Abstract
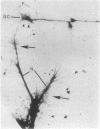
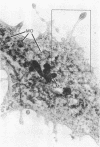
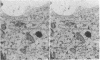
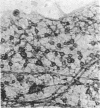
We have examined growth cones and neurites of cultured central nervous system neurons by high-voltage electron microscopy. Embryonic chicken retina cells were cultured on polylysine-treated and Formvar-coated gold grids for 2-6 days, fixed, and critical point dried. Growth cones and neurites were examined as unembedded whole mounts. Three-dimensional images from stereo-pair electron micrographs of these regions showed a high degree of ultrastructural articulation, with distinct, non-tapering filaments (5-9 nm in diameter) joining both cytoskeletal and membranous components. In the central regions of growth cones, interconnected structures included microtubules, large membranous sacs (up to 400 nm), and irregular vesicles (25-75 nm). A denser filamentous network was prevalent at the edges of growth cones. This network, which frequently adjoined the surface membrane, linked vesicles of uniform size (35-40 nm). Such vesicles often were seen densely packed in growth cone protrusions that were about the size of small synaptic boutons. Prevalent structural interconnections within growth cones conceivably could play a logistic role in specific membrane assembly, intracellular transport, endocytosis, and secretion. Because such processes are not unique to growth cones, the extensive linkages we have observed may have implications for cytoplasmic structure in general.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Davis J., Fowler W. E. Brain spectrin, a membrane-associated protein related in structure and function to erythrocyte spectrin. Nature. 1982 Sep 9;299(5879):126–131. doi: 10.1038/299126a0. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Mackey M. C., Weldon P. R. Organelle formation from pinocytotic elements in neurites of cultured sympathetic ganglia. J Neurocytol. 1972 Dec;1(4):311–340. doi: 10.1007/BF01102938. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D., Bunge M. B. Serial analysis of microtubules in cultured rat sensory axons. J Neurocytol. 1981 Aug;10(4):589–605. doi: 10.1007/BF01262592. [DOI] [PubMed] [Google Scholar]
- Bray D. Surface movements during the growth of single explanted neurons. Proc Natl Acad Sci U S A. 1970 Apr;65(4):905–910. doi: 10.1073/pnas.65.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge M. B. Initial endocytosis of perioxidase or ferritin by growth cones of cultured nerve cells. J Neurocytol. 1977 Aug;6(4):407–439. doi: 10.1007/BF01178226. [DOI] [PubMed] [Google Scholar]
- Droz B., Rambourg A., Koenig H. L. The smooth endoplasmic reticulum: structure and role in the renewal of axonal membrane and synaptic vesicles by fast axonal transport. Brain Res. 1975 Jul 25;93(1):1–13. doi: 10.1016/0006-8993(75)90282-6. [DOI] [PubMed] [Google Scholar]
- Ellisman M. H., Porter K. R. Microtrabecular structure of the axoplasmic matrix: visualization of cross-linking structures and their distribution. J Cell Biol. 1980 Nov;87(2 Pt 1):464–479. doi: 10.1083/jcb.87.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman E. L., Axelrod D., Schwartz M., Heacock A. M., Agranoff B. W. Studies on the localization of newly added membrane in growing neurites. J Neurobiol. 1981 Nov;12(6):591–598. doi: 10.1002/neu.480120607. [DOI] [PubMed] [Google Scholar]
- Frank E., Fischbach G. D. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979 Oct;83(1):143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Kirschner M. W. Filament organization revealed in platinum replicas of freeze-dried cytoskeletons. J Cell Biol. 1980 Jul;86(1):212–234. doi: 10.1083/jcb.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol. 1982 Jul;94(1):129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Yeh E. Initial synaptic transmission at the growth cone in Xenopus nerve-muscle cultures. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6727–6731. doi: 10.1073/pnas.79.21.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H., Wolosewick J. J., Pappas G. D. The microtrabecular lattice of the adrenal medulla revealed by polyethylene glycol embedding and stereo electron microscopy. J Neurosci. 1982 Jan;2(1):57–65. doi: 10.1523/JNEUROSCI.02-01-00057.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski E. R., Rosenbaum J. L. Studies on the organization and localization of actin and myosin in neurons. J Cell Biol. 1979 Feb;80(2):356–371. doi: 10.1083/jcb.80.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavail J. H., Rapisardi S., Sugino I. K. Evidence against the smooth endoplasmic reticulum as a continuous channel for the retrograde axonal transport of horseradish peroxidase. Brain Res. 1980 Jun 2;191(1):3–20. doi: 10.1016/0006-8993(80)90311-x. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C. Cell-substratum adhesion of neurite growth cones, and its role in neurite elongation. Exp Cell Res. 1979 Nov;124(1):127–138. doi: 10.1016/0014-4827(79)90263-5. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C. Immunocytochemical evidence for colocalization in neurite growth cones of actin and myosin and their relationship to cell--substratum adhesions. Dev Biol. 1981 Jul 15;85(1):113–122. doi: 10.1016/0012-1606(81)90240-2. [DOI] [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin-Szamier P., Pollard T. D. Actin filament destruction by osmium tetroxide. J Cell Biol. 1978 Jun;77(3):837–852. doi: 10.1083/jcb.77.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAI J. Dissociated dorsal root ganglia in tissue culture. Am J Anat. 1956 Jul;99(1):81–129. doi: 10.1002/aja.1000990105. [DOI] [PubMed] [Google Scholar]
- Pfenninger K. H., Maylié-Pfenninger M. F. Lectin labeling of sprouting neurons. II. Relative movement and appearance of glycoconjugates during plasmalemmal expansion. J Cell Biol. 1981 Jun;89(3):547–559. doi: 10.1083/jcb.89.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro D. G., De Mello F. G., Nirenberg M. Synapse turnover: the formation and termination of transient synapses. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4977–4981. doi: 10.1073/pnas.74.11.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A., Droz B. Smooth endoplasmic reticulum and axonal transport. J Neurochem. 1980 Jul;35(1):16–25. doi: 10.1111/j.1471-4159.1980.tb12484.x. [DOI] [PubMed] [Google Scholar]
- Rees R. P., Reese T. S. New structural features of freeze-substituted neuritic growth cones. Neuroscience. 1981;6(2):247–254. doi: 10.1016/0306-4522(81)90060-9. [DOI] [PubMed] [Google Scholar]
- Shaw G., Osborn M., Weber K. Arrangement of neurofilaments, microtubules and microfilament-associated proteins in cultured dorsal root ganglia cells. Eur J Cell Biol. 1981 Apr;24(1):20–27. [PubMed] [Google Scholar]
- Siman R. G., Klein W. L. Differential regulation of muscarinic and nicotinic receptors by cholinergic stimulation in cultured avian retina cells. Brain Res. 1983 Feb 28;262(1):99–108. doi: 10.1016/0006-8993(83)90473-0. [DOI] [PubMed] [Google Scholar]
- Small J. V. Organization of actin in the leading edge of cultured cells: influence of osmium tetroxide and dehydration on the ultrastructure of actin meshworks. J Cell Biol. 1981 Dec;91(3 Pt 1):695–705. doi: 10.1083/jcb.91.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Ishikawa H. The movement of membranous organelles in axons. Electron microscopic identification of anterogradely and retrogradely transported organelles. J Cell Biol. 1980 Mar;84(3):513–530. doi: 10.1083/jcb.84.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Usukura J., Tsukita S., Ishikawa H. The cytoskeleton in myelinated axons: a freeze-etch replica study. Neuroscience. 1982;7(9):2135–2147. doi: 10.1016/0306-4522(82)90125-7. [DOI] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol. 1979 Jul;82(1):114–139. doi: 10.1083/jcb.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Stereo high-voltage electron microscopy of whole cells of the human diploid line, WI-38. Am J Anat. 1976 Nov;147(3):303–323. doi: 10.1002/aja.1001470305. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol. 1971 Jun;49(3):614–635. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner B. A., Shooter E. M. The biology and mechanism of action of nerve growth factor. Annu Rev Biochem. 1982;51:845–868. doi: 10.1146/annurev.bi.51.070182.004213. [DOI] [PubMed] [Google Scholar]