Abstract
(R)-Thalidomide was oxidized to 5-hydroxythalidomide and 5’-hydroxythalidomide by NADPH-fortified liver microsomes from humans and monkeys. (R)-Thalidomide was hydroxylated more efficiently than (S)-thalidomide. Recombinant human P450s 3A4, 3A5, and 3A7 and monkey P450s 3A8 and 3A5 (co-expressed with NADPH-P450 reductase in bacterial membranes) also catalyzed (R)-thalidomide 5-hydroxylation. Purified human P450s 2C19, 3A4, and 3A5 mediated (R)-thalidomide 5-hydroxylation at similar rates in reconstituted systems. P450 2C19 showed a rather non-saturable substrate-velocity curve; however, P450s 3A4 and 3A5 showed sigmoidal curves. P450 also oxidized 5-hydroxythalidomide to an epoxide or dihydroxy compound. Liquid chromatography-mass spectrometry analysis revealed formation of a glutathione conjugate from (R)- and (S)-5-hydroxythalidomide, catalyzed by liver microsomal P450s 3A4 and 3A5 in the presence of glutathione (assigned as a conjugate of 5-hydroxythalidomide formed on the phenyl ring). These results indicate that human P450s 3A4 and 3A5 mediate thalidomide 5-hydroxylation and further oxidation leading to a glutathione conjugate, which may be of relevance in the pharmacological and toxicological actions of thalidomide.
Introduction
P450 comprises a superfamily of enzymes involved in the oxidation of a large number of endogenous and exogenous compounds and contributes to many of their pharmacological or toxicological actions (1). P450 3A4 is the major P450 enzyme in human liver and small intestine (2); however, the importance of polymorphic P450 3A5 has been recently suggested, particularly in drug oxidations in Asian populations (3, 4). P450 2C19 is a relatively minor P450 in the liver, in terms of the amount of the protein (5) but catalyzes the oxidation of many marketed drugs (6).
Thalidomide [α-(N-phthalimido)glutarimide] was withdrawn from clinical use in the early 1960s because of its teratogenic effects in humans but has been approved for the treatment of refractory multiple myeloma by the United States and Japan since 2000 (7, 8). The CYP2C19 genotype has been reported to be associated with cancer treatment outcome using thalidomide (9), and the clinical response rate in 62 patients on thalidomide (with dexamethasone) treatment was twice as high in extensive metabolizers than in poor metabolizers (9). Decreased formation of active thalidomide metabolites (if those contribute to therapy) would be expected with defective P450 2C19 alleles compared to wild-type (9). No evidence has been obtained for drug interactions between thalidomide and hormonal contraceptives (10, 11). In contrast, we found that thalidomide inhibited P450 2C19-dependent (S)-mephenytoin 4'-hydroxylation at high concentrations but enhanced P450 3A5-dependent midazolam hydroxylation and cyclosporine A clearance at clinically relevant concentrations (12). Two hydroxylated metabolites of thalidomide have been reported to be formed at very low concentrations after incubation with recombinant human P450 2C19 (13), but little other information regarding thalidomide metabolism by P450s in animal and human liver is available.
The purpose of this study was to characterize thalidomide metabolism by human P450 enzymes and also to better understand the activation of thalidomide and any interactions with GSH. Although ligand cooperativity with P450 3A5 has been observed (14), we report homotropic cooperativity of P450 3A5 with thalidomide herein. We also describe the characterization of a GSH conjugate of an oxidation product of 5-hydroxythalidomide, indicating that activation of thalidomide occurs to produce an electrophilic product.
Experimental Procedures
Chemicals
(R)-(+)-Thalidomide and (S)-(−)-thalidomide (Sigma-Aldrich, St. Louis, MO), GSH (Wako Pure Chemicals, Osaka, Japan), and L-cysteine (Fluka Chemical, Milwaukee, WI) were purchased from the indicated sources. 5- and 5’-Hydroxythalidomide were synthesized as reported previously (15). Other chemicals and reagents used in this study were obtained from the sources described previously (16, 17) or were of the highest quality commercially available.
Enzyme Preparations
Pooled liver microsomes from humans, cynomolgus monkeys, Yucatan minipigs, and New Zealand White rabbits were obtained from BD Biosciences (Woburn, MA). Pooled liver microsomes from male micro-minipigs (8 months old, Fuji Micra Inc, Fujinomiya, Japan) and rats (7 weeks old, Japan SLC, Hamamatsu, Japan) were prepared in 10 mM Tris-HCl buffer (pH 7.4) containing 0.10 mM EDTA and 20% (v/v) glycerol, as described previously (17, 18). The use of animal livers for this study was approved by the Ethics Committees of Showa Pharmaceutical University. Each recombinant human or monkey P450, co-expressed with human NADPH-P450 reductase (EC 1.6.2.4) in Escherichia coli membranes, was prepared as described earlier (16, 19). Human P450s 2C19, 3A4, and 3A5 and NADPH-P450 reductase were purified as described previously (16). Microsomal protein concentrations were estimated using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). Concentrations of total P450 (20) and NADPH-P450 reductase (16) were estimated as described previously.
Hydroxylation of Thalidomide
Thalidomide hydroxylation activities were determined using HPLC (13). Briefly, a typical incubation mixture (total volume of 0.20 mL) contained microsomal protein (1.0 mg mL−1) or recombinant P450 (0.10 µM) in bacterial membranes or in reconstituted systems (16), an NADPH-generating system (0.25 mM NADP+, 2.5 mM glucose 6-phosphate, and 0.25 unit mL−1 yeast glucose 6-phosphate dehydrogenase), and thalidomide (1.0 mM) in 0.10 M potassium phosphate buffer (pH 7.4), unless otherwise specified. For P450 activity determinations, incubations were carried out at 37 °C for 30–60 min. Incubations were terminated by adding 0.20 mL of ice-cold Cl3CCO2H. The aqueous supernatant was centrifuged at 2 × 103 g for 10 min and analyzed using a Shimadzu HPLC or Waters LC/MS system, vide infra (21).
Hydroxylation of (R) 5-Hydroxythalidomide by P450 3A4
A typical incubation mixture (total volume of 100 µL) contained recombinant P450 3A4 (1.0 µM) in bicistronic bacterial membranes (16), an NADPH-generating system (0.25 mM NADP+, 2.5 mM glucose 6-phosphate, and 0.25 unit mL−1 yeast glucose 6-phosphate dehydrogenase), and (R)-5-hydroxythalidomide (0–1.0 mM) in 100 mM potassium phosphate buffer (pH 7.4). Incubations were carried out at 37 °C for 30 min and terminated by adding 5 µL of 10 M ice-cold Cl3CCO2H. The solution was centrifuged at 2 × 103 g for 10 min and the supernatant was analyzed using LC-MS/MS, vide infra (21).
GSH Adduct Formation from 5-Hydroxythalidomide Oxidation Products
A typical incubation mixture (total volume of 0.5–5 mL) contained recombinant P450 3A4 (1.0 µM) in bicistronic bacterial membranes (16), an NADPH-generating system (0.25–1 mM NADP+, 2.5–10 mM glucose 6-phosphate, and 0.25–1 unit mL−1 yeast glucose 6-phosphate dehydrogenase), (R)- or (S)-5-hydroxythalidomide (0.1–0.2 mM), and GSH (5–20 mM) in 100 mM potassium phosphate buffer (pH 7.4). Incubations were carried out at 37 °C for 3 h and terminated by adding equal volume of cold CH3CN. The solution was centrifuged at 2 × 103 g for 10 min and the supernatant was dried under a stream of N2. The reaction product was then dissolved in H2O and loaded on a C18 Bakerbond SPE column (Mallinckrodt, Inc., Phillipsburg, NJ) that had been washed with 2 volumes of CH3OH and 6 volumes of H2O. After adding the supernatant the columns were washed with 6 volumes of H2O and finally eluted with 1 volume of CH3CN. The eluant was dried under a stream of N2, dissolved in H2O, and analyzed using LC-MS, vide infra.
LC-UV Assays
The liquid chromatography system consisted of a pump and UV detector (Shimadzu, Kyoto, Japan) with an analytical octadecylsilane (C18) reversed-phase column (5 µm, 4.6 mm × 250 mm, Mightysil RP-18 GP, Kanto Chemicals, Tokyo, Japan). The gradient mobile phase consisted of CH3CN in 100 mM sodium phosphate buffer (pH 3.0) with the following gradient: 0–10 min with 20–25% CH3CN (v/v) in the phosphate buffer; 10–11 min with 25% CH3CN (v/v); 11–12 min with 80% CH3CN (v/v); 12–18 min with 20% CH3CN (v/v), at a flow rate of 1.0 mL min−1. The UV detector wavelength was set at 220 nm (for 5’-hydroxythalidomide) or 236 nm (for 5-hydroxythalidomide) unless otherwise specified. The HPLC column was operated at 40 °C.
LC-MS Assays
In preliminary analysis, a Quattro micro API mass analyzer was used for metabolite analysis (Waters, Tokyo, Japan). The instrument was operated in the electrospray negative ionization mode and was directly coupled to a Waters LC 2695 system with an octadecylsilane C18 column (Atlantis, 3 µm, 2.1 mm × 100 mm) and MassLynx NT4.1 software for data acquisition (Waters). To tune the mass spectrometer, the cone voltage was optimized to maximize the intensity of the precursor ions for thalidomide m/z 257. The collision energy was then adjusted to optimize the signal. Typical tuning conditions were as follow: electrospray capillary voltage, 3.2 kV; sample cone voltage, 25 V; and collision energy, 20 eV at a collision gas (Ar) pressure of 1.6 × 10−4 kPa. The gradient mobile phase consisted of H2O and CH3CN in 0.1 % CH3CO2H (v/v): 0–3 min with 0–95% CH3CN (v/v) with 0.1% CH3CO2H (v/v); 3–8 min with 95% CH3CN (v/v); 8–10.5 min with 95–0% CH3CN with 0.1% CH3CO2H (v/v); 10.5–13 min with 0.1% CH3CO2H (v/v), all at a flow rate of 0.25 mL min−1.
LC-MS/MS analyses of the oxidation product of 5-hydroxythalidomide were performed on a Waters Acquity UPLC system (Waters, Milford, MA) connected to a Thermo LTQ mass spectrometer (Thermo Fisher, Waltham, MA) using an Aquity UPLC BEH octadecylsilane (C18) column (2.1 mm × 100 mm). LC conditions were as follows: buffer A contained 10 mM NH4CH3CO2 (pH 4.0) in 2% CH3CN and 98% H2O (v/v), and buffer B contained 10 mM NH4CH3CO2 (pH 4.0) in 95% CH3CN and 5% H2O (v/v). The following gradient program was used, with a flow rate of 0.4 mL min−1: 0–5 min, linear gradient from 100% A to 75% A (v/v); 5–5.5 min, linear gradient to 100% B; 5.5–7.5 min, hold at 100% B; 7.5–8 min, linear gradient to 100% A; 8–10 min, hold at 100% A. The temperature of the column was maintained at 40 °C. Samples (20 µL) were infused with an auto-sampler. MS analyses were performed in the negative ion mode. The mass spectrometer was tuned using 5-hydroxythalidomide. Product ion spectra were acquired over the range m/z 100–500. Dihydroxythalidomide was quantified using the m/z 289→177 transition, based on a standard curve of the m/z 273→161 transition of 5-hydroxythalidomide.
LC-MSn analyses of the 5-hydroxythalidomide-GSH conjugate were performed on a Waters Acquity UPLC system (Waters, Milford, MA) connected to a Thermo LTQ mass spectrometer (Thermo Fisher, Waltham, MA) using either an Aquity UPLC BEH octadecylsilane (C18) column (2.1 mm × 100 mm) or a YMC C18 column (4.6 mm × 250 mm). LC conditions were as follows: buffer A contained 2% CH3CN in H2O (v/v), and buffer B contained 95% CH3CN (v/v) with each having 0.1% HCO2H. For the UPLC column the following gradient program was used, with a flow rate of 0.4 mL min−1: 0–5 min, linear gradient from 100% A to 75% A (v/v); 5–5.5 min, linear gradient to 100% B; 5.5–7.5 min, hold at 100% B; 7.5–8 min, linear gradient to 100% A; 8–10 min, hold at 100% A. The temperature of the column was maintained at 40 °C. For the analytical column the following gradient program was used, with a flow rate of 0.8 mL min−1: 0–15 min, linear gradient from 100% A to 75% A (v/v); 15–17 min, linear gradient to 100% B; 17–22 min, hold at 100% B; 22–24 min, linear gradient to 100% A; 24–30 min, hold at 100% A. Samples (10–20 µL) were infused with an auto-sampler. MS analyses were performed in the positive ion mode. The mass spectrometer was tuned using GSH.
LC-HRMS was performed on a Waters Acquity UPLC system and a Waters Synapt hybrid quadropole/OA-TOF mass spectrometer (Waters Corporation, Milford, MA) equipped with a dual chemical ionization/electrospray source (ESCI) source. LC conditions were same as mentioned in the previous section. MS analyses were performed in the positive ion mode. Electrospray ionization (ESI) conditions were as follows: capillary voltage 2.59 V, sampling cone 30, extraction cone 4.1, source temp 125 °C, desolvation temperature 325 °C, and Trap CE of 6. Ion spectra were acquired over the range m/z 50–500 using the waters MassLynx V4.1 software.
Kinetic Analysis
Kinetic analysis was done using nonlinear regression analysis programs (Prism, GraphPad Software, La Jolla, CA). The Michaelis constant Km and S50 values were determined using hyperbolic or sigmoidal substrate concentration-dependent velocity curves.
Results
Thalidomide Hydroxylation Catalyzed by Human and Monkey Liver Microsomes and Recombinant P450 3A Enzymes
(R)-Thalidomide was oxidized to 5- and 5’-hydroxythalidomide by human liver microsomes (Table 1): the retention times of 5'- and 5-hydroxythalidomide were 7.6 and 8.3 min, respectively, under these conditions (data not shown) (13, 22). The presence of the peak was found to be dependent upon the presence of both P450 and NADPH. Further evidence was obtained in a separate experiment where incubation of (R)-thalidomide with recombinant human P450s 3A4 (co-expressed with NADPH-P450 reductase in bacterial membranes) followed by LC-MS analysis resulted in the detection of a peak with m/z 273 and corresponding to hydroxythalidomide. CID of the m/z 273 peak gave fragment ions that are consistent with 5-hydroxythalidomide (Supporting Information Figure S1) and high resolution MS (Waters QTof) yielded m/z 273.0526 (calculated for C13H9N2O5, 273.0511).
Table 1.
Thalidomide hydroxylation catalyzed by human or monkey liver microsomes, recombinant human or monkey P450s expressed in E. coli membranes, and purified human P450s in reconstituted systems.
| product | |||||
|---|---|---|---|---|---|
| enzyme source | P450 | (R)-thalidomide | (S)-thalidomide | ||
| 5-hydroxythalidomide | 5’-hydroxythalidomide | 5-hydroxythalidomide | 5’-hydroxythalidomide | ||
| pmol formed min−1 (mg protein)−1 | |||||
| human liver microsomes | 0.08 | 0.59 | 0.03 | < 0.01 | |
| monkey liver microsomes | 0.21 | 2.28 | 0.07 | 0.18 | |
| pmol min−1 (nmol P450)−1 | |||||
| E. coli membranes | 3A4 | 2.0 | < 0.1 | < 0.1 | < 0.1 |
| human | 3A5 | 2.3 | < 0.1 | < 0.1 | < 0.1 |
| 3A7 | 2.8 | < 0.1 | < 0.1 | < 0.1 | |
| monkey | 3A8 | 12 | < 0.1 | 3.4 | < 0.1 |
| 3A5 | 9.2 | < 0.1 | 2.9 | < 0.1 | |
| reconstituted system | 2C19 | 2.4 | 1.7 | 1.1 | < 0.1 |
| human | 3A4 | 2.0 | < 0.1 | 0.9 | < 0.1 |
| 3A5 | 2.5 | < 0.1 | 0.7 | < 0.1 | |
Thalidomide (1000 µM) was incubated with liver microsomes (1.0 mg protein mL−1) or P450 2C19, 3A4, 3A5, 3A7, and 3A8 (100 nM P450) at 37 °C for 60 min. Results are presented as means of results of duplicate determination.
Recombinant human P450s 3A4, 3A5, and 3A7 (co-expressed with NADPH-P450 reductase in bacterial membranes) also catalyzed (R)-thalidomide 5-hydroxylation. Recombinant monkey P450 3A8 and 3A5 catalyzed both (R)- and (S)-thalidomide 5-hydroxylation under these conditions. Purified human P450s 2C19, 3A4, and 3A5 (used in the reconstituted systems) mediated (R)-thalidomide 5-hydroxylation at similar rates (Table 1).
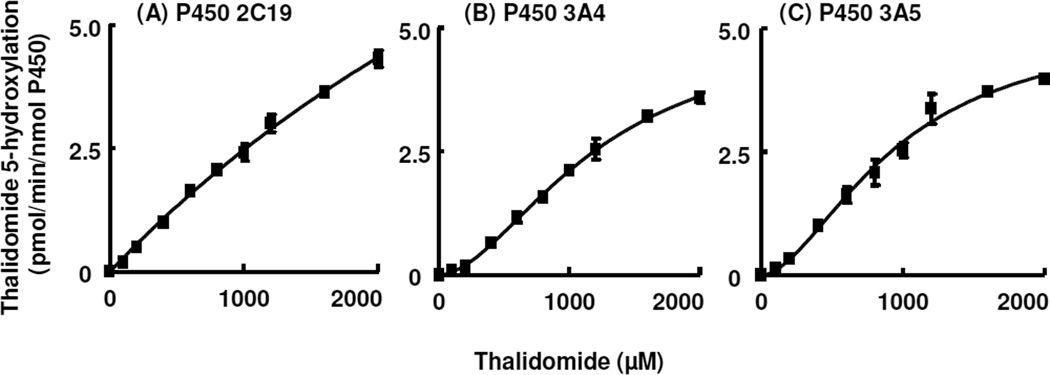
Kinetic analyses were conducted with purified human P450s 2C19, 3A4, and 3A5 (Figure 1). In the reconstituted systems, linearity was seen within a P450 concentration range of 50–150 nM and incubation times of 30–60 min (for (R)-thalidomide 5-hydroxylation). P450 2C19 yielded a rather linear substrate-dependent velocity curve with little saturation (Figure 1A). Apparent kcat and Km values for P450 2C19-mediated (R)-thalidomide 5-hydroxylation were calculated to be 18 ± 3 pmol min−1 (nmol P450 2C19)−1 and 6.1 ± 1.5 mM (mean ± SE), respectively, although the slope is probably more useful as a measure of the reaction efficiency because of the lack of saturation. In contrast, P450 3A4 and 3A5 showed sigmoidal curves (Figures 1B and 1C), although at higher concentrations these also showed little saturation. The calculated Hill coefficients for P450 3A4 and 3A5 were 1.8 ± 0.1 and 1.7 ± 0.2, respectively. Apparent S50 and kcat values for P450 3A4- and 3A5-mediated (R)-thalidomide 5-hydroxylation were calculated to be 1.2 ± 0.1 mM and 0.95 ± 0.14 mM and 5.1 ± 0.3 and 5.2 ± 0.5 pmol min−1 (nmol P450)−1, respectively. Apparent kcat/Km or kcat/S50 values were low and similar (0.003–0.004 min−1 mM−1 P450) for these three P450 enzymes.
Figure 1. Thalidomide 5-hydroxylation activity catalyzed by purified human P450s as a function of substrate concentration.
(A) P450 2C19, (B) P450 3A4, (C) P450 3A5. Results are presented as means ± SD of triplicate determinations.
Hydroxylation of 5-Hydroxythalidomide by P450 3A4
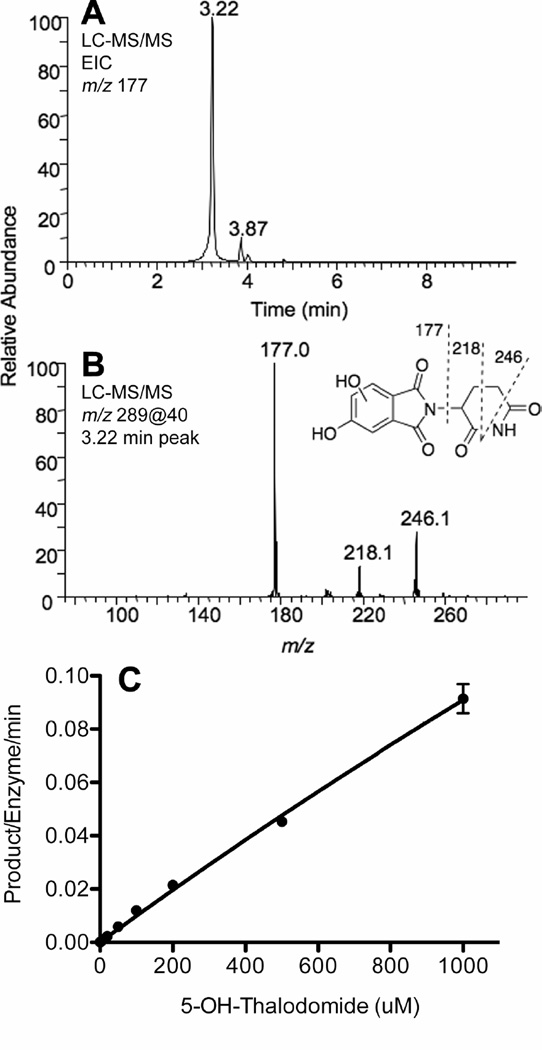
P450 3A4 was found to further oxidize 5-hydroxythalidomide to a dihydroxythalidomide product, as detected by LC-MS and LC-MS/MS analysis. This new peak had a parent ion at m/z 289, 16 a.m.u. higher than 5-hydroxythalidomide. CID of the parent ion (m/z 289) in the positive ion mode yielded fragment ions with m/z 177, 218, and 246 that are consistent with the presence of a dihydroxythalidomide compound with the two hydroxy groups being present in the phenyl ring of thalidomide (Figure 2B). Much less of this compound was detected with liver microsomes, suggesting that this metabolite (or a precursor, e.g. an epoxide) may be unstable in a complex system, presumably reacting with necleophilic groups of proteins. However, its production could be quantified by LC-MS/MS, although lowering the pH was necessary to obtain sharp peaks, as might be expected for a phenol or dihydroxy compound (Figure 2A). As in the case of the conversion of thalidomide to 5-hydroxythalidomide by P450 3A4 (or 3A5) (Figure 1B), little saturation was observed in plots of v vs. S (Figure 2C). The parameters kcat = 1.0 nmol product formed min−1 (nmol P450)−1 and Km = 10 mM could be estimated, but the slope (~kcat/Km) is a better estimate of enzyme efficiency, i.e. 0.1 min−1 mM−1 (100 min−1 M−1), which is ~ 25-fold higher than the rate of 5-hydroxylation of thalidomide by the same enzyme.
Figure 2. 5-Hydroxythalidomide hydroxylation activity catalyzed by purified human P450 3A4.
(A) Extracted ion chromatogram of the product ion with m/z 177. (B) MS/MS spectrum of dihydroxythalidomide (MH+ 289). (C) v vs. S plot.
GSH Adduct Formation from 5-Hydroxythalidomide Oxidation Products
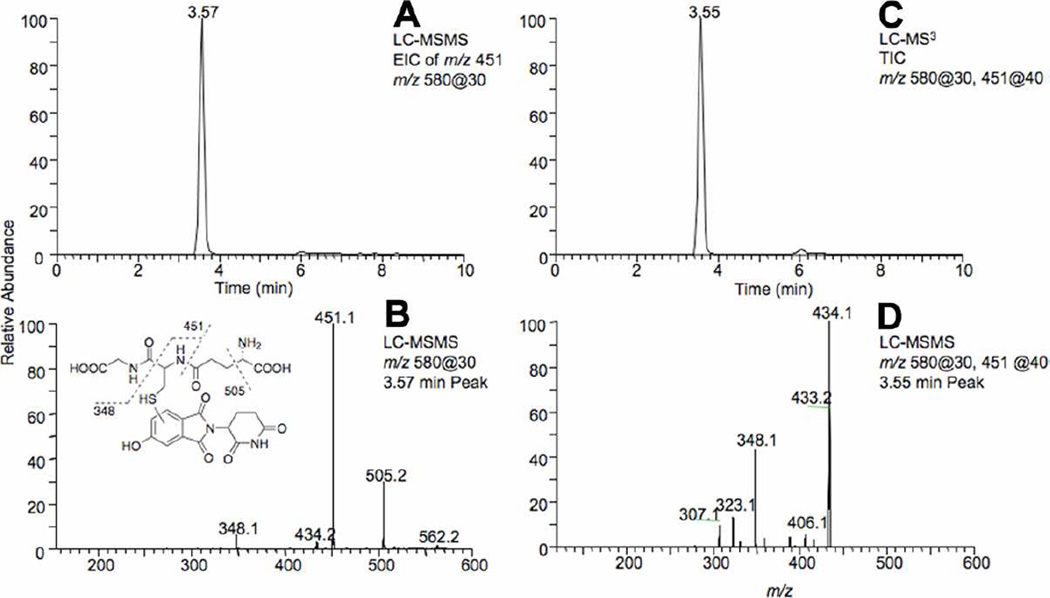
Further analyses of the oxidation of 5-hydroxythalidomide by P450 3A4 in the presence of GSH, using LC-MS and LC-MS/MS in the positive ion mode, led to the detection of a peak at tR ~3.5 min (tR ~14 min when the YMC analytical column was used with a different LC method) with a mass of 579 that is consistent with the presence of a GSH conjugate of 5-hydroxythalidomide (Figures 3A and 4A and Figure S2, Supporting Information).). Using LC-HRMS, the mass of the [MH]+ ion of the 3.5 min peak was found to be 580.1344 (calculated [MH]+ for a 580.1344) (Figure S2, Supporting Information). CID of the peak at tR ~3.5 min in the positive ion mode yielded fragment ions with m/z 505 (loss of 75) and 451 (loss of 129) that are typical of GSH conjugates (Figures 3B and 4B). Together these results clearly indicate that oxidation of 5-hydroxythalidomide by P450 3A4 generates a reactive intermediate that, in the presence of GSH, results in the formation of a GSH conjugate. The formula (C23H25N5O11S) of the conjugate indicates that the GSH addition must happen following a formal 2-electron oxidation, not direct attack of GSH on 5-hydroxythalidomide.
Figure 3. LC-MS/MS chromatograms and CID spectra showing the presence of the GSH conjugate of 5-hydroxythalidomide formed in the presence of 5 mM GSH.
(A) Extracted ion chromatogram of the product ion with m/z 451 of the 5-hydroxythalidomide-GSH conjugate. (B) MS/MS spectrum of the 5-hydroxythalidomide-GSH conjugate (MH+ 580). (C) Total ion chromatogram of the CID of the m/z 451 product ion. (D) MS3 spectrum of the 5-hydroxythalidomide-GSH conjugate (m/z 580@30, 451@40). 5-hydroxythalidomide (100 µM) was incubated with the P450 3A4 bicistronic membranes (1 µM) in the presence of GSH (5 mM) and an NADPH-generating system for 3 h.
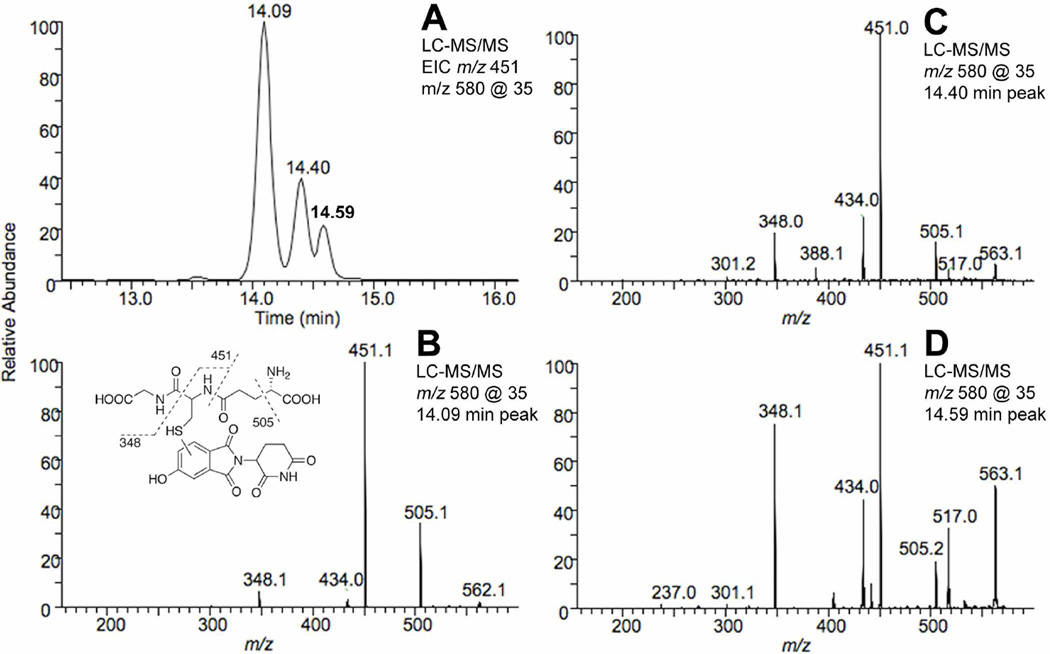
Figure 4. LC-MS/MS chromatograms and CID spectra showing the presence of the GSH conjugates of 5-hydroxythalidomide formed in the presence of 20 mM GSH.
(A) Extracted ion chromatogram of the product ion with m/z 451 of the 5-hydroxythalidomide-GSH conjugate. (B) MS/MS spectrum of the 5-hydroxythalidomide-GSH conjugate (MH+ 580) eluting at 14.09 min. (C) MS/MS spectrum of the 5-hydroxythalidomide-GSH conjugate (MH+ 580) eluting at 14.40 min. (D) MS/MS spectrum of the 5-hydroxythalidomide-GSH conjugate (MH+ 580) eluting at 14.59 min. 5-Hydroxythalidomide (200 µM) was incubated with the P450 3A4 bicistronic membranes (1 µM) in the presence of GSH (20 mM) and an NADPH-generating system for 3 h.
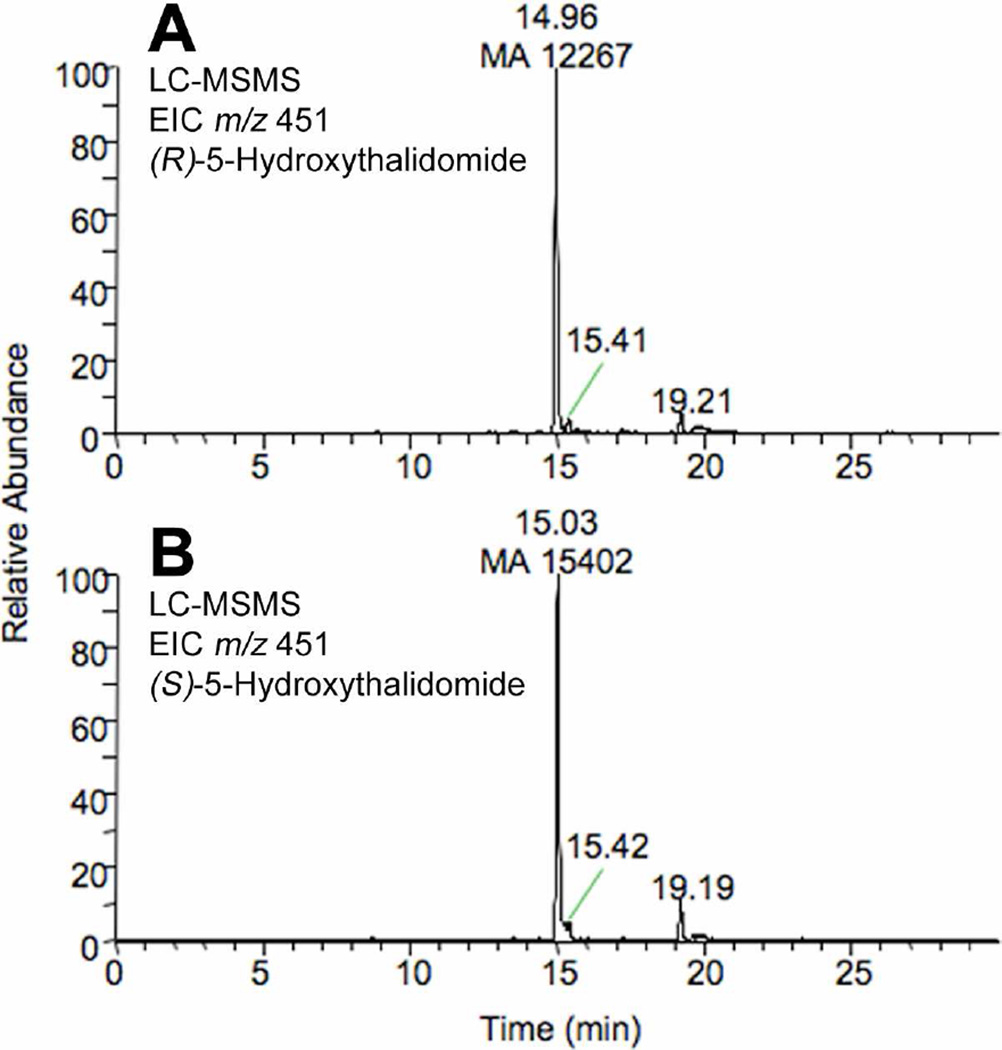
Interestingly, when the reactions were performed in the presence of 20 mM GSH three peaks (tR ~14, 14.4 and 14.6 min and a characteristic m/z 580→451 transition) were obtained (CID also showed the presence of m/z 505, a loss of 75 (a. m. u.) for all three peaks) with yields of 65, 23, and 11% (Figure 4). In contrast, when a more physiological concentration (5 mM) of GSH was used in the reaction mixture the presence of the minor peaks (tR ~14.4 and 14.6 min) was negligible (although detectible) (Figures 3A and 5A). The (R)- and (S)-enantiomers were compared for formation of the GSH conjugate (Figure 4); the yields of the GSH conjugate for the (R)- and (S)-enantiomer were very similar as judged by the m/z 580→451 transition in the LC-MS/MS experiments (Figure 5).
Figure 5. LC-MS/MS chromatograms showing the presence and relative yield of the GSH conjugate of 5-hydroxythalidomide formed from the (R)- and (S)-thalidomide enantiomers.
(A) (R)-thalidomide; (B) (S)-thalidomide. (R)- or (S)-5-hydroxythalidomide (100 µM) was incubated with the P450 3A4 bicistronic membranes (1 µM P450) in the presence of GSH (5 mM) and an NADPH-generating system for 60 min. Extracted ion chromatograms showing the m/z 580→451 transition are shown. MA: mass area.
Characterization of the 5-Hydroxythalidomide-GSH Conjugate
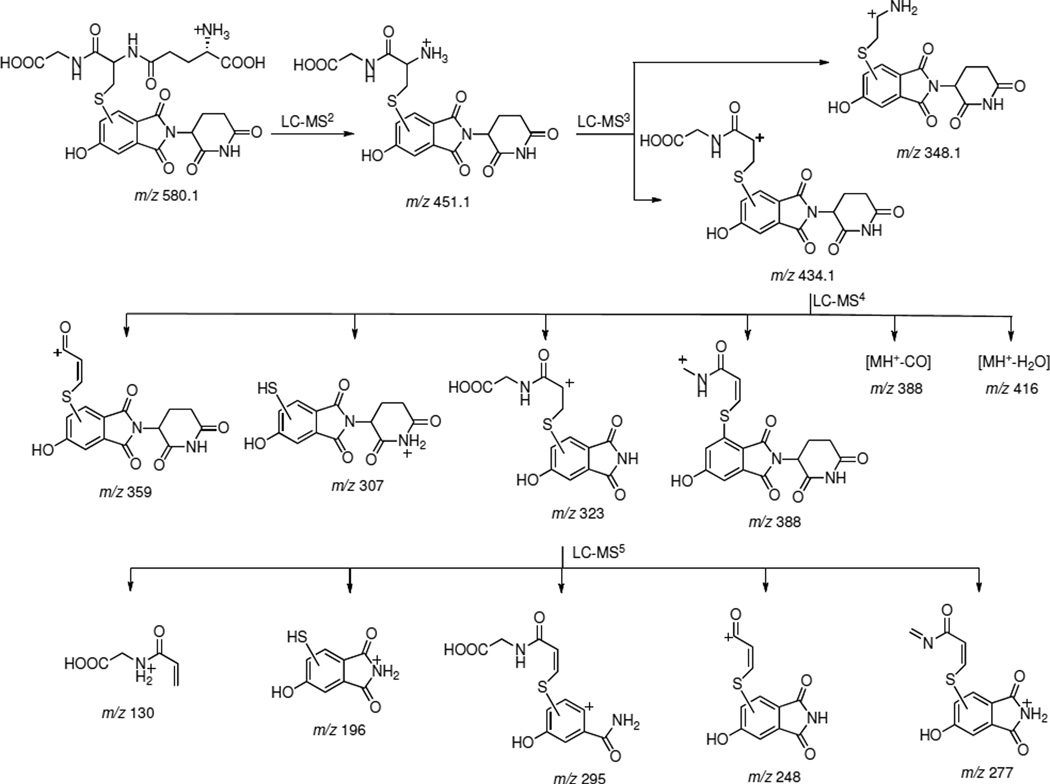
Our initial attempts to characterize the structure of the 5-hydroxythalidomide-GSH conjugate using 1- and 2-dimensional NMR were not successful because of the limited yield of the product. Accordingly we used tandem LC-MS to gain insight regarding the structure of the conjugate. Fragment ions and their proposed structures obtained as a result of tandem CID of the m/z 580 species and subsequent major product ions are shown in Scheme 1 and Figure S3 (Supporting Information). CID of the m/z 580 ion gave a loss of 129 with m/z 451 as the major peak (Figure 3B) that upon further fragmentation gave m/z 434 (loss of NH3) as the major ion (Figure 3D). LC-MS4 of m/z 434 resulted in the formation of an ion with m/z 323, consistent with the loss of the piperidine dione moiety. The assigned structure of the m/z 323 species was confirmed by further fragmentation (Figure S3, Supporting Information). The detection of this m/z 323 species clearly suggests that GSH is attached to the phenyl ring of the thalidomide molecule. However, based on the tandem fragmentation data, the linkage of the GSH molecule to a particular atom of thalidomide could not be established unambiguously at this point, although it did clearly indicate that the m/z 580 species is a 5-hydroxythalidomide-GSH conjugate.
Scheme 1. Scheme showing the proposed structures of the various fragmentation products of the 5-hydroxythalidomide-GSH conjugate.
Discussion
Thalidomide has been the subject of numerous studies following the teratogenicity problems with its use 50 years ago (7, 23). However, relatively few studies have been done on the metabolism of thalidomide. With the renewed interest in this drug for other indications where teratology is not an issue (7, 8), several studies have been reported. Early work had shown the presence of phenolic products produced by rabbits, a sensitive species, but not rats (24, 25). Subsequent work has confirmed that the 4-and 5-hydroxy products are formed in various species (24, 26). In humans the major metabolites are 5- and (cis) 5’-hydroxythalidomide (11, 13, 22, 26).
We recently reported the heterotropic cooperativity of human P450 3A5 in a study of drug interactions of thalidomide with midazolam and cyclosporine A (12). In the present study, the homotropic cooperativity of human P450s 3A4 and 3A5 was observed in thalidomide 5-hydroxylation (Figure 1). In general, the substrate specificity of P450 3A5 is considered to be similar to that of P450 3A4, in large part because P450 3A5 has 83% sequence identity with P450 3A4. In terms of species differences in thalidomide hydroxylation, monkey P450 3A8 and 3A5 also showed selective (R)-thalidomide hydroxylation and higher activities compared with human P450s 3A4 and 3A5 (Table 1). In liver microsomes, minipigs (or micro-minipigs) and rabbits had similar (R)- vs. (S)-thalidomide 5-hydroxylation regioselectivity compared to humans and monkeys but, of the animals tested in this study, only rats had different properties (results not shown).
It has been reported that decreased formation of thalidomide metabolites would be expected with defective alleles of P450 2C19, compared to wild type (2C19*1), on clinical treatment with thalidomide plus dexamethasone (9). The present study demonstrated that metabolically activated thalidomide reacts with GSH (Figures 3–5, Scheme 1).
A number of possible mechanisms have been proposed for the toxicity of thalidomide, including biological acylation (27) and, more recently, various types of oxidative stress (28–32). However, concerns about the oxidative stress proposals include the very low rates of oxidation and the lack of chemical proclivity of metabolites to autoxidation. A role for reactive metabolites has been entertained previously (33): using an in vitro activation/toxicity assay, the toxicity of thalidomide was reduced by the addition of purified epoxide hydrolase and increased by the addition of the epoxide hydrolase inhibitor 1,1,1-trichloropropylene oxide (33). These results notwithstanding, no direct evidence for an epoxide intermediate was demonstrated. Incubations of P450 3A4 or 3A5 with thalidomide and GSH led to the formation of a GSH conjugate. The MS characteristics show clearly that this must have arisen from a 2-electron oxidized product of thalidomide (e.g. 5-hydroxy product), followed by a further (2-electron) oxidation (i.e. MH+ 580) (Figure S2, Supporting Information). Possible mechanisms are presented in the Supporting Information, Scheme S1. The epoxide pathway would be consistent with the previous results of Gordon et al. (33).
The relevance of the oxidation pathways can be questioned in the context of the low kcat and high Km values. Very recently Ito et al. (34) reported the (non-covalent) binding of thalidomide to the protein cereblon, part of a protein complex postulated to be important in development. However, most of the experiments in that study were done with 300 to 400 µM concentrations of thalidomide, and in the context the high P450 Km values (Figures 1, 2) and the covalent binding may also be important.
The formation of a GSH conjugate is considered evidence for reactive metabolites, particularly in the absence of GSH transferases, and of possible relevance to toxicity (35). The extent of oxidation of thalidomide is very low. It is known that only the (S)-enantiomer of thalidomide is teratogenic (7, 8). We made a direct comparison of the formation of the GSH conjugate from (R)- and (S)-thalidomide (Figure 5) and found that the yields are similar. However, a preferential reaction of one diasteromeric epoxide with a specific protein cannot be ruled out.
The participation of human P450 2C19 in thalidomide oxidation has been reported (13), but significant roles of P450 3A4 and 3A5 were indicated in our study. In conclusion, the present study suggests that human P450 3A4 and 3A5 effectively mediate thalidomide 5-hydroxylation and lead to GSH conjugation, which may be relevant to its pharmacological and toxicological actions.
Supplementary Material
Acknowledgments
This work was supported in part by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (H.Y.) and United States Public Health Service grants R37 CA090426, R01 ES010546, and P30 ES000267 (F.P.G.).
Abbreviations
- CID
collision-induced dissociation
- ESI
electrospray ionization
- UPLC
ultra-performance liquid chromatography
References
- 1.Guengerich FP. Cytochrome P450 and chemical toxicology. Chem. Res. Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- 2.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 3.Yamaori S, Yamazaki H, Iwano S, Kiyotani K, Matsumura K, Honda G, Nakagawa K, Ishizaki T, Kamataki T. CYP3A5 contributes significantly to CYP3A-mediated drug oxidations in liver microsomes from Japanese subjects. Drug Metab. Pharmacokin. 2004;19:120–129. doi: 10.2133/dmpk.19.120. [DOI] [PubMed] [Google Scholar]
- 4.Niwa T, Murayama N, Emoto C, Yamazaki H. Comparison of kinetic parameters for drug oxidation rates and substrate inhibition potential mediated by cytochrome P450 3A4 and 3A5. Curr. Drug Metab. 2008;9:20–33. doi: 10.2174/138920008783331121. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K, Yamazaki H, Imiya K, Akasaka S, Guengerich FP, Shimada T. Relationship between CYP2C9 and 2C19 genotypes and tolbutamide methyl hydroxylation and S-mephenytoin 4'-hydroxylation activities in livers of Japanese and Caucasian populations. Pharmacogenetics. 1997;7:103–113. doi: 10.1097/00008571-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Wienkers LC, Heath TG. Predicting in vivo drug interactions from in vitro drug discovery data. Nat. Rev. Drug Discov. 2005;4:825–833. doi: 10.1038/nrd1851. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese L, Resztak K. Thalidomide revisited: pharmacology and clinical applications. Expert. Opin. Investig. Drugs. 1998;7:2043–2060. doi: 10.1517/13543784.7.12.2043. [DOI] [PubMed] [Google Scholar]
- 8.Sembongi K, Tanaka M, Sakurada K, Kobayashi M, Itagaki S, Hirano T, Iseki K. A new method for determination of both thalidomide enantiomers using HPLC systems. Biol. Pharm. Bull. 2008;31:497–500. doi: 10.1248/bpb.31.497. [DOI] [PubMed] [Google Scholar]
- 9.Li YH, Hou J. Effect of CYP2C19 gene polymorphism on efficacy of thalidomide-based regimens for the treatment of multiple myeloma . Zhonghua Xue. Ye. Xue. Za Zhi. 2007;28:651–654. [PubMed] [Google Scholar]
- 10.Trapnell CB, Donahue SR, Collins JM, Flockhart DA, Thacker D, Abernethy DR. Thalidomide does not alter the pharmacokinetics of ethinyl estradiol and norethindrone. Clin. Pharmacol. Ther. 1998;64:597–602. doi: 10.1016/S0009-9236(98)90050-9. [DOI] [PubMed] [Google Scholar]
- 11.Teo SK, Sabourin PJ, O'Brien K, Kook KA, Thomas SD. Metabolism of thalidomide in human microsomes, cloned human cytochrome P-450 isozymes, and Hansen's disease patients. J. Biochem. Mol. Toxicol. 2000;14:140–147. doi: 10.1002/(sici)1099-0461(2000)14:3<140::aid-jbt3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.Okada Y, Murayama N, Yanagida C, Shimizu M, Guengerich FP, Yamazaki H. Drug interactions of thalidomide with midazolam and cyclosporine A: heterotropic cooperativity of human cytochrome P450 3A5. Drug Metab Dispos. 2009;37:18–23. doi: 10.1124/dmd.108.024679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ando Y, Fuse E, Figg WD. Thalidomide metabolism by the CYP2C subfamily. Clin. Cancer Res. 2002;8:1964–1973. [PubMed] [Google Scholar]
- 14.Niwa T, Murayama N, Yamazaki H. Heterotropic cooperativity in oxidation mediated by cytochrome P450. Curr. Drug Metab. 2008;9:453–462. doi: 10.2174/138920008784746364. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Shibata N, Sukeguchi D, Takashima M, Nakamura S, Toru T, Matsunaga N, Hara H, Tanaka M, Obata T, Sasaki T. Synthesis, configurational stability and stereochemical biological evaluations of (S)- and (R)-5-hydroxythalidomides. Bioorg. Med. Chem. Lett. 2009;19:3973–3976. doi: 10.1016/j.bmcl.2009.02.108. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki H, Nakamura M, Komatsu T, Ohyama K, Hatanaka N, Asahi S, Shimada N, Guengerich FP, Shimada T, Nakajima M, Yokoi T. Roles of NADPH-P450 reductase and apo- and holo-cytochrome b5 on xenobiotic oxidations catalyzed by 12 recombinant human cytochrome P450s expressed in membranes of Escherichia coli. Protein Express. Purif. 2002;24:329–337. doi: 10.1006/prep.2001.1578. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki H, Okayama A, Imai N, Guengerich FP, Shimizu M. Interindividual variation of cytochrome P450 2J2 expression and catalytic activities in liver microsomes from Japanese and Caucasian populations. Xenobiotica. 2006;36:1201–1209. doi: 10.1080/00498250600944318. [DOI] [PubMed] [Google Scholar]
- 18.Murayama M, Kaneko N, Horiuchi K, Ohyama K, Shimizu M, Ito K, Yamazaki H. Cytochrome P450-dependent drug oxidation activities of liver microsomes from microminipigs, a possible new animal model for humans in non-clinical study. Drug Metab Pharmacokinet. 24:404–408. doi: 10.2133/dmpk.24.404. [DOI] [PubMed] [Google Scholar]
- 19.Uno Y, Hosaka S, Matsuno K, Nakamura C, Kito G, Kamataki T, Nagata R. Characterization of cynomolgus monkey cytochrome P450 (CYP) cDNAs: is CYP2C76 the only monkey-specific CYP gene responsible for species differences in drug metabolism? Arch. Biochem. Biophys. 2007;466:98–105. doi: 10.1016/j.abb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 21.Murayama N, Imai N, Nakane T, Shimizu M, Yamazaki H. Roles of CYP3A4 and CYP2C19 in methylhydroxylated and N-oxidized metabolite formation from voriconazole, a new anti-fungal agent, in human liver microsomes. Biochem. Pharmacol. 2007;73:2020–2026. doi: 10.1016/j.bcp.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Ando Y, Price DK, Dahut WL, Cox MC, Reed E, Figg WD. Pharmacogenetic associations of CYP2C19 genotype with in vivo metabolisms and pharmacological effects of thalidomide. Cancer Biol. Ther. 2002;1:669–673. doi: 10.4161/cbt.318. [DOI] [PubMed] [Google Scholar]
- 23.Bosch ME, Sanchez AJ, Rojas FS, Ojeda CB. Recent advances in analytical determination of thalidomide and its metabolites. J. Pharm. Biomedi. Anal. 2008;46:9–17. doi: 10.1016/j.jpba.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson T, Bjorkman S, Roth B, Bjork H, Hoglund P. Hydroxylated metabolites of thalidomide: formation in-vitro and in-vivo in man. J. Pharm. Pharmacol. 1998;50:1409–1416. doi: 10.1111/j.2042-7158.1998.tb03368.x. [DOI] [PubMed] [Google Scholar]
- 25.Schumacher H, Smith RL, Williams RT. The metabolism of thalidomide: the fate of thalidomide and some of its hydrolysis products in various species. Br. J. Pharmacol. Chemother. 1965;25:338–351. doi: 10.1111/j.1476-5381.1965.tb02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J, Helsby N, Palmer BD, Tingle M, Baguley BC, Kestell P, Ching LM. Metabolism of thalidomide in liver microsomes of mice, rabbits, and humans. J. Pharmacol. Exp. Ther. 2004;310:571–577. doi: 10.1124/jpet.104.067793. [DOI] [PubMed] [Google Scholar]
- 27.Fabro S, Smith RL, Williams RT. Thalidomide as a possible biological acylating agent. Nature. 1965;208:1208–1209. doi: 10.1038/2081208a0. [DOI] [PubMed] [Google Scholar]
- 28.Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat. Med. 1999;5:582–585. doi: 10.1038/8466. [DOI] [PubMed] [Google Scholar]
- 29.Hansen JM, Carney EW, Harris C. Differential alteration by thalidomide of the glutathione content of rat vs. rabbit conceptuses in vitro. Reprod. Toxicol. 1999;13:547–554. doi: 10.1016/s0890-6238(99)00053-2. [DOI] [PubMed] [Google Scholar]
- 30.Hansen JM, Harris KK, Philbert MA, Harris C. Thalidomide modulates nuclear redox status and preferentially depletes glutathione in rabbit limb versus rat limb. J. Pharmacol. Exp. Ther. 2002;300:768–776. doi: 10.1124/jpet.300.3.768. [DOI] [PubMed] [Google Scholar]
- 31.Lv P, Luo HS, Zhou XP, Chireyath PS, Xiao YJ, Si XM, Liu SQ. Thalidomide prevents rat liver cirrhosis via inhibition of oxidative stress. Pathol. Res Pract. 2006;202:777–788. doi: 10.1016/j.prp.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Knobloch J, Reimann K, Klotz LO, Ruther U. Thalidomide resistance is based on the capacity of the glutathione-dependent antioxidant defense. Mol. Pharm. 2008;5:1138–1144. doi: 10.1021/mp8001232. [DOI] [PubMed] [Google Scholar]
- 33.Gordon GB, Spielberg SP, Blake DA, Balasubramanian V. Thalidomide teratogenesis: evidence for a toxic arene oxide metabolite. Proc. Natl. Acad. Sci. U. S. A. 1981;78:2545–2548. doi: 10.1073/pnas.78.4.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 35.Evans DC, Watt AP, Nicoll-Griffith DA, Baillie TA. Drug-protein adducts: An industry perspective on minimizing the potential for drug bioactivation in drug discovery and development. Chem. Res. Toxicol. 2004;17:3–16. doi: 10.1021/tx034170b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.