Abstract
Many hypotheses for the specificity of connections in the nervous system postulate the presence of surface chemical differences between neurons. Hybridoma technology offers a potential route to identify such surface antigenic differences between neurons. Monoclonal antibody Cat-301 was one of a panel of antibodies generated by immunizing mice with homogenized adult cat spinal cord. At the light microscopic level, Cat-301 recognizes a subset of neurons in many areas of the vertebrate central nervous system. This report shows at the ultrastructural level that Cat-301 binds to a surface antigen on neurons in the intact vertebrate central nervous system. Cat-301-positive neurons carry the antigen on cell bodies and proximal dendrites but not on axons. Using secondary antibody labeled with horseradish peroxidase, we show that antibody binding sites are present along the surfaces of neurons and extend around presynaptic profiles but are excluded from the synaptic cleft. The distribution of the Cat-301 antigen at central synapses is similar to that described for some components of the extracellular matrix of the neuromuscular junction. This study demonstrates that a specific surface antigen is found on a subset of neurons and suggests that other surface markers may be present on other subsets of mammalian central nervous system neurons. Antibodies against this antigen and other surface antigens may allow insight into the mechanisms involved in the formation and maintenance of synaptic connections in the central nervous system.
Full text
PDF
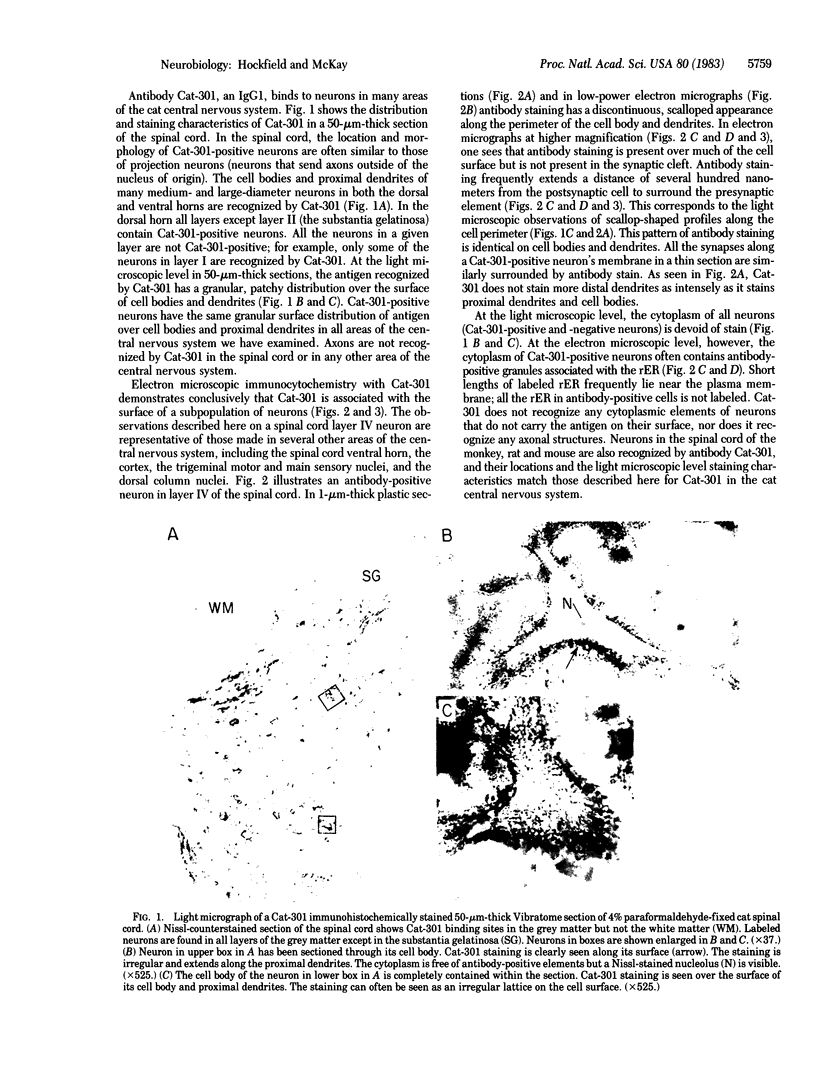
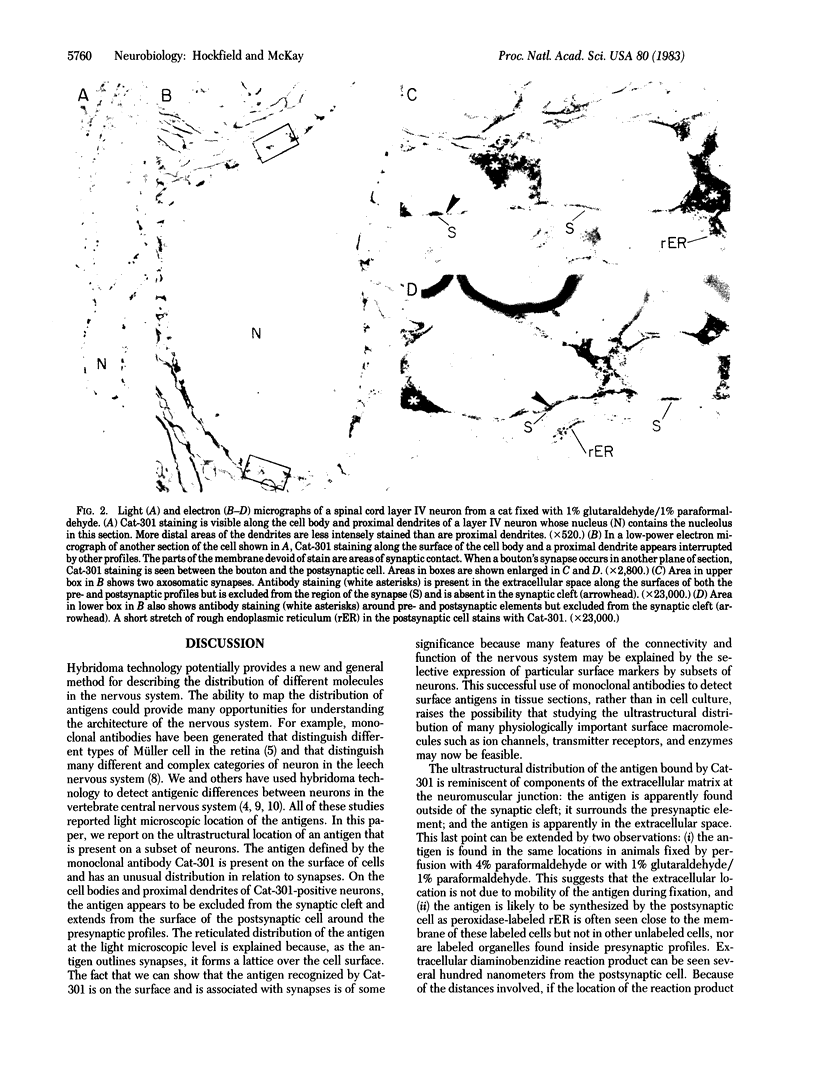

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J. Monoclonal antibodies which recognize different cell types in the rat retina. Nature. 1980 Jul 17;286(5770):231–235. doi: 10.1038/286231a0. [DOI] [PubMed] [Google Scholar]
- Betz W., Sakmann B. Effects of proteolytic enzymes on function and structure of frog neuromuscular junctions. J Physiol. 1973 May;230(3):673–688. doi: 10.1113/jphysiol.1973.sp010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden S. J., Sargent P. B., McMahan U. J. Acetylcholine receptors in regenerating muscle accumulate at original synaptic sites in the absence of the nerve. J Cell Biol. 1979 Aug;82(2):412–425. doi: 10.1083/jcb.82.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Matus A. Monoclonal antibodies identify novel neural antigens. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2410–2414. doi: 10.1073/pnas.79.7.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Salpeter S. R. Organization of acetylcholine receptors in quick-frozen, deep-etched, and rotary-replicated Torpedo postsynaptic membrane. J Cell Biol. 1979 Jul;82(1):150–173. doi: 10.1083/jcb.82.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockfield S., McKay R. Monoclonal antibodies demonstrate the organization of axons in the leech. J Neurosci. 1983 Feb;3(2):369–375. doi: 10.1523/JNEUROSCI.03-02-00369.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay R. D., Hockfield S. J. Monoclonal antibodies distinguish antigenically discrete neuronal types in the vertebrate central nervous system. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6747–6751. doi: 10.1073/pnas.79.21.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. P., Fritz L. C., Raftery M. A., Brockes J. P. Isolation and characterization of a monoclonal antibody against the saxitoxin-binding component from the electric organ of the eel Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1673–1677. doi: 10.1073/pnas.79.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R. Roles of extracellular matrix in neural development. Annu Rev Physiol. 1983;45:581–600. doi: 10.1146/annurev.ph.45.030183.003053. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Harwell L. W., Sternberger N. H. Neurotypy: regional individuality in rat brain detected by immunocytochemistry with monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1326–1330. doi: 10.1073/pnas.79.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisler G. D., Schneider M. D., Nirenberg M. A topographic gradient of molecules in retina can be used to identify neuron position. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2145–2149. doi: 10.1073/pnas.78.4.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Lindstrom J. M. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci U S A. 1980 Feb;77(2):755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. N., Hudson L., Jessell T. M., Yamamoto M. A monoclonal antibody defining antigenic determinants on subpopulations of mammalian neurones and Trypanosoma cruzi parasites. Nature. 1982 Mar 4;296(5852):34–38. doi: 10.1038/296034a0. [DOI] [PubMed] [Google Scholar]
- Zipser B., McKay R. Monoclonal antibodies distinguish identifiable neurones in the leech. Nature. 1981 Feb 12;289(5798):549–554. doi: 10.1038/289549a0. [DOI] [PubMed] [Google Scholar]