Abstract
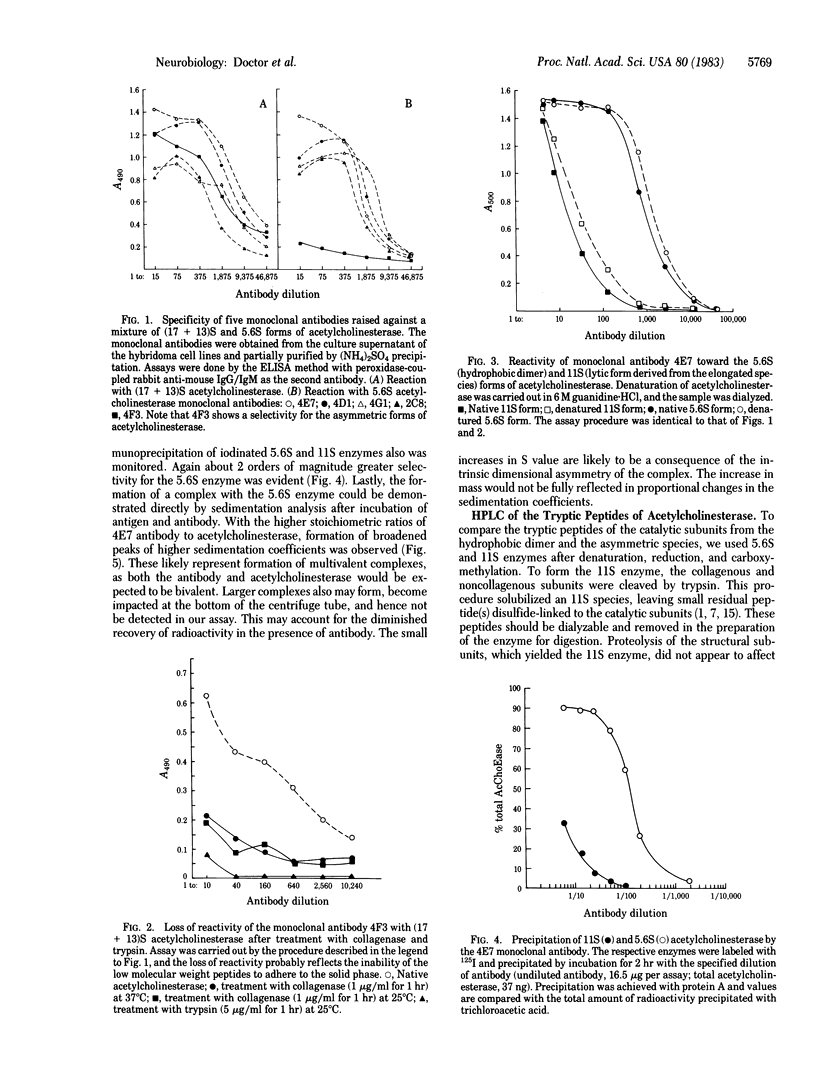
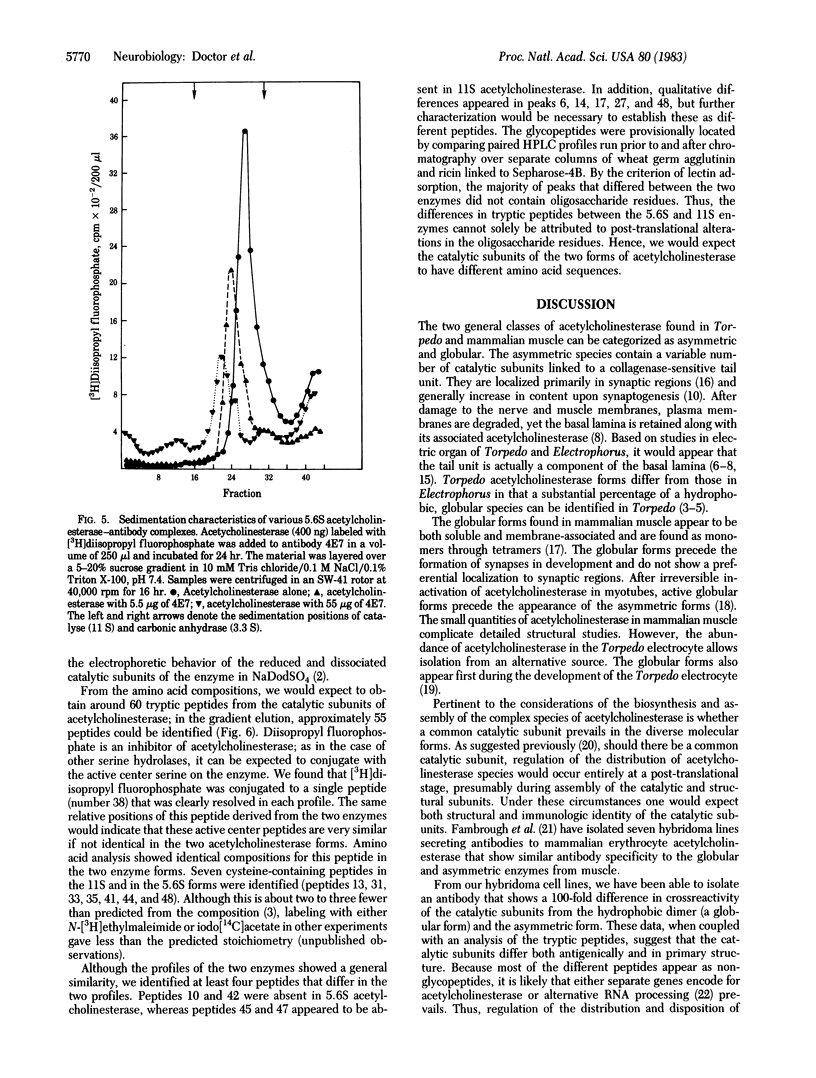
A mixture of the 5.6S hydrophobic dimer and the asymmetric, tail-containing (17 + 13)S forms of acetylcholinesterase (acetylcholine acetylhydrolase, EC 3.1.1.7) from Torpedo californica was used to immunize mice, and spleen cells from these mice were used to produce nine hybridoma lines secreting antibodies against acetylcholinesterase. Antibodies from one of the lines showed a 100-fold greater affinity for the 5.6S species when compared with the catalytic subunits of the (17 + 13)S species. This difference in specificity was retained after denaturation of the two acetylcholinesterase species. Another line produced antibody directed only to structural subunits of the (17 + 13)S species, whereas the remaining seven antibodies exhibited nearly equivalent crossreactivity for all of the forms of acetylcholinesterase. Tryptic peptides were generated from the catalytic subunits of the 5.6S and tail-containing acetylcholinesterase species, and high-pressure liquid chromatographic profiles show at least two distinct peptides in the catalytic subunits for each enzyme species. Some of these peptides exhibit retention times different from those of the identified glycopeptides. Thus, it is likely that the catalytic subunits of two molecular forms of acetylcholinesterase differ in primary structure and sites of antigenicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bon S., Vigny M., Massoulié J. Asymmetric and globular forms of acetylcholinesterase in mammals and birds. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2546–2550. doi: 10.1073/pnas.76.6.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Engel A. G., Rosenberry T. L. Acetylcholinesterase of human erythrocytes and neuromuscular junctions: homologies revealed by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1078–1082. doi: 10.1073/pnas.79.4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry M. K., Henchal E. A., McCown J. M., Brandt W. E., Dalrymple J. M. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. Am J Trop Med Hyg. 1982 May;31(3 Pt 1):548–555. doi: 10.4269/ajtmh.1982.31.548. [DOI] [PubMed] [Google Scholar]
- Koenig J., Vigny M. Neural induction of the 16S acetylcholinesterase in muscle cell cultures. Nature. 1978 Jan 5;271(5640):75–77. doi: 10.1038/271075a0. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Camp S. J., Taylor P. Characterization of a hydrophobic, dimeric form of acetylcholinesterase from Torpedo. J Biol Chem. 1982 Oct 25;257(20):12302–12309. [PubMed] [Google Scholar]
- Lee S. L., Heinemann S., Taylor P. Structural characterization of the asymmetric (17 + 13) S forms of acetylcholinesterase from Torpedo. I. Analysis of subunit composition. J Biol Chem. 1982 Oct 25;257(20):12282–12291. [PubMed] [Google Scholar]
- Lee S. L., Taylor P. Structural characterization of the asymmetric (17 + 13) S species of acetylcholinesterase from Torpedo. II. Component peptides obtained by selective proteolysis and disulfide bond reduction. J Biol Chem. 1982 Oct 25;257(20):12292–12301. [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S., Lappi S., Taylor P. Molecular forms of acetylcholinesterase from Torpedo californica: their relationship to synaptic membranes. Biochemistry. 1976 Apr 6;15(7):1425–1434. doi: 10.1021/bi00652a012. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Mays C., Rosenberry T. L. Characterization of pepsin-resistant collagen-like tail subunit fragments of 18S and 14S acetylcholinesterase from Electrophorus electricus. Biochemistry. 1981 May 12;20(10):2810–2817. doi: 10.1021/bi00513a016. [DOI] [PubMed] [Google Scholar]
- Ott P., Brodbeck U. Multiple molecular forms of acetylcholinesterase from human erythrocyte membranes. Interconversion and subunit composition of forms separated by density gradient centrifugation in a zonal rotor. Eur J Biochem. 1978 Jul 17;88(1):119–125. doi: 10.1111/j.1432-1033.1978.tb12428.x. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Richardson J. M. Structure of 18S and 14S acetylcholinesterase. Identification of collagen-like subunits that are linked by disulfide bonds to catalytic subunits. Biochemistry. 1977 Aug 9;16(16):3550–3558. doi: 10.1021/bi00635a008. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Marshall L. M., McMahan U. J. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J Cell Biol. 1978 Jul;78(1):176–198. doi: 10.1083/jcb.78.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viratelle O. M., Bernhard S. A. Major component of acetylcholinesterase in Torpedo electroplax is not basal lamina associated. Biochemistry. 1980 Oct 28;19(22):4999–5007. doi: 10.1021/bi00563a011. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Boustead C. Changes in acetylcholinesterase molecular forms during the embryonic development of Torpedo marmorata. J Neurochem. 1982 Sep;39(3):747–755. doi: 10.1111/j.1471-4159.1982.tb07956.x. [DOI] [PubMed] [Google Scholar]
- Younkin S. G., Rosenstein C., Collins P. L., Rosenberry T. L. Cellular localization of the molecular forms of acetylcholinesterase in rat diaphragm. J Biol Chem. 1982 Nov 25;257(22):13630–13637. [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]


