Abstract
Objective:
Because some recent studies suggest increased risk for suicide-related behavior (SRB; ideation, attempts) among those receiving antiepileptic drugs (AEDs), we examined the temporal relationship between new AED exposure and SRB in a cohort of older veterans.
Methods:
We used national Veterans Health Administration databases to identify veterans aged ≥65 years who received a new AED prescription in 2004–2006. All instances of SRB were identified using ICD-9-CM codes 1 year before and after the AED exposure (index) date. We also identified comorbid conditions and medication associated with SRB in prior research. We used generalized estimating equations with a logit link to examine the association between new AED exposure and SRB during 30-day intervals during the year before and after the index date, controlling for potential confounders.
Results:
In this cohort of 90,263 older veterans, the likelihood of SRB the month prior to AED exposure was significantly higher than in other time periods even after adjusting for potential confounders. Although there were 87 SRB events (74 individuals) the year before and 106 SRB events (92 individuals) after, approximately 22% (n = 16) of those also had SRB before the index date. Moreover, the rate of SRB after AED start was gradually reduced over time.
Conclusions:
The temporal pattern of AED exposure and SRB suggests that, in clinical practice, the peak in SRB is prior to exposure. While speculative, the rate of gradual reduction in SRB thereafter suggests that symptoms may prompt AED prescription.
Since the US Food and Drug Administration (FDA) warning linking antiepileptic drugs (AEDs) to suicide-related behavior (SRB) in 2008,1 study findings examining SRB among AED users have been inconsistent. Some found increased risk for different individual AEDs,2,3 while others found that increased risk associated with AED exposure was primarily associated with depression,4 was not significant, or was attenuated after controlling for psychiatric comorbidity.3,5–7 These findings suggest the possibility of confounding by indication, where those at high risk (e.g., bipolar disorder, depression, chronic pain) are prescribed AEDs to address symptoms of these conditions.8–11 Simon et al.12 conducted a temporal analysis examining patterns of SRB before and after initiation of 3 types of therapy—antidepressant therapy in primary care, antidepressant therapy in psychiatric specialty care, or psychotherapy without antidepressant—and found that SRB peaked just before the initiation of therapy under all 3 conditions, supporting the possibility of confounding by indication in studies linking antidepressant prescriptions to SRB.12
In a recent study, we examined the association of new AED exposure and SRB in an older population since the FDA meta-analysis was unable to adequately address differences in SRB in the geriatric population.1 We found a significant association between AED exposure and SRB even after controlling for mood disorders and prior SRB.8 However, we did not systematically examine the temporal relationship between SRB and AED exposure. This study used a technique similar to the approach of Simon et al. to examine the temporal relationship between SRB and AED exposure in that cohort of older veterans.12
METHODS
We conducted a retrospective cohort study to examine the temporal relationship between new AED monotherapy exposure and SRB in older veterans.13 Given the limitations inherent in observational data analysis, we selected a new user monotherapy design, assessing the SRB 1 year before and 1 year after the AED exposure date among new AED users. This design can reduce the potential “survivor” bias that occurs when patients with chronic use are more likely to better tolerate a drug, and remain in the cohort.13 Moreover, new exposure designs allow the study to assign an index date to those with exposure, thus avoiding “chronology” bias similar to development of an inception cohort in disease epidemiology.13 While the characteristics of the new user design are a strength for these reasons, it does not really allow assessment of polytherapy combinations unless that combination is prescribed on the index treatment day, which occurred in fewer than 1% of new users in this cohort.
Standard protocol approvals, registrations, and patient consents.
Institutional review board approval was obtained from the University of Texas Health Science Center San Antonio.
Data source.
Data included national Veterans Health Administration (VA) pharmacy and diagnostic data for individuals aged 65 years or older receiving care between fiscal year (FY) 2004 (October 1, 2003–September 30, 2006) and FY2006. To ensure accuracy in identifying comorbid conditions and medication use, we required that participants have at least 1 year of VA care prior to the index date (first AED initiation during the study period). In order to allow 1-year follow-up after all exposures, our data also included FY2007. Thus, data included in this study were from FY2003 to FY2007.
Exposure to seizure medications.
We used the VA product name to identify prescription of any of the following AEDs included in the VA Pharmacy Benefits Management database between FY2004 and 2006: phenobarbital, phenytoin, carbamazepine, valproate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, tiagabine, topiramate, and pregabalin. Individuals receiving a first (monotherapy) prescription for any of these AEDs in the VA pharmacy data between FY2004 and 2006 were included in the study. While we cannot guarantee that no AED prescriptions occurred ever prior to our study period, given the 1-year clean period, this index date is hereafter termed first AED exposure. Individuals with prior use of AEDs in the year before the study period and those prescribed multiple AEDs during the study period were excluded from the cohort.
Suicide-related behavior.
SRB was defined using ICD-9-CM codes (V62.84: suicidal ideation, E950–E958: attempt, completed suicide, and self-inflicted injury) in VA inpatient and outpatient data files 1 year prior to and 1 year after the index date. We identified the date of all SRB episodes and identified SRB for participants as a dichotomous variable during 24 periods (1 for each 30-day period before and after the index date). Consistent with the procedure used by Simon et al.12 and others,3 the index date was included in the period 30 days prior to the index date. However, we also conducted analyses (not reported here) including the index day in the period 30 days after the index date to determine whether results were consistent.
Demographic covariates.
We used VA inpatient and outpatient data to identify demographic characteristics associated with AED exposure, including age (65–74 years, 75–84 years, 85 years and older), sex, race/ethnicity (non-Hispanic white, black, Hispanic, other), and an indicator of poverty where those with very low income do not have copayment (VA means test).14
Clinical covariates.
Baseline clinical characteristics were identified during the year prior to AED exposure. We included indicators of disease states or conditions that have previously been associated with SRB. These included the following psychiatric diagnoses: depression, anxiety, bipolar disorder, posttraumatic stress disorder (PTSD), substance abuse or dependence, and schizophrenia using previously validated ICD-9-CM codes.14–16 Finally, we evaluated other conditions that have been previously associated with AED use or SRB, including epilepsy, chronic pain conditions (migraine, back pain, neuropathic pain), and dementia.17–20 Additional indicators of psychiatric comorbidity included in previous analyses (prior prescription of any antidepressant or antipsychotic medication and prior psychiatric hospitalization)6 were not included in this analysis due to collinearity with the temporal assessment of SRB and mental health diagnosis.
Analysis.
We first described characteristics of individuals with new AED exposure including the proportion of individuals who had SRB before and after the index date using frequencies and percentages for categorical variables or means and SDs for continuous variables. General estimating equations (GEE) analyses21 compared the likelihood of SRB during each 30-day period, using the period 30 days after the index date as the reference period. GEE was used to account for the correlation among the repeated measures of SRB within each individual. We modeled the log-odds of SRB occurrence as a linear function of time period and covariates described above and report the adjusted odds ratios (ORs) and 95% confidence intervals. Analyses were conducted with Proc Genmod using SAS (SAS 9.2 Ts Level 2M3, 2002–2003, SAS Institute, Cary, NC).
RESULTS
From the overall population aged 65 years and older receiving VA care FY2004–2006 (n = 2,430,186), 283,012 were excluded due to chronic/multiple AED use or inadequate data (e.g., no VA care each year of the study period) prior to meeting age or AED exposure criteria. Of those remaining, 90,230 individuals (4.2% of the overall population) received a first AED monotherapy prescription between FY2004 and FY2006 without a previous AED prescription in FY2003. Table 1 shows descriptive statistics overall for those with a first AED exposure. Participants were primarily male (97.3%) with a mean age of 75.1 years (SD 6.1). The majority (76.2%) of those receiving a first AED monotherapy received gabapentin (n = 68,725). Valproate (n = 5,833) was second most common, followed by phenobarbital/primidone (n = 5,289), phenytoin (n = 4,136), carbamazepine (n = 2,976), and topiramate (n = 1,516). Fewer than 0.1% of the cohort received each of the following AEDs: lamotrigine (n = 815), levetiracetam (n = 636), oxcarbazepine (n = 169), pregabalin (n = 83), tiagabine (n = 53), and zonisamide (n = 32).
Table 1.
Characteristics of those who received a new antiepileptic drug monotherapy between FY2004 and 2006
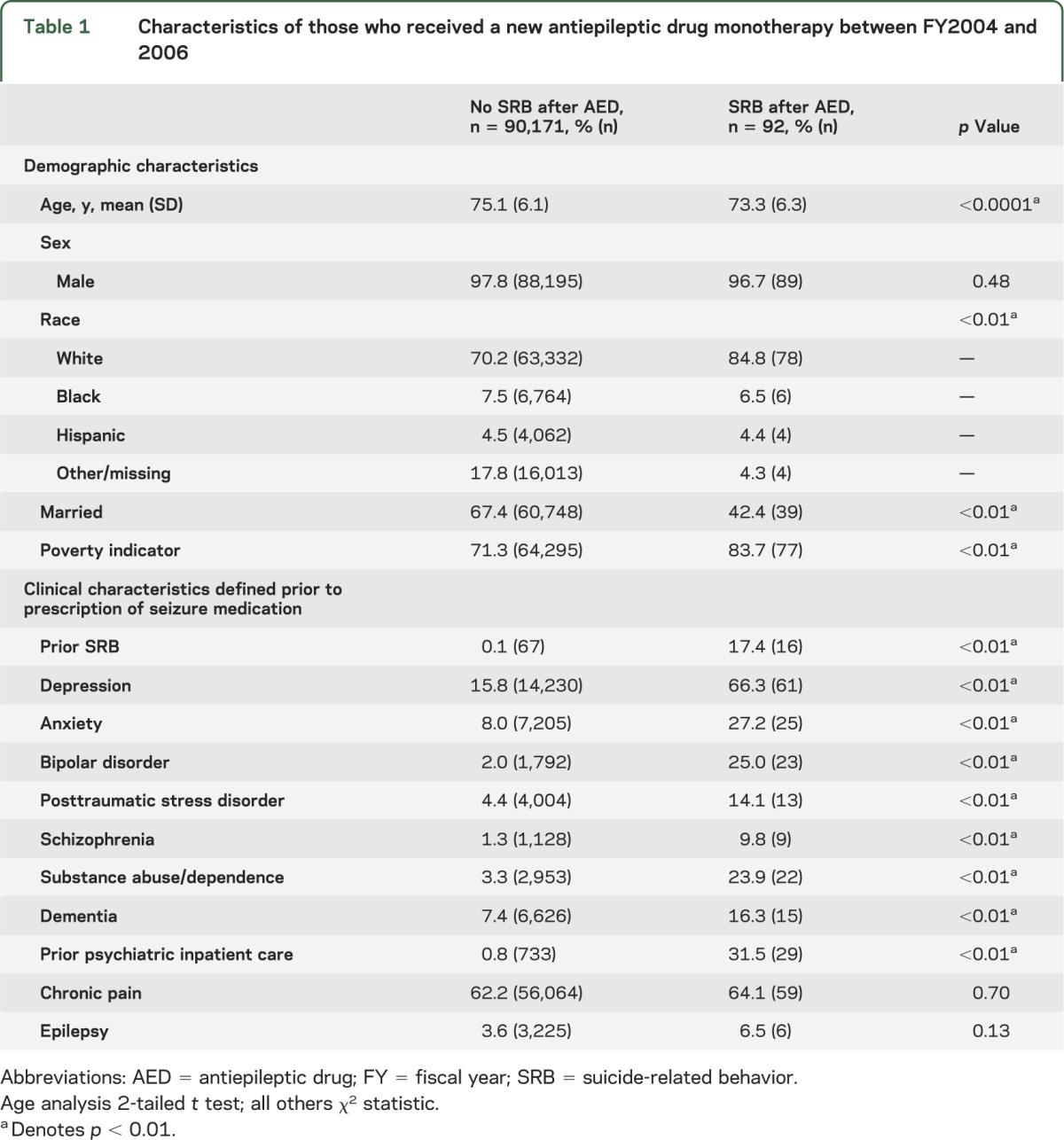
Individuals with a first AED monotherapy commonly had previous diagnoses of SRB, depression, anxiety, bipolar disorder, PTSD, schizophrenia, substance abuse/dependence, conditions associated with chronic pain, and dementia. Psychiatric hospitalization and prescriptions for antidepressant or antipsychotic medications in the previous year were also common. These rates were significantly higher than found in similar veterans without AED exposure (data not shown).22
Table 1 shows bivariate statistics comparing characteristics of individuals with and without SRB after first AED prescription. Those with subsequent SRB were more likely to be younger, white, unmarried, and with income below the VA poverty indicator. These individuals also were more likely to have diagnoses of depression, anxiety, bipolar disorder, PTSD, schizophrenia, substance abuse, and dementia. They were also more likely to have prior SRB during the year prior to AED prescription.
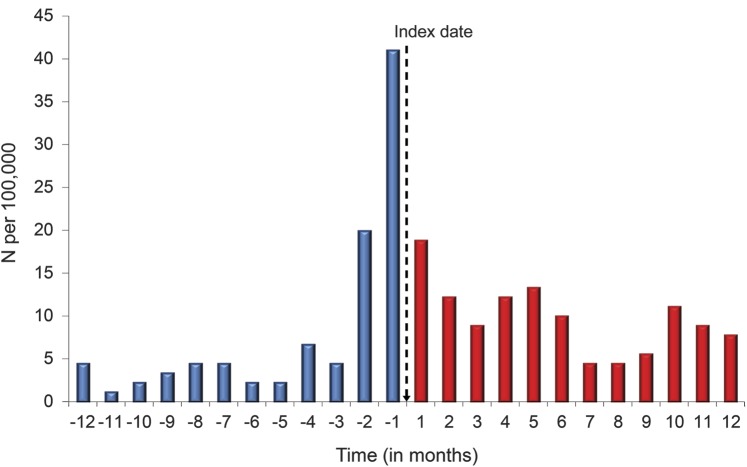
Figure 1 shows the temporal patterns of SRB among those with a first AED exposure. The clear peaks of SRB prior to AED exposure suggest that the SRB was most common prior to the AED prescription. However, SRB continued to occur after AED exposure. Overall, there were more SRB attempts after the index date (n = 106 episodes in 92 individuals) than before the index date (n = 84 episodes in 74 individuals). Approximately 22% of those who had SRB prior to the index date also had SRB after the index date (16 of 74; OR = 327 compared to those without prior SRB).
Figure 1. Temporal relationship of seizure medication index date and suicide-related behavior.
Suicide-related behavior (SRB) before and after receipt of antiepileptic drugs (AEDs) among older veterans. The index date (hashed line) indicates the patient's receipt of AEDs. Values to the left of the index date show the incidence of SRB up to 12 months before receipt of AEDs. Values to the right of the index date show incidence of SRB up to 12 months after receipt of AEDs.
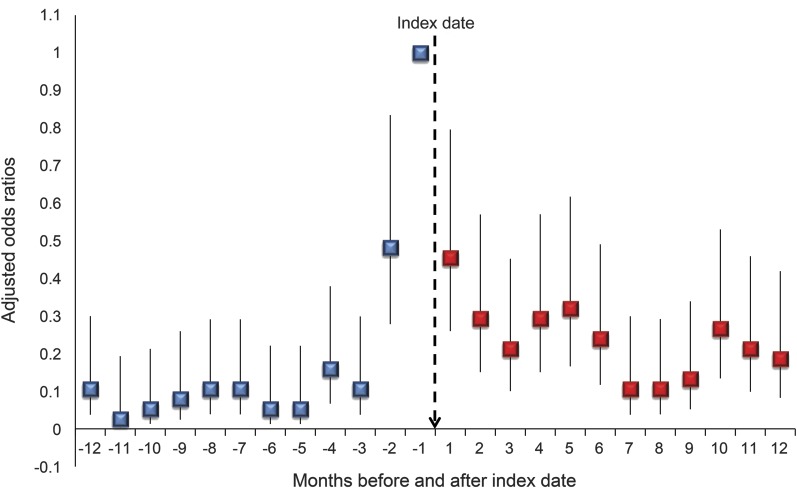
GEE analyses compared the likelihood of SRB during the 24 time periods controlling for demographic and clinical characteristics, using the period 30 days after the index date as the reference time period. Among those with a first AED exposure, SRB was significantly less likely in every time period compared to the index period (figure 2). Findings were similar when the index date was included in the 30 days after exposure.
Figure 2. Suicide-related behavior in relation to the month before seizure medication prescription.
Graphed adjusted odds ratios show the likelihood of suicide-related behavior before and after the index period (30 days prior to and including the date of first antiepileptic drug prescription); odds ratios adjusted for variables described in table 1.
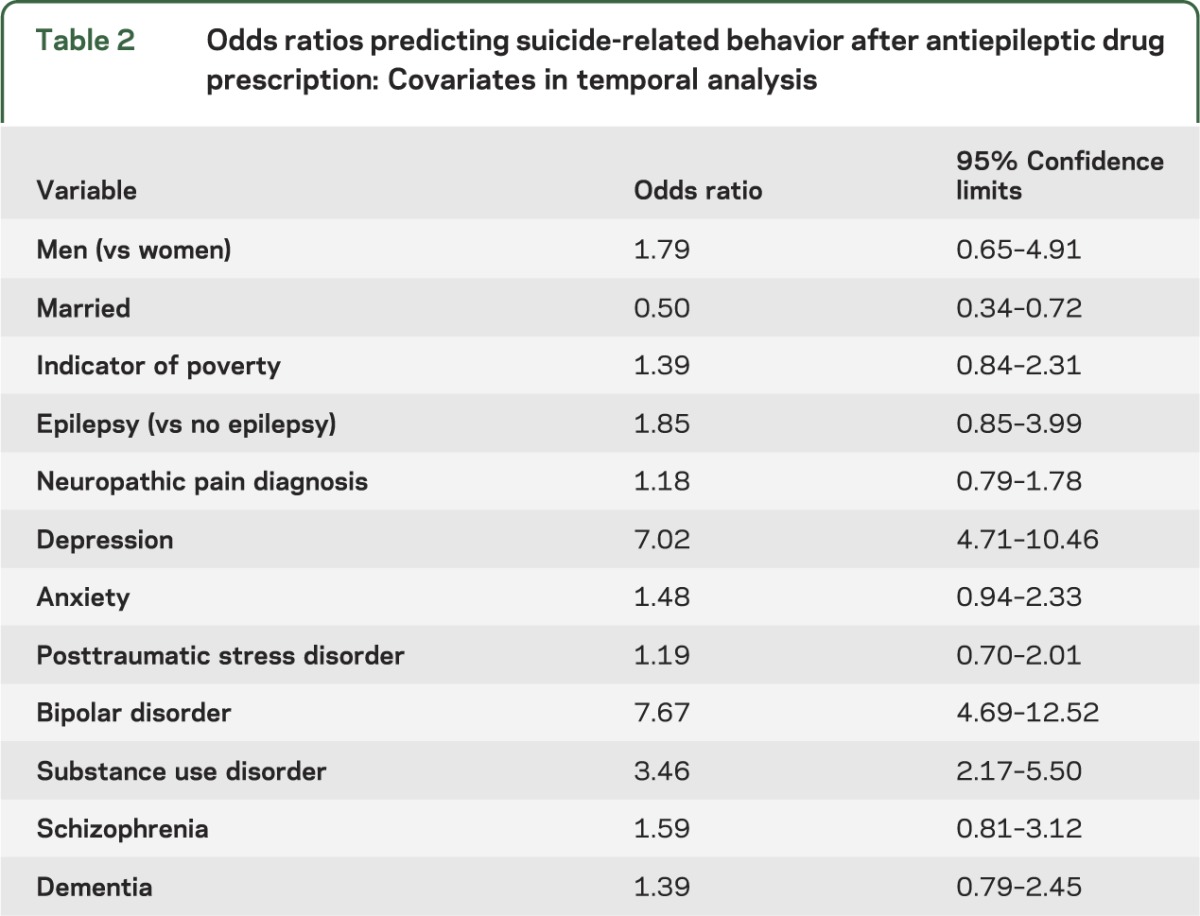
Table 2 shows the ORs for the demographic and clinical characteristics included in the GEE analysis of SRB over time. These findings suggest that depression and bipolar disorder remained significant predictors of SRB in the multivariable temporal model.
Table 2.
Odds ratios predicting suicide-related behavior after antiepileptic drug prescription: Covariates in temporal analysis
DISCUSSION
We found that individuals receiving a first AED during our study period were more likely to have SRB during the 30 days prior to AED exposure than any other time period in the year before and after exposure. These findings are similar to those of Simon et al.12 in a sample of patients with depression. That study found that, regardless of the type of treatment (antidepressant or psychotherapy) or type of provider (primary care, psychiatrist, psychologist), SRB was most common prior to the onset of treatment with a gradual reduction in SRB after the effects of the treatment were realized.
In a prior analysis, we found that individuals with a first AED exposure had increased risk of SRB after exposure22 even when controlling for prior SRB. This is consistent with our current findings, in which we found that there were more episodes of SRB after exposure than before exposure. However, in our current analysis, we found that SRB peaked prior to AED prescription and that rates of SRB were significantly lower in all periods before and after the index date, which included the 30 days before initiating treatment as well as the first day of treatment. Moreover, the only statistically significant clinical conditions in the multivariate model were depression and bipolar disorder. Rather than highlighting the association between SRB and AED exposure, these data suggest that individuals who received the AED were at greater risk of SRB both before and after initiation of AED, even after controlling for psychiatric comorbidity.
This is also consistent with the findings of Gibbons and colleagues,7 who found that the risk of SRB was not increased for nonpsychiatric populations (e.g., those with epilepsy or pain indications). Moreover, they found that SRB decreased after treatment with gabapentin. Additionally, our findings account for methodologic considerations not included in Gibbons et al.7 that limited their work23: standardized assessment of suicidality (i.e., ICD-9-CM codes were documented in medical records only if the patient sought care for the specific condition) and full definition of suicidiality (i.e., our definition of SRB included ideation, attempts, completed suicides, and self-inflicted injury).
These findings for epilepsy are, however, contrary to the FDA analysis and other studies where epilepsy was a risk factor for SRB, though methodologic differences may help explain this finding. The outcome for this study is SRB throughout a 24-month study period—12 months before AED prescription and 12 months after AED prescription. Epilepsy patients included in the clinical trials of AEDs were individuals with chronic, uncontrolled epilepsy, and the AED was tested as add-on therapy. These data suggest that new-onset epilepsy may incur less SRB risk than chronic epilepsy.
Finally, while a number of other demographic characteristics were significant in bivariate analyses, only marital status (a proxy measure of psychosocial status) was statistically significant in the multivariable model, which controlled for comorbidity previously associated with SRB.6
Our findings must be interpreted in light of several limitations. First, we were not able to identify the specialty of the AED prescriber. Second, our approach combined ICD-9-CM codes for suicidal ideation and attempts. We did not have other data that may have identified additional suicide completions, which is substantially rarer than suicidal ideation or attempt,24 so our analysis may be less informative when trying to understand whether AEDs are associated with suicide completion. Moreover, the administrative data may have limited our ability to identify all cases of SRB where chart review, death index review, or interview methods might have revealed more patients with this outcome. Kim and colleagues25 found that the specificity of these codes was high (>90%) but sensitivity was poor. Additionally, administrative datasets provided limited insight about psychosocial patient factors. In this case, we found that those who were married were significantly less likely to have SRB, but those under the poverty limit were not at increased risk compared to those above the poverty limit. Our data could not account for other psychosocial factors (e.g., living conditions/psychosocial stressors, or changes in such factors) around SRB.
Our new user design restricted the number of individuals included, thus limiting our ability to examine difference by specific AED. It also excluded those who received multiple AEDs. As described earlier, a chronic use cohort introduces several types of bias that limit the ability to evaluate the association of a specific drug exposure on outcomes.13 Our use of a 1-year clean period for AEDs for study inclusion likely resulted in the inclusion of individuals who had been previously exposed to AEDs. Though the exact proportion is unknown, our prior results are similar even after adjusting for prior use of AEDs.
Finally, the study population consisted of older VA patients who were primarily men. The geriatric population is a strength since the FDA meta-analysis was limited by inclusion of few older patients in clinical trials. However, prior research of antidepressants and AEDs have found reduced risk of SRB in the elderly.26 Thus, findings may not generalize to younger patients or women. The racial composition of the sample was similar to the racial composition of older Americans more broadly.27
Although prior studies have raised concerns regarding increased risk for SRB among those receiving AEDs, our findings, and those of other studies that have found recurrence of SRB ranging from 20% to 50% overall and 11% to 14% for those over age 50, it is appropriate to be cautious in interpreting studies that do not examine and address SRB occurring prior to AEDs.28–30 As the risk for recurrent SRB was 22% in individuals with SRB prior to AEDs, these patients should be followed closely to prevent recurrent SRB.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Kathleen Franklin for manuscript preparation. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
GLOSSARY
- AED
antiepileptic drug
- FDA
US Food and Drug Administration
- FY
fiscal year
- GEE
general estimating equations
- ICD-9-CM
International Classification of Diseases, 9th revision, Clinical Modification
- OR
odds ratio
- PTSD
posttraumatic stress disorder
- SRB
suicide-related behavior
- VA
Veterans Health Administration
Footnotes
Editorial, page 1889
AUTHOR CONTRIBUTIONS
Mary Jo V. Pugh: designed the study, obtained funding, interpretation of analysis, and drafted manuscript. Dale Hesdorffer: interpretation of data and revised manuscript. Chen-Pin Wang: study design, data analysis, critical review of manuscript. Megan E. Amuan: data analysis, data acquisition, interpretation of analysis. Jeffrey Tabares: interpretation of data and revised manuscript. Erin P. Finley: interpretation of data and revised manuscript. Joyce A.: interpretation of data and revised manuscript. Andres M. Kanner: interpretation of data and revised manuscript. Craig J. Bryan: conceptualization of study, study design, interpretation of analysis, and critical review of manuscript.
STUDY FUNDING
Supported by the South Texas Veterans Healthcare System/Audie L. Murphy Division and the VERDICT research program; and the VA Health Services Research and Development Service IIR 06-062. The funding organizations had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
DISCLOSURE
M. Pugh reports no disclosures. D. Hesdorffer serves on the editorial board of Epilepsy and Behavior and Epilepsy Research and as contributing editor to Epilepsy Currents. She co-chairs the Commission on Epidemiology for the International League Against Epilepsy. She is a consultant for the Mount Sinai Medical Center, Injury Prevention Center. Dr. Hesdorffer received a travel award from GlaxoSmithKline in 2010. In 2012, she participated in advisory boards for UCB, UpsherSmith, and Esai. She is funded by grants from CDC, DP002209, PI, 2009–2014; AUCD, RT01, Co-I (PI of Columbia subcontract), 2008–2012; National Institute of Neurological Disorders and Stroke, NS31146, Co-I (PI of Columbia subcontract), 2007–2014; National Institute of Neurological Disorders and Stroke, NS043209, Co-I (PI of Columbia subcontract), 2003–2013; CDC, MM1002, Co-I, 2006–2010; National Institute of Neurological Disorders and Stroke, 5U01NS04911, Co-I (PI of Columbia subcontract), 2011–2012; National Institute of Neurological Disorders and Stroke, NS078419, Co-I, 2012–2015; and the Epilepsy Foundation of America 2010–2012. C. Wang, M. Amuan, J. Tabares, E. Finley, and J. Cramer report no disclosures. A. Kanner reports the following disclosures: Consultant honorarium from Neuropace (2013). Member of Data safety Board: Vertex Laboratories (2012, 2013). Member Editorial Boards: Epilepsy Currents, Epilepsy & Behavior, CNS Spectrum, and Epileptology. Research grants from GlaxoSmithKline (last one in 2010), Novartis (2010), and Pfizer (2011). Royalties for Psychiatric Issues in Epilepsy, Second Edition: A Practical Guide to Diagnosis and Treatment (Lippincott Williams & Wilkins, 2006); Controversial Issues in Psychiatric Aspects of Epilepsy (Elsevier, 2008); and Depression in Neurologic Disorder (Wylie & Blackwell 2013). C. Bryan reports grant funding from the Department of Defense and the Department of the Air Force; consulting fees from Kognito Interactive; consulting fees from Intelligent Automation, Inc.; honoraria from CMI Education; and royalties from Springer Publishing. Go to Neurology.org for full disclosures.
REFERENCES
- 1.U.S. Food and Drug Administration: Center for Drug Evaluation and Research Information for healthcare professionals: suicidality and antiepileptic drugs [online]. Available at: http://www.fda.gov/cder/drug/InfoSheets/HCP/antiepilepticsHCP.htm. Accessed July 18, 2008
- 2.Olesen JB, Hansen PR, Erdal J, et al. Antiepileptic drugs and risk of suicide: a nationwide study. Pharmacoepidemiol Drug Saf 2010;19:518–524 [DOI] [PubMed] [Google Scholar]
- 3.Patorno E, Bohn RL, Wahl PM, et al. Anticonvulsant medications and the risk of suicide, attempted suicide, or violent death. JAMA 2010;303:1401–1409 [DOI] [PubMed] [Google Scholar]
- 4.Arana A, Wentworth CE, Ayuso-Mateos JL, Arellano FM. Suicide-related events in patients treated with antiepileptic drugs. N Engl J Med 2010;363:542–551 [DOI] [PubMed] [Google Scholar]
- 5.Machado RA, Espinosa AG, Melendrez D, Gonzalez YR, Garcia VF, Rodriguez YQ. Suicidal risk and suicide attempts in people treated with antiepileptic drugs for epilepsy. Seizure 2011;20:280–284 [DOI] [PubMed] [Google Scholar]
- 6.VanCott AC, Cramer JA, Copeland LA, et al. Suicide-related behaviors in older patients with new anti-epileptic drug use: data from the VA hospital system. BMC Med 2010;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibbons RD, Hur K, Brown CH, Mann JJ. Relationship between antiepileptic drugs and suicide attempts in patients with bipolar disorder. Arch Gen Psychiatry 2009;66:1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesdorffer DC, Kanner AM. The FDA alert on suicidality and antiepileptic drugs: fire or false alarm? Epilepsia 2009;50:978–986 [DOI] [PubMed] [Google Scholar]
- 9.Mula M, Hesdorffer DC. Suicidal behavior and antiepileptic drugs in epilepsy: analysis of the emerging evidence. Drug Healthc Patient Saf 2011;3:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosco JL, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol 2010;63:64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Psaty BM, Koepsell TD, Lin D, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc 1999;47:749–754 [DOI] [PubMed] [Google Scholar]
- 12.Simon GE, Savarino J, Operskalski B, Wang PS. Suicide risk during antidepressant treatment. Am J Psychiatry 2006;163:41–47 [DOI] [PubMed] [Google Scholar]
- 13.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915–920 [DOI] [PubMed] [Google Scholar]
- 14.Pugh MJ, Copeland LA, Zeber JE, et al. The impact of epilepsy on health status among younger and older adults. Epilepsia 2005;46:1820–1827 [DOI] [PubMed] [Google Scholar]
- 15.Selim AJ, Berlowitz DR, Ren XS, et al. The comorbidity index. In: Davies M, ed. Measuring and Managing Health Care Quality. New York: Aspen Publishers; 2002: 91–94 [Google Scholar]
- 16.Selim A, Fincke G, Ren X, et al. Comorbidity assessments based on patient report: results from the Veterans Health Study. J Ambul Care Manage 2004;27:281–295 [DOI] [PubMed] [Google Scholar]
- 17.Hope OA, Zeber JE, Kressin NR, et al. New-onset geriatric epilepsy care: race, setting of diagnosis, and choice of antiepileptic drug. Epilepsia 2009;50:1085–1093 [DOI] [PubMed] [Google Scholar]
- 18.Juurlink DN, Herrmann N, Szalai JP, Kopp A, Redelmeier DA. Medical illness and the risk of suicide in the elderly. Arch Intern Med 2004;164:1179–1184 [DOI] [PubMed] [Google Scholar]
- 19.Conwell Y, Brent D. Suicide and aging: I: patterns of psychiatric diagnosis. Int Psychogeriatr 1995;7:149–164 [DOI] [PubMed] [Google Scholar]
- 20.Kaplan MS, Huguet N, McFarland BH, Newsom JT. Suicide among male veterans: a prospective population-based study. J Epidemiol Community Health 2007;61:619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardin JW, Hilbe JM, eds. Generalized Estimating Equations, 1st ed New York: Chapman & Hall/CRC; 2003 [Google Scholar]
- 22.Pugh MJ, Copeland LA, Zeber JE, et al. Antiepileptic drug monotherapy exposure and suicide-related behavior in older veterans. J Am Geriatr Soc 2012;60:2042–2047 [DOI] [PubMed] [Google Scholar]
- 23.Hesdorffer DC, Berg AT, Kanner AM. An update on antiepileptic drugs and suicide: are there definitive answers yet? Epilepsy Curr 2010;10:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawton K, Harriss L. How often does deliberate self-harm occur relative to each suicide? A study of variations by gender and age. Suicide Life Threat Behav 2008;38:650–660 [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Smith E, Stano C, et al. Validation of key behaviourally based mental health diagnoses in administrative data: suicide attempt, alcohol abuse, illicit drug abuse and tobacco use. BMC Health Serv Res 2012;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isacsson G, Holmgren P, Druid H, Bergman U. Psychotropics and suicide prevention. Implications from toxicological screening of 5281 suicides in Sweden 1992-1994. Br J Psychiatry 1999;174:259–265 [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC) The State of Aging and Health in America 2007. Whitehouse Station, NJ: The Merck Company Foundation; 2007 [Google Scholar]
- 28.American Foundation for Suicide Prevention Risk factors for suicide [online]. Available at: http://www.afsp.org/index.cfm?page_id=05147440-E24E-E376-BDF4BF8BA6444E76. Accessed January 22, 2013
- 29.Kapur N, Cooper J, King-Hele S, et al. The repetition of suicidal behavior: a multicenter cohort study. J Clin Psychiatry 2006;67:1599–1609 [DOI] [PubMed] [Google Scholar]
- 30.Scoliers G, Portzky G, van Heeringen K, Audenaert K. Sociodemographic and psychopathological risk factors for repetition of attempted suicide: a 5-year follow-up study. Arch Suicide Res 2009;13:201–213 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.