, Shehabeldin A, Migon E, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O’Reilly L, Mason K, Gross O, Ma S, Guarda G, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Wallach D, Kang TB, Rajput A, Kim JC, Bogdanov K, Yang SH, Kovalenko A. Anti-inflammatory functions of the “apoptotic” caspases. Ann N Y Acad Sci. 2010;1209:17–22. doi: 10.1111/j.1749-6632.2010.05742.x. [DOI] [PubMed] [Google Scholar]
- Weinlich R, Dillon CP, Green DR. Ripped to death. Trends Cell Biol. 2011;21:630–637. doi: 10.1016/j.tcb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.