Abstract
Gd(III) associated with carbon nanomaterials relaxes water proton spins at an effectiveness that approaches or exceeds the theoretical limit for a single bound water molecule. These Gd(III)-labeled materials represent a potential breakthrough in sensitivity for Gd(III)-based contrast agents used for magnetic resonance imaging (MRI). However, their mechanism of action remains unclear. A gadographene library encompassing GdCl3, two different Gd(III)-complexes, graphene oxide (GO), and graphene suspended by two different surfactants and subjected to varying degrees of sonication was prepared and characterized for their relaxometric properties. Gadographene was found to perform comparably to other Gd(III)-carbon nanomaterials; its longitudinal (r1) and transverse (r2) relaxivity is modulated between 12–85 mM−1s−1 and 24–115 mM−1s−1, respectively, depending on the Gd(III)-carbon backbone combination. The unusually large relaxivity and its variance can be understood under the modified Florence model incorporating the Lipari-Szabo approach. Changes in hydration number (q), water residence time (τM), molecular tumbling rate (τR), and local motion (τfast) sufficiently explain most of the measured relaxivities. Furthermore, results implicated the coupling between graphene and Gd(III) as a minor contributor to proton spin relaxation.
Keywords: gadolinium, graphene, graphene oxide, relaxivity, modified Florence NMRD program
Introduction
The effectiveness of Gd(III)-carbon nanomaterials1–16 in mediating water proton spin relaxation is rarely matched by other Gd(III)-nanomaterial constructs.17,18 Whether the carbon scaffold has unique properties that contribute to the relaxation process is not yet fully understood. Accelerating proton relaxation underlies the function of contrast agents used in magnetic resonance imaging (MRI).19–36 Thus, a better elucidation of the mechanism of action of Gd(III)-carbon nanomaterials may provide an opportunity to increase the sensitivity of Gd(III) agents beyond what is currently accessible by Gd(III)-complexes.
Gd(III) relaxes proton spins by creating a fluctuating magnetic field via electronic spin relaxation (T1e and T2e) and molecular motion as described by the Solomon-Bloembergen-Morgan (SBM) theory37–39 and several more advanced theories that account for static zero field splitting (ZFS), namely the slow motion theory,40–42 the Grenoble approach,43,44 the Florence model,45 and the modified Florence model.46,47 The effectiveness of this process is measured by the relaxation enhancement induced per unit Gd(III) concentration in a quantity known as relaxivity (mM−1s−1). The relaxivity of Gd(III)-complexes at 20–60 MHz is typically 4 mM−1s−1 and increases to 10–40 mM−1s−1 when the complex is conjugated to a slow-tumbling nanomaterial or macromolecule, with the majority of the examples falling between 10–20 mM−1s−1.17,18,37,38,48–54 Exceptional cases include agents that utilize human serum albumin,55 apoferritin,56 gold nanoparticle,57 and viral capsid58 with reported relaxivities of up to 84 (20 MHz), 80 (20 MHz), 60 (30 MHz), and 202 mM−1s−1 (61 MHz), respectively. Gd(III)-carbon nanomaterials such as gadofullerenes,7,10,12–16 gadonanotubes,2,6,8,9 and Gd(III)-nanodiamonds1 studied over the past decade rank among the most potent relaxation agents reported to date. As a class, these materials have achieved relaxivities of up to 50–170 mM−1s−1, approaching or even exceeding the theoretical limit for a single water molecule bound to a Gd(III) ion (Figure S1).37,59
The impressive relaxivities of Gd(III)-carbon nanomaterials raise speculation regarding the potential role of carbon in mediating unique mechanisms that contribute to proton relaxation.4,8,12 The theoretical limit for Gd(III) relaxivity is approximately 45 mM−1s−1 and 120 mM−1s−1 at 60 MHz and 20 MHz, respectively, when assuming isolated paramagnetic centers, single water coordination per metal (q = 1), and negligible contribution from second- and outer-sphere waters (Figure S1).37,59 Of the literature examples that approach or exceed these limits, the viral capsid agent likely has a q larger than 1, the albumin and the gold nanoparticle agents are known to have a q of 2, and the apoferritin agent has large second- and outer-sphere relaxivity. Whether the extraordinary relaxivities of Gd(III)-carbon nanomaterials can be similarly explained is not completely clear due to difficulties in their characterization and structural control. Suggestions have been made that unusual magnetic properties at least partially contribute to the observed relaxivities in some cases.2,4,8,12 If true, the unique ability of carbon nanomaterials in modulating magnetism would sharply differentiate them from other scaffold materials and present an opportunity to engineer the sensitivity of Gd(III) contrast agents beyond what is currently possible.
The different Gd(III)-carbon nanomaterials are understood to varying degrees with respect to their mechanism of spin relaxation induction. For endohedral gadofullerenes (r1 up to 98 mM−1s−1 at 60 MHz),60 the relaxivity is recognized to derive largely from the exchangeable protons on the fullerene surface and the interstitial water confined between gadofullerenes in an aggregate.60,61 However, the carbon cage is thought to possess a ½ spin that contributes to relaxivity due to transfer of the 5d1 and 6s2 electrons from the entrapped Gd(III).62 For Gd(III)-nanodiamonds (r1 = 59 mM−1s−1 at 60 MHz),1 the mechanism of action is unknown. Potential explanations include slow tumbling rate, nanodiamond clustering, a unique nanoscopic hydration layer,1 and the two water coordination sites on the Gd(III)-complex. For carbon nanotubes non-covalently functionalized with amphiphilic Gd(III)-complexes (r1 up to 50 mM−1s−1 at 20 MHz), the magnetic susceptibility of the carbon nanomaterial was speculated to contribute to proton relaxation,8 but no mechanistic study has been performed. For gadonanotubes (r1 = 170 mM−1s−1 at 60 MHz)2 utilizing GdCl3, confinement of the Gd(III) ions has been suggested to induce a superparamagnetic state that enhances the relaxivity of this material.2 Similar phenomenon seems to have been recapitulated in HiPco single-walled carbon nanotubes4 and graphene materials with trace Mn(II) (r1 up to 63 mM−1s−1 at 20 MHz).63 However, direct evidence that establishes a causative link between the superparamagnetism and relaxivity has not yet been reported. The question as to whether the carbon scaffold plays a significant role in the unusual relaxivities of Gd(III)-carbon nanomaterials remains unclear.
To better understand the basis of high relaxivity in Gd(III)-carbon nanomaterials, a gadographene library was prepared and studied (Figure 1, Table S1). Graphene is considered to be the parent material of carbon fullerenes and carbon nanotubes; its electronic structure is easily modulated by oxidation64 and sonication,65 making it an ideal system of investigation. By systematically changing the carbon backbone, the potential role of the itinerant electrons66,67 in modulating relaxivity can be clarified. In addition, the different carbon backbones were functionalized with either GdCl3, Gd(III)-DTPA-NH2 (q=1), or Gd(III)-DO3A-NH2 (q=2). This assortment of materials allowed probing of the contribution from second- and outer-sphere waters and the significance of the mode of association between Gd(III) and the carbon nanomaterial. The gadographenes were carefully checked for metal impurities by ICP-MS (Table S1) and their relaxivities were measured at varying field strengths in Nuclear Magnetic Relaxation Dispersion (NMRD) experiments. For one of the gadographenes, direct measurement of q and magnetic susceptibility (χ) was possible on Gd(III) in its solution state at room temperature, marking the first time these important mechanistic parameters were directly obtained for a Gd(III)-carbon nanomaterial in solution.
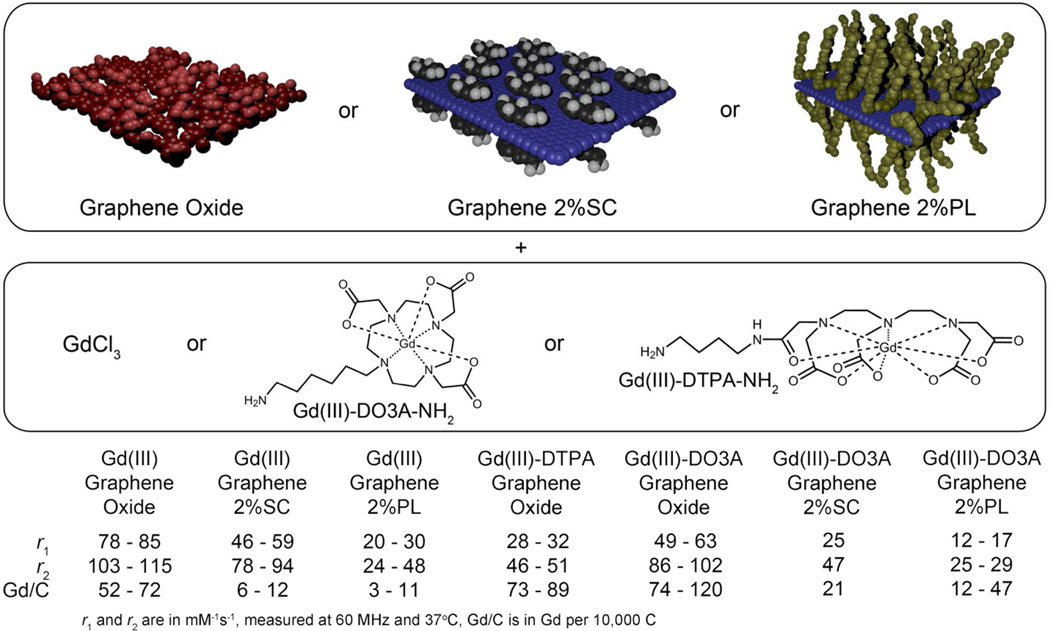
Figure 1.
Overview of gadographene materials prepared and investigated. Gadographenes have unusually large relaxivities that are comparable to other Gd(III)-carbon nanomaterials and greater than most nanomaterial or macromolecular agents based on Gd(III). SC = sodium cholate. PL = Pluronic F108NF. r1 = longitudinal relaxivity. r2 = transverse relaxivity.
Materials and Methods
Preparation of Graphene, GO, and reduced GO
The preparation of Graphene, GO, and reduced GO follows previously published methods68–71 and is described in more detail in Supporting Information. Briefly, sodium cholate (SC)-dispersed graphene was prepared by ultrasonication of graphite (3061 grade, Asbury Graphite Mills) at 40 – 70 W for 45 min – 16 h and purified by removal of large particles via centrifugation (~4,620 g for 30 min), followed by repeated washing and centrifugation in DI water. GO was prepared using a modified Hummer’s method,70 which calls for the treatment of graphite by sulfuric acid and KMnO4, followed by the addition of DI water and 30% H2O2. The resulting GO was washed by 1:10 HCl solution and DI water on a vacuum filter, ultrasonicated at ~10 W for 1 h, and separated from large aggregates by centrifugation (~21,000 g for 10 min). Reduced GO was prepared by treating dispersed GO solution with 1.5M NaOH for 6 hours under bath sonication,71 followed by repeated washing and centrifugation in DI water, 1 h ultrasonication at ~10 W, and further centrifugation to remove large particles. Studies by Rourke et al.72 and Fernandez-Merino, et al.73 show that the treatment of GO by strong base strips the GO of oxidative debris to achieve a partially reduced, conductive material.
Preparation of Gadographenes
Functionalization of graphene and GO by GdCl3 follows a previously reported method for gadonanotubes.2 Briefly, 8 mL of 1 mg/mL GdCl3 solution was mixed with 8 mg of graphene or GO and bath sonicated for 1 h. The sample was left undisturbed for 12 h, and then washed by repeated centrifugation and re-suspension in DI water. The resulting material was dispersed in either 2%SC/2%PL solution or DI water by ultrasonication for 1 h at ~10 W. Large aggregates were removed by centrifugation to complete the processing. Functionalization of graphene and GO by Gd(III)-complexes (Gd(III)-DO3A-NH2 or Gd(III)-DTPA-NH2) was performed by using 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide1 and basic pH,74–77 respectively. Details of the Gd(III)-complex syntheses and reaction conditions are available in Supporting Information. Excess Gd(III)-complexes and activating agents were removed by repeated washing and centrifugation. The same functionalization procedures were followed for both GO and reduced GO.
NMRD and 60 MHz Relaxivity Measurments
The T1 of water protons at 0.01–40 MHz Larmor frequency was measured by a Stelar Spinmaster FFC-2000-1T fast field cycling relaxometer. A Bruker mq60 minispec relaxometer was used to measure the relaxivity at 60 MHz. T1 measurements were performed by an inversion recovery pulse sequence. T2 measurements were performed by a Carr-Purcell-Meiboom-Gill pulse sequence with 1 ms 90°–180° pulse separation. Relaxivity was obtained by linear fitting 1/T1,2 against Gd(III) concentration. Measurement on graphene, GO, 2%SC, and 2%PL controls, and methods used to correct for contributions from impurities are detailed in Supporting Information.
Gd(III) Concentration Determination
Gd(III) concentration was measured by Inductively Coupled Plasmon-Mass Spectrometry (ICP-MS) and cross-validated by ICP-Optical Emission Spectroscopy (ICP-OES) across two sample digestion protocols (Table S7). Sample digestion was performed by incubation with ≥ 69% HNO3 at 60°C overnight, or alternatively by substituting HNO3 with 3:2 HNO3:H2O2 (30% w/w). Iron impurity concentration was measured in addition to Gd(III) concentration. Other metal impurities were characterized by the ICP-MS survey scan, but not measured quantitatively.
Results and Discussion
The gadographene library consists of seven different materials (Figure 1, Table S1). Each of graphene oxide (GO), graphene in 2% F108NF pluronic acid (PL), or graphene in 2% sodium cholate (SC) was functionalized with either GdCl3 or Gd(III)-DO3A-NH2; additionally, a GO with Gd(III)-DTPA-NH2 was prepared. Whether the Gd(III)-complexes are covalently linked or simply adsorbed to the carbon nanomaterial is unclear, but the uncertainty does not detract from the interpretation of the results. The relaxivity (measured at 60 MHz) of gadographene is modulated by sonication and the choice of Gd(III)-carbon combination. In some cases, gadographene recapitulates the high relaxivity of other Gd(III)-carbon nanomaterials, validating its relevance as a system for mechanistic investigation.
Relaxivity Trends
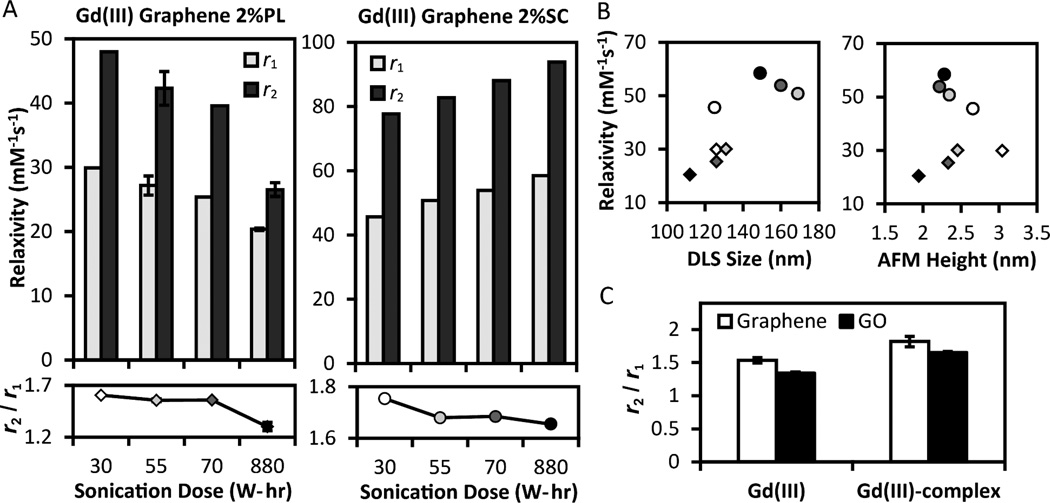
Several relaxivity trends (Figure 2) in gadographene suggest a possibly unique role of the carbon scaffold in modulating relaxivity. The first indication is the opposing trends between Gd(III) Graphene 2%PL and Gd(III) Graphene 2%SC as sonication increases (Figure 2A). According to SBM equations (see Supporting Information), relaxivity at 60 MHz decreases with smaller scaffolds and faster molecular tumbling (small τR) under the typical scenario of τR < 5 ns and T1e >> τR (Figure S1). Since graphene flake size tends to decrease with sonication, the decreasing trend in the 2%PL samples is consistent with theory. However, the same explanation does not apply to the increasing trend in the 2%SC samples. To rationalize this result, the special case of τR, τM > 10 ns may be considered (τM is the water residence time). In this regime, the theoretical r1 at 60 MHz increases with smaller flake size to match the experimental measurements (Figure S2). However, while this hypothetical scenario explains the observed r1 trend, it fails to account for the increasing r2 trend. Therefore, the trending of relaxivity with sonication is not consistent with an interpretation using flake size. Further confirmation was obtained when experiments showed an absence of pronounced correlation between relaxivity and graphene size as measured by both Dynamic Light Scattering (DLS) and Atomic Force Microscopy (AFM) (Figure 2B, Figure S3). Hence, contrary to expectation, there is little evidence to support molecular tumbling as the primary explanatory factor for the correlation between relaxivity and sonication.
Figure 2.
(A) Relaxivity (r1 and r2) at 60 MHz as a function of sonication dose for Gd(III) Graphene 2%PL and 2%SC. The trend is opposite for the two surfactants. (B) Sonication results in thinner graphene stacks, as measured by DLS and AFM height, but no clear positive correlation between relaxivity and graphene size is detected. Symbols and shades in (B) indicate surfactant choice and sonication dose as in (A). More pristine gadographenes possess larger r2/r1 ratios than more damaged gadographenes (A) and materials based on graphene oxide (C) for both GdCl3 and Gd(III)-complexes (Gd(III)-DO3A-NH2 and Gd(III)-DTPA-NH2). Where available, error bars represent standard error.
Alternatively, it is possible that the relative portion of Gd(III) distributed to the graphene planes increases with sonication as compared to the portion distributed to the edges. With one exception (Gd(III) Graphene 2%SC 30 W-hr), the DLS data shows that graphene size decreases with sonication (Figure 2B). The origin of this decrease is likely due to a decrease in thickness (Figure 2B) as no correlation was observed between graphene area and sonication (Figure S3). When sonication is increased, relatively more Gd(III) would be distributed to the planes as a result of the thinner graphene stacks. If the relaxivity of the planar Gd(III) population is larger than that of the edge population in the 2%SC samples (and vice versa in the 2%PL samples), then the observed relaxivity trends can be explained.
A further explanation may be that graphene damage modulates relaxivity in a surfactant-dependent manner. Damage in the form of sonication-induced defects and oxidation can alter the τM or the effective correlation time (τc−1 = τR−1 + τM−1 + T1e−1) of gadographene, or modulate the electronic, and in turn, the magnetic properties of the material.67 Intriguingly, trends in the transverse-to-longitudinal relaxivity ratio (r2/r1) correlate with damage (Figure 2A, C). Gd(III) Graphenes possess r2/r1 ratios that exceed 1.2, suggesting that their τc and τM are longer than 1 ns (Figure S2). One interpretation is that as damage increased, the r2/r1 correspondingly decreased to imply a decline in τc or τM. However, this interpretation may be flawed for Gd(III) Graphene 2%SC because the material’s decreasing trend in r2/r1 cannot be reconciled with its increasing trend in r1 and r2 under any combination of τR and τM (Figure S2). Consequently, a different interpretation of the r2/r1 data is desirable.
Large r2/r1 that exceed those typically reported for paramagnetic ions is a notable property of gadonanotubes2,11 and graphene with Mn(II) impurities63 (3–9 compared to ~ 1 for Gd(III)-complexes). Both of these materials possess unusual r1 NMRD profiles beyond the explanatory power of SBM theory. While the r2/r1 of gadographenes are not as pronounced, samples utilizing more pristine graphene had consistently larger ratios when compared to more sonicated graphene and GO, regardless of whether GdCl3 or Gd(III)-complexes were used (Figure 2A, C). By drawing analogy to other Gd(III)-carbon nanomaterials, these trends in r2/r1 suggest the possibility that graphene damage may influence relaxivity through contributions unaccounted for by current theory. If the effect of graphene damage interacts with surfactant choice, then the opposite effect of sonication on Gd(III) Graphene 2%SC and Gd(III) Graphene 2%PL can be understood.
Given the empirical trends that circumstantially implicate graphene as a unique modulator of relaxivity, magnetic properties that can originate from the graphene itinerant electrons66,67 were examined more closely. Materials based on non-conductive GO64 were compared to materials based on graphene. For both GdCl3 and Gd(III)-complexes, higher relaxivity was achieved using GO, indicating that the presence of itinerant electrons is not crucial for gadographene relaxivity (Figure 1, S4). Further investigation of the potential magnetic coupling between Gd(III) and the carbon backbone was performed by comparing gadographene to analogs that use other metals of large magnetic moment, namely iron and terbium. The relaxivities of these analogs decreased by more than 10 fold when compared to gadographene (Figure S4), suggesting that any magnetic coupling between Gd(III) and graphitic carbon does not generalize to other metal ions even if it were present. These results imply that magnetism or other intrinsic mechanisms in graphene are not the determinant of gadographene relaxivity, although they may play a secondary role.
Similar to the ideas proposed for other Gd(III)-carbon nanomaterials, suggestive evidence was found implicating graphene as a unique modulator of relaxivity. At the same time, the results indicate that the contribution is at most minor, leaving the mechanism for the unusually large relaxivities of gadographene up for further elucidation. Additionally, the basis of the difference in relaxivity among agents utilizing GO, 2% PL graphene, and 2% SC graphene, and the reason for Gd(III)-DO3A GO to have a comparable relaxivity to Gd(III) GO despite a lower expected q (Figure 1) pose further questions. To gain a more detailed mechanistic understanding of gadographene relaxivity, NMRDs were acquired and analyzed by the modified Florence program (SI Materials and Methods).26,27 This more advanced model was used for NMRD analysis because SBM theory does not take into account static ZFS that determines the spin energy levels at low fields (< 10 MHz). At high fields, Zeeman interactions dominate, and the two theories converge.
Gd(III) Graphene Oxide
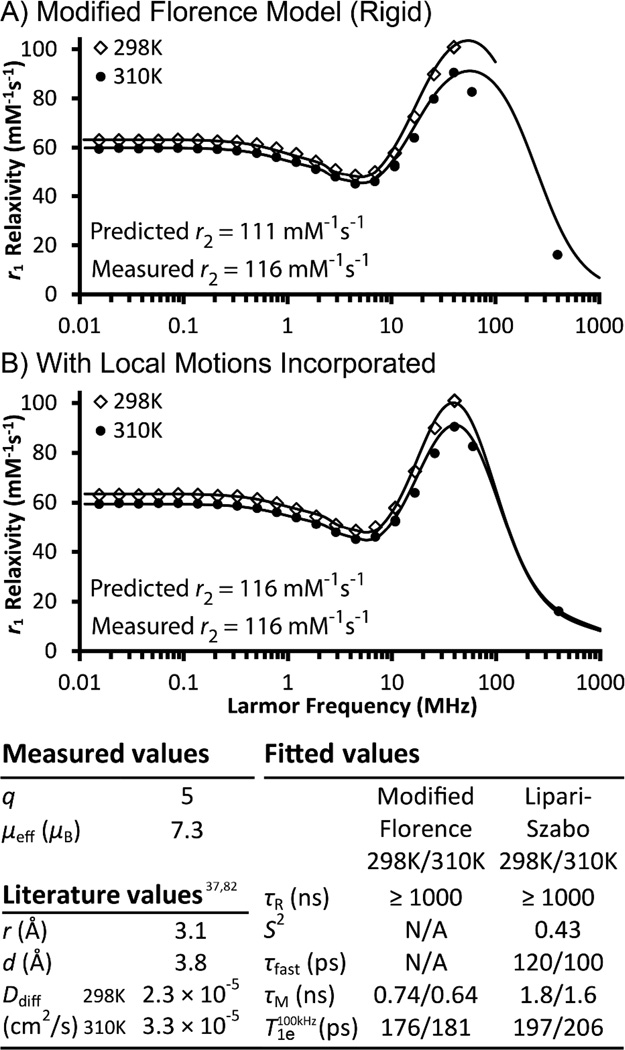
Within the gadographene library, Gd(III) GO is the most well characterized and understood. Its high Gd(III) loading allowed for preparation of samples with sub-millimolar Gd concentration to enable the measurement of q and χ by 17O NMR.78 These mechanistic parameters have never been directly measured for a Gd(III)-carbon nanomaterial due to incompatibility with conventional methods. Typically, the fluorescence lifetime of Eu(III) or Tb(III) analogs of a Gd(III)-complex is used to measure its q.79 However, whether q is conserved across analogs beyond well-defined coordination complexes is unknown. Furthermore, carbon nanomaterials often quench fluorescence80 and are difficult to prepare at sufficiently high concentrations for the measurement. For χ, a Semiconducting Quantum Interference Device (SQUID) is usually employed, but the samples must be in solid state. Here, by observing the 17O chemical shift with and without field locking against a GO control by NMR, the q and χ of Gd(III) GO is directly measured in solution at 296 K and 353 K. The q measured was 5 (Figure 3, Table S2), consistent with the measured dissociation constant of 10−8 – 10−10 M to indicate weakly coordinated Gd(III) ions (Figure S5). The effective magnetic moment (µeff) measured was 7.3 (Figure 3, Table S2), compared to the theoretical value of 7.981 to imply a χ that is consistent with paramagnetic Gd(III) ions. In other words, no unusual magnetic properties were observed with Gd(III) GO.
Figure 3.
Mechanism of Gd(III) Graphene Oxide-induced proton relaxation. Gd(III) was found to experience local mobility based on analysis of the NMRD profiles at 298 and 310K; better fits were obtained when the modified Florence model (A) incorporated the Lipari-Szabo order parameter S2 (B). The observed high relaxivity is derived from a q of 5 and a τR ≥ 1000 ns while fast local motions (τfast and S2) and a τM < 10 ns act as limiting factors. The fitted parameters accurately predicted the transverse relaxivity (r2) measured at 60 MHz, 310K. q = hydration number, µeff = effective magnetic moment, µB = Bohr magneton, r = Gd(III)-proton distance, d = distance of closest approach, Ddiff = diffusion coefficient, τR = rotational correlation time, S2 = order paramter, τfast = fast motion correlation time, τM = mean water residence time, T1e = longitudinal electronic relaxation time.
To further understand the relaxivity of Gd(III) GO, its τM and τR were characterized. For τM, there is no established method available for direct measurement on Gd(III) agents that are slow-tumbling (τR > 1 ns). Nevertheless, τM was determined to be in the fast-exchange regime (≤ 10 ns) by comparing NMRD measurements at two temperatures. Water exchange is defined to be fast or slow in relation to the relaxation time of the water protons dipole-dipole coupled to Gd(III).82 When τM is in the slow-exchange regime, the faster molecular motion and water exchange at higher temperature have opposite effects on relaxivity. The two effects can cancel to result in a weak temperature-dependence of the NMRD profiles, but more often, the outcome is an increase of r1 with increasing temperature due to the dominant effect of water exchange. When τM is in the fast-exchange regime, τR is the main limiting factor, and higher temperature results in lower relaxivities due to faster molecular tumbling. Based on these expectations, the data for Gd(III) GO indicates that its τM is in the fast-exchange regime (Figure 3). To further confirm this result, quantitative fits of the NMRD profiles were obtained. The fitted τM values were 0.74 ns and 0.64 ns at 298 K and 310 K, respectively (Figure 3), approximating the τM of Gd(III) aqua ions (0.94 ns at 310 K)37 and consistent with the expected temperature dependence.
For τR, HYDRONMR83 simulation of a graphene model was used to provide guidance in selecting a range of reasonable values. Result showed the expected τR to be greater than 1000 ns for a perfectly rigid graphene flake of the dimensions used in this study (SI Materials and Methods). In this range, τR does not affect NMRD fitting, and therefore was fixed in our analysis. However, using the modified Florence program, the fitted profiles exhibited high field (> 10 MHz) peaks that were too broad for the collected data points (Figure 3A). This feature persisted even when best-fits based on smaller τR were forced (unpublished results). When the Lipari-Szabo approach84 was incorporated into the modified Florence model85 as an alternative, the fits improved markedly (Figure 3B).
The Lipari-Szabo formalism treats a system as having two correlation times (τfast and τR) related to two completely uncoupled motions without assuming their precise nature. The order parameter S2 models the degree of spatial restriction of the fast motion, with 0 being no restriction and 1 being complete restriction. For Gd(III) GO, the two correlation times do not correspond to anisotropic tumbling. When a graphene stack of 3.6 × 150 × 150 Å3 was modeled by HYDRONMR, all five components of the rotational diffusion tensor were calculated to be greater than 100 ns despite the model being ten times smaller than the experimental samples studied. This prediction is inconsistent with the fitted τfast that fell below 0.5 ns regardless of the τR chosen, indicating that the captured NMRD feature is not related to anisotropy. Therefore, τfast most likely reflects fast local motions related to either the mobility of the Gd(III) ions with respect to the carbon scaffold,86 the swapping of coordinated waters, or the pseudorotation rearrangement of the Gd(III) hydration shell.87 These fast motions cause incomplete reorientation of the proton–Gd(III) vector in timescales faster than the overall tumbling time of the system.
Results of fitting after incorporation of the Lipari-Szabo approach showed τfast to be approximately 100 ps with a S2 of 0.43. This timescale is similar to that of free-tumbling small molecule Gd(III)-complexes. As expected, the adjusted τM (1.6 ns at 310 K) remained close to that of Gd(III) aqua ions. For secondary validation, the best-fit parameters were used to calculate the theoretical r2. The measured and predicted values agreed with each other, confirming that the obtained parameters are indeed reasonable (Figure 3).
In summary, the high relaxivity of Gd(III) GO results from the combination of q = 5 and large τR, and is limited by short water residence time and fast local motion. Based on HYDRONMR simulation, GO may be an attractive scaffold for high relaxivity Gd(III) agents because its sheet geometry results in a τR that is larger than what a spherical scaffold of the same mass would exhibit. No evidence suggested any unusual magnetic behavior. The NMRD of Gd(III) GO can be completely interpreted within the available theory.
Gd(III) Graphene
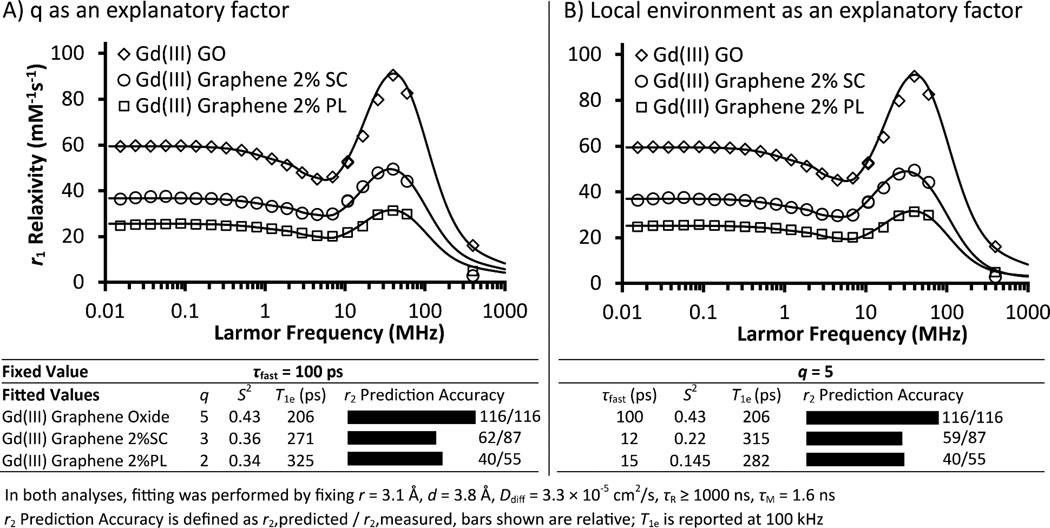
To further elucidate whether the relaxivity of gadographene is influenced by the carbon scaffold beyond its τR properties, materials utilizing different graphene backbones were investigated. Specifically, Gd(III) GO, Gd(III) Graphene 2%SC, and Gd(III) Graphene 2%PL were compared, and materials receiving different doses of sonication were analyzed. Results showed that most of the relaxivity variations can be explained by the modified Florence model, but not all. The small, unexplained portion of the variation should not be overlooked because it points to processes related to pristine graphene that can potentially be engineered to further enhance relaxivity. Unlike Gd(III) GO, Gd(III) Graphenes were not amenable to 17O NMR studies due to their lower Gd(III) loading. Consequently, findings from Gd(III) GO were relied upon as anchoring points for data interpretation. The comparable Gd(III) dissociation constants of the three agents suggest that this approach is reasonable (Figure S5). Similar to Gd(III) GO, NMRD profiles of Gd(III) graphene consistently found better fits with the Lipari-Szabo approach than without, indicating the likely existence of fast local motions (Figure S6). Beyond this conclusion, it was only possible to interpret the data by several equally plausible scenarios because solutions from NMRD fitting are not unique without independent measurements of the fitting parameters q and τM.
The different relaxivities of Gd(III) GO, Gd(III) Graphene 2%SC, and Gd(III) Graphene 2%PL at 60 MHz may be accounted for by one of three possible explanations: 1) q, 2) τfast and S2, or 3) τM. For q, the three agents may differ as a result of the suspending surfactants. Due to the carboxyl group on SC and the polymeric nature of PL (Figure S7), it is conceivable that transient interactions could change the effective q of Gd(III) when the surfactants are used at 2% w/v concentrations. Quantitative analysis showed that the idea is plausible (Figure 4A). When τR, τfast, and τM were fixed to the values obtained for Gd(III) GO, satisfactory NMRD fits were obtained for the GO, SC, and PL samples at a q of 5, 3, and 2, respectively; additionally, a small difference in S2 was noted between the GO and the graphene samples.
Figure 4.
Mechanism of Gd(III) Graphene-induced proton relaxation. Similar to Gd(III) GO, local motions were found to be an important feature of Gd(III) Graphene. Their NMRD profiles were analyzed by the modified Florence model incorporating the Lipari-Szabo approach. The relaxivity of Gd(III) Graphene depended on the choice of surfactant (SC or PL) and was reduced compared to Gd(III) GO. Possible explanations include 1) decreased q by transient surfactant coordination to Gd(III), 2) a difference in Gd(III) microenvironments that resulted in different local motions and S2, 3) reduced water access due to a surfactant-posed diffusion barrier, or some combination of the three factors. The plausibility of the first two explanations were supported by NMRD fitting in (A) and (B), respectively. Theory consistently underestimated the r2 of Gd(III) Graphene, as shown by the r2 prediction accuracy.
Alternatively, the observed relaxivity differences could be attributed to the dissimilar local Gd(III) environments in the GO, SC, and PL samples. Although the details are difficult to pinpoint, the surfactants and the different surface groups on GO and graphene can both contribute to creating unique microenvironments for the Gd(III) ions in each of the three agents. These differences would manifest in the τfast and S2 values in NMRD analysis. Global tumbling was ruled out as a factor in controlling Gd(III) Graphene relaxivity because the graphene flakes studied more than doubled the size of the models used in HYDRONMR simulation. Therefore, τR ≥ 1000 ns was maintained in the NMRD fits. Results showed that τfast and S2 are indeed sufficient to explain the relaxivity differences, with the graphene samples exhibiting smaller order parameters and faster local motions than the GO sample (Figure 4B).
Finally, τM provides a third possible explanation for the different relaxivities of Gd(III) GO and SC- and PL-suspended Gd(III) Graphene. Intuitively, surfactants in Gd(III) Graphene reduce relaxivity by posing a diffusion barrier. To test this idea, two-temperature NMRD experiments were performed to probe τM (Figure S8). Unfortunately, while larger relaxivities were observed at the lower temperature, the difference was small. Therefore, it is ambiguous whether τM shifted into the slow-exchange regime as a result of the suspending surfactants in Gd(III) Graphene.
The observed relaxivity differences among Gd(III) GO and Gd(III) Graphenes can be attributed to the individual or combined effects of q, τfast, S2, and τM. Regardless of the actual mechanism, all of the proposed possibilities are understandable within the current theoretical framework. NMRD analysis of Gd(III) Graphenes at varying sonication dose suggests that graphene properties additionally contribute to the relaxivity differences observed (Figure S9). First, the unpaired Gd(III) electron T1e at 100 kHz negatively correlates with sonication and is longer in Gd(III) Graphene (~ 300 ps) than in Gd(III) GO (~ 200 ps), suggesting that graphene damage and/or surfactants may be modulating the electronic relaxation process (Figure S9). Second, NMRD further confirmed the previous analysis that the opposite 60 MHz relaxivity trends between the SC- and PL-suspended Gd(III) Graphenes cannot be interpreted by flake size (Figure 2A, Figure S9). Third, unlike for Gd(III) GO, theoretical predictions consistently underestimated the measured r2 for Gd(III) Graphenes (Figure 4, Figure S9). Theory miscalculated by 32% for the most pristine graphene and predicted with increasing accuracy for the more damaged samples, consistent with the noted trends in r2/r1 ratio. Unusually large scalar interactions or a chemical shift difference between water molecules confined within graphene stacks and the bulk can be speculated to provide an interpretation of the data and are discussed further in Supplementary Note S1. Trivial explanations related to the residual Fe ions in the samples were ruled out by correcting for their contributions to r2 (SI Materials and Methods). The data suggests that there are hidden parameters (including those related to intrinsic mechanisms of graphene) contributing to the relaxivity of Gd(III) Graphene but not that of Gd(III) GO.
Gd(III)-Complex Graphene Oxide
Gd(III)-DTPA-NH2 (q=1) and Gd(III)-DO3A-NH2 (q=2) conjugated to GO and partially reduced GO (rGO)72,73 are valuable agents in the mechanistic investigation of gadographene relaxivity for two reasons: 1) their q are systematically controlled and 2) their Gd(III) ions couple into the carbon backbone indirectly via a coordinating complex, in contrast to the ionic gadographenes. Beyond their fundamental interest, these agents may be of practical value because the use of complexes makes them potentially compatible with in vivo applications.
By comparing gadographenes based on the two different complexes, inferences can be made about the relative contributions of inner- and outer-sphere effects to relaxivity. The relaxivity of the q=2 complex (Gd(III)-DO3A-NH2) was found to be almost exactly double that of the q=1 complex (Gd(III)-DTPA-NH2) for both GO and rGO (Figure 1). This result implies that gadographene relaxivity derives mostly from inner-sphere effects with only minor contributions from outer-sphere effects, in agreement with the analyses performed for Gd(III) GO and Gd(III) Graphene.
By comparing the complexed version and the ionic version of the same agent, we examined if the mode of association between Gd(III) ions and the carbon backbone influences relaxivity. Magnetic interactions mediated by the carbon itinerant electrons66,67 could potentially amplify relaxivity, and the mode of association may modulate these interactions. Intriguingly, results showed the relaxivities of Gd(III)-complex GO to be much closer to Gd(III) GO than would be expected given their lower q (Figure 1). In the absence of significant outer-sphere effects, this result indicates that the Gd(III) ions are more efficient at relaxing water protons on a per-coordination-site basis when they are complexed compared to when they are associated directly with the carbon backbone in gadographene.
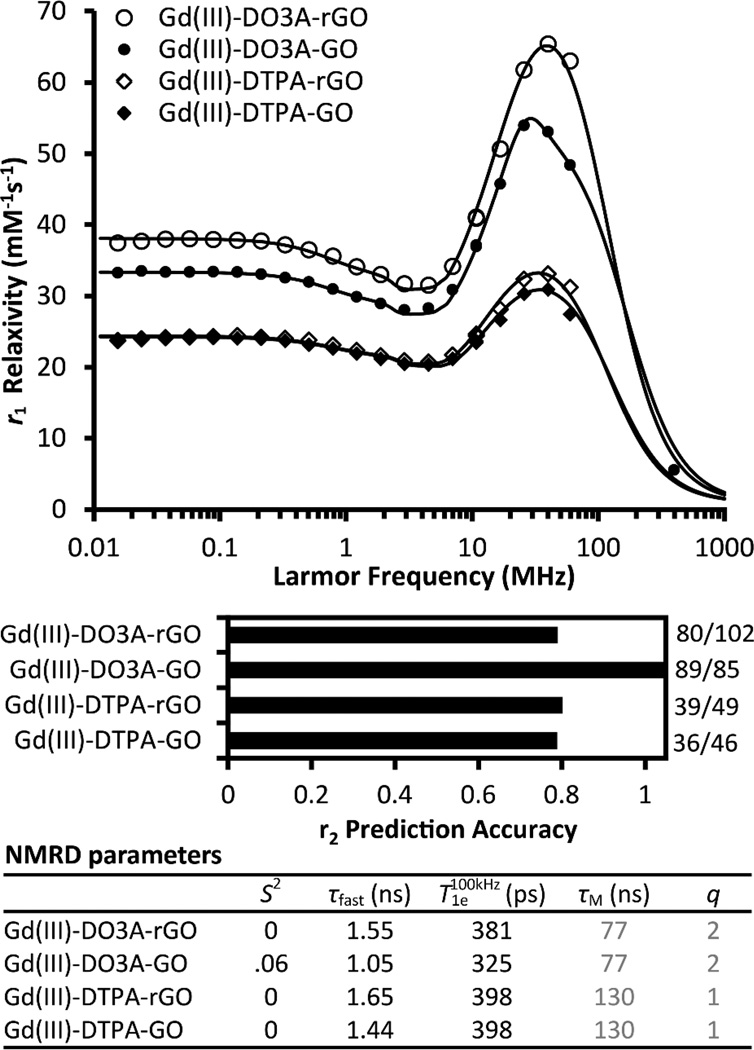
To better understand the origin of this observation, NMRD profiles of Gd(III)-complex GO were acquired and analyzed (Figure 5). Assuming τR > 1000 ns as before and that the τM of the complexes37 remain unchanged post-conjugation, the high relaxation efficiency of Gd(III)-complex GO can be satisfactorily explained by their optimized correlation times (for local motion, τcl−1 = τR−1 + τM−1 + T1e -1 + τfast−1)84,88 without having to evoke unusual magnetic properties. The use of complexes prolonged τM by approximately two orders of magnitude and τfast by one order of magnitude compared to Gd(III) ions. As a result, τcl increased towards the optimal condition of τcl = 1/νH with a concomitant increase in relaxivity (νH is the proton Larmor frequency). Interestingly, although the τfast of the agent is about ten times slower than the τR of a free complex, the near-zero S2 of the fits suggest that the complexes experience isotropic tumbling independent of the motions of the GO scaffold. Therefore, in addition to slowing down water exchange and eliminating the type of local motions associated with Gd(III) ions, Gd(III)-complexes evidently undergo slow reorientation on the GO surface and consequently experience an enhancement in relaxivity.
Figure 5.
Mechanism of Gd(III)-Complex Graphene Oxide-induced proton relaxation. Gd(III)-DO3A-NH2 (q=2) or Gd(III)-DTPA-NH2 (q=1) was conjugated to either GO or reduced GO (rGO). Analysis of the NMRD profiles shows that these agents have higher relaxivities per q compared to Gd(III) GO as a result of their more optimized τM and slower local motion. The near-zero values of S2 suggest isotropic tumbling of Gd(III)-complexes on the GO surface. Consistent with the findings in Gd(III) Graphene, r2 prediction (r2,predicted / r2,measured ) using the fitted parameters is more accurate for Gd(III)-DO3A-GO than for its rGO counterpart. τM and q of the complexes were obtained from literature37 and assumed to remain unchanged post-conjugation.
To further examine the effect that the carbon backbone may have on relaxivity beyond contributing slow global motion, the GO and the rGO version of the agent were compared. Consistent with the findings in Gd(III) Graphene, the best-fit parameters obtained from NMRD analysis did not accurately predict the measured r2 (60 MHz) of all agents (Figure 5). Notably, theory underestimated the r2 of Gd(III)-DO3A-NH2 when rGO (but not when GO) was used as the carbon scaffold. rGO is known to more closely mimic graphene with an increased conductivity when compared to GO.72,73 These results suggest that pristine graphene contributes weakly to relaxivity, consistent with the observation in comparing Gd(III) Graphene with Gd(III) GO.
In summary, gadographenes utilizing Gd(III)-complexes are more efficient at relaxing the proton spins of each coordinated water compared to analogs utilizing Gd(III) ions. Based on NMRD analysis, the likely reason for this result is that the complexes used have relatively optimal τM and eliminate fast local motions that Gd(III) ions experience; these effects are completely inner-sphere. Although no unusual magnetic properties were required to explain most of the measured relaxivities, the r2 of these materials could not be consistently predicted and implicate graphene-related processes as a weak contributor to relaxivity. Gd(III)-complex GO achieved high relaxivity despite near-zero dependence on the global tumbling of GO. Thus, an opportunity may exist for this class of agents to achieve the maximum theoretical relaxivity if local mobility can be removed.
Conclusions
Gadographenes are a suitable model system for elucidating the mechanistic origin of high relaxivity exhibited by Gd(III)-carbon nanomaterials. In particular, the question as to whether graphene magnetism contributes to gadographene relaxivity was examined. It was found that the agents can be described using the modified Florence model of relaxation without having to evoke significant unusual magnetic behaviors or second- or outer-sphere effects. Specifically, the high relaxivities of the ionic gadographenes were attributed to q > 1 and slow tumbling, while those of the complexed samples were further enhanced per coordinated water due to optimal water exchange and slow local motion. Additionally, several relaxivity trends suggest pristine graphene as a potential weak contributor to gadographene relaxivity and present future opportunities for further investigation and engineering.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R01EB014806, the National Cancer Institute Center for Cancer Nanotechnology Excellence initiative at Northwestern University Award No. U54CA151880, the Ente Cassa di Risparmio di Firenze, and the European Commission contract Bio-NMR no. 261863, and a Cooperative Agreement between the National Science Foundation and the Environmental Protection Agency (DBI 0830117). The authors thank the Northwestern University Integrated Molecular Structure Education and Research Center (IMSERC); a description of the facility and full funding disclosure can be found at http://aslloginsrv.chem.northwestern.edu/analyticalserviceslab/asl.htm.
Footnotes
Supporting Information
Preparation of all gadographene materials, detailed descriptions of the characterization methods, a comprehensive list of all samples studied, validation of 17O NMR and [Gd] measurements, full tables of fitted NMRD parameters, theoretical simulations of relaxivities, AFM size histograms, data from dissociation constant experiments, and additional NMRD analyses and discussions are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
References
- 1.Manus LM, Mastarone DJ, Waters EA, Zhang XQ, Schultz-Sikma EA, MacRenaris KW, Ho D, Meade TJ. Gd(III)-Nanodiamond Conjugates for MRI Contrast Enhancement. Nano Lett. 2010;10:484–489. doi: 10.1021/nl903264h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sitharaman B, Kissell KR, Hartman KB, Tran LA, Baikalov A, Rusakova I, Sun Y, Khant HA, Ludtke SJ, Chiu W, Laus S, Toth E, Helm L, Merbach AE, Wilson LJ. Superparamagnetic Gadonanotubes Are High-Performance MRI Contrast Agents. Chem. Commun. 2005:3915–3917. doi: 10.1039/b504435a. [DOI] [PubMed] [Google Scholar]
- 3.Sitharaman B, Wilson LJ. Gadofullerenes and Gadonanotubes: A New Paradigm for High-Performance Magnetic Resonance Imaging Contrast Agent Probes. J. Biomed. Nanotechnol. 2007;3:342–352. [Google Scholar]
- 4.Ananta JS, Matson ML, Tang AM, Mandal T, Lin S, Wong K, Wong ST, Wilson LJ. Single-Walled Carbon Nanotube Materials as T2-Weighted MRI Contrast Agents. J. Phys. Chem. C. 2009;113:19369–19372. [Google Scholar]
- 5.Chan BTY, Hassan AA, Hartman KB, Ananta JS, Mackeyev Y, Hu L, Pautler RG, Wilson LJ, Lee AV. Utilization of Serine-Derivatized Gadonanotubes as Magnetic Nanoprobes for Intracellular Labeling of MCF-7 Human Breast Cancer Cell. Cancer Res. 2009;69:766s–766s. doi: 10.1002/cmmi.293. [DOI] [PubMed] [Google Scholar]
- 6.Hartman KB, Laus S, Bolskar RD, Muthupillai R, Helm L, Toth E, Merbach AE, Wilson LJ. Gadonanotubes as Ultrasensitive pH-Smart Probes for Magnetic Resonance Imaging. Nano Lett. 2008;8:415–419. doi: 10.1021/nl0720408. [DOI] [PubMed] [Google Scholar]
- 7.Kato H, Kanazawa Y, Okumura M, Taninaka A, Yokawa T, Shinohara H. Lanthanoid Endohedral Metallofullerenols for MRI Contrast Agents. J. Am. Chem. Soc. 2003;125:4391–4397. doi: 10.1021/ja027555+. [DOI] [PubMed] [Google Scholar]
- 8.Richard C, Doan BT, Beloeil JC, Bessodes M, Toth E, Scherman D. Noncovalent Functionalization of Carbon Nanotubes with Amphiphilic Gd3+ Chelates: Toward Powerful T1 and T2 MRI Contrast Agents. Nano Lett. 2008;8:232–236. doi: 10.1021/nl072509z. [DOI] [PubMed] [Google Scholar]
- 9.Sethi R, Mackeyev Y, Wilson LJ. The Gadonanotubes Revisited: A New Frontier in MRI Contrast Agent Design. Inorg. Chim. Acta. 2012;393:165–172. [Google Scholar]
- 10.Shu CY, Corwin FD, Zhang JF, Chen ZJ, Reid JE, Sun MH, Xu W, Sim JH, Wang CR, Fatouros PP, Esker AR, Gibson HW, Dorn HC. Facile Preparation of a New Gadofullerene-Based Magnetic Resonance Imaging Contrast Agent with High 1H Relaxivity. Bioconjugate Chem. 2009;20:1186–1193. doi: 10.1021/bc900051d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitharaman B, Van Der Zande M, Ananta JS, Shi XF, Veltien A, Walboomers XF, Wilson LJ, Mikos AG, Heerschap A, Jansen JA. Magnetic Resonance Imaging Studies on Gadonanotube-Reinforced Biodegradable Polymer Nanocomposites. J. Biomed. Mater. Res. A. 2010;93A:1454–1462. doi: 10.1002/jbm.a.32650. [DOI] [PubMed] [Google Scholar]
- 12.Toth E, Bolskar RD, Borel A, Gonzalez G, Helm L, Merbach AE, Sitharaman B, Wilson LJ. Water-Soluble Gadofullerenes: Toward High-Relaxivity, pH-Responsive MRI Contrast Agents. J. Am. Chem. Soc. 2005;127:799–805. doi: 10.1021/ja044688h. [DOI] [PubMed] [Google Scholar]
- 13.Zhang JF, Fatouros PP, Shu CY, Reid J, Owens LS, Cai T, Gibson HW, Long GL, Corwin FD, Chen ZJ, Dorn HC. High Relaxivity Trimetallic Nitride (Gd3n) Metallofullerene MRI Contrast Agents with Optimized Functionality. Bioconjugate Chem. 2010;21:610–615. doi: 10.1021/bc900375n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolskar RD. Gadofullerene MRI Contrast Agents. Nanomedicine. 2008;3:201–213. doi: 10.2217/17435889.3.2.201. [DOI] [PubMed] [Google Scholar]
- 15.Mikawa M, Kato H, Okumura M, Narazaki M, Kanazawa Y, Miwa N, Shinohara H. Paramagnetic Water-Soluble Metallofullerenes Having the Highest Relaxivity for MRI Contrast Agents. Bioconjugate Chem. 2001;12:510–514. doi: 10.1021/bc000136m. [DOI] [PubMed] [Google Scholar]
- 16.Bolskar RD, Benedetto AF, Husebo LO, Price RE, Jackson EF, Wallace S, Wilson LJ, Alford JM. First Soluble M@C60 Derivatives Provide Enhanced Access to Metallofullerenes and Permit in Vivo Evaluation of Gd@C60[C(COOH)(2)](10) as a MRI Contrast Agent. J. Am. Chem. Soc. 2003;125:5471–5478. doi: 10.1021/ja0340984. [DOI] [PubMed] [Google Scholar]
- 17.Botta M, Tei L. Relaxivity Enhancement in Macromolecular and Nanosized Gd(III)-Based MRI Contrast Agents. Eur. J. Inorg. Chem. 2012:1945–1960. [Google Scholar]
- 18.Liu YJ, Zhang N. Gadolinium Loaded Nanoparticles in Theranostic Magnetic Resonance Imaging. Biomaterials. 2012;33:5363–5375. doi: 10.1016/j.biomaterials.2012.03.084. [DOI] [PubMed] [Google Scholar]
- 19.Allen MJ, Meade TJ. Synthesis and Visualization of a Membrane-Permeable MRI Contrast Agent. J. Biol. Inorg. Chem. 2003;8:746–750. doi: 10.1007/s00775-003-0475-2. [DOI] [PubMed] [Google Scholar]
- 20.Manus LM, Strauch RC, Hung AH, Eckermann AL, Meade TJ. Analytical Methods for Characterizing Magnetic Resonance Probes. Anal. Chem. 2012;84:6278–6287. doi: 10.1021/ac300527z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell DH, NiDhubhghaill OM, Pubanz D, Helm L, Lebedev YS, Schlaepfer W, Merbach AE. Structural and Dynamic Parameters Obtained from 17O NMR, EPR, and NMRD Studies of Monomeric and Dimeric Gd3+ Complexes of Interest in Magnetic Resonance Imaging: An Integrated and Theoretically Self Consistent Approach. J. Am. Chem. Soc. 1996;118:9333–9346. [Google Scholar]
- 22.Mastarone DJ, Harrison VSR, Eckermann AL, Parigi G, Luchinat C, Meade TJ. A Modular System for the Synthesis of Multiplexed Magnetic Resonance Probes. J. Am. Chem. Soc. 2011;133:5329–5337. doi: 10.1021/ja1099616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin J, Laurent S, Jo YS, Roch A, Mikhaylova M, Bhujwalla ZM, Muller RN, Muhammed M. A High-Performance Magnetic Resonance Imaging T2 Contrast Agent. Adv. Mater. 2007;19:1874–1878. [Google Scholar]
- 24.Matosziuk LM, Harney AS, MacRenaris KW, Meade TJ. Synthesis, Characterization, and in Vitro Testing of a Bacteria-Targeted MR Contrast Agent. Eur. J. Inorg. Chem. 2012:2099–2107. doi: 10.1002/ejic.201101362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherry AD, Caravan P, Lenkinski RE. Primer on Gadolinium Chemistry. J. Magn. Reson. Imaging. 2009;30:1240–1248. doi: 10.1002/jmri.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang SR, Wu KC, Sherry AD. A Novel pH-Sensitive MRI Contrast Agent. Angew. Chem. Int. Ed. 1999;38:3192–3194. [PubMed] [Google Scholar]
- 27.Li WH, Fraser SE, Meade TJ. A Calcium-Sensitive Magnetic Resonance Imaging Contrast Agent. J. Am. Chem. Soc. 1999;121:1413–1414. [Google Scholar]
- 28.De Leon-Rodriguez LM, Lubag AJM, Malloy CR, Martinez GV, Gillies RJ, Sherry AD. Responsive MRI Agents for Sensing Metabolism in Vivo. Acc. Chem. Res. 2009;42:948–957. doi: 10.1021/ar800237f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Major JL, Parigi G, Luchinat C, Meade TJ. The Synthesis and in Vitro Testing of a Zinc-Activated MRI Contrast Agent. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13881–13886. doi: 10.1073/pnas.0706247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Y, Kohlmeir EK, Meade TJ. Synthesis of Multimeric MR Contrast Agents for Cellular Imaging. J. Am. Chem. Soc. 2008;130:6662–6663. doi: 10.1021/ja0777990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In Vivo Visualization of Gene Expression Using Magnetic Resonance Imaging. Nat. Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 32.Roch A, Muller RN, Gillis P. Theory of Proton Relaxation Induced by Superparamagnetic Particles. J. Chem. Phys. 1999;110:5403–5411. [Google Scholar]
- 33.Sukerkar PA, MacRenaris KW, Meade TJ, Burdettes JE. A Steroid- Conjugated Magnetic Resonance Probe Enhances Contrast in Progesterone Receptor Expressing Organs and Tumors in Vivo. Mol. Pharm. 2011;8:1390–1400. doi: 10.1021/mp200219e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esqueda AC, Lopez JA, Andreu-De-Riquer G, Alvarado-Monzon JC, Ratnakar J, Lubag AJM, Sherry AD, De Leon-Rodriguez LM. A New Gadolinium- Based MRI Zinc Sensor. J. Am. Chem. Soc. 2009;131:11387–11391. doi: 10.1021/ja901875v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strauch RC, Mastarone DJ, Sukerkar PA, Song Y, Ipsaro JJ, Meade TJ. Reporter Protein-Targeted Probes for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2011;133:16346–16349. doi: 10.1021/ja206134b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu FQ, MacRenaris KW, Waters EA, Liang TY, Schultz-Sikma EA, Eckermann AL, Meade TJ. Ultrasmall, Water-Soluble Magnetite Nanoparticles with High Relaxivity for Magnetic Resonance Imaging. J. Phys. Chem. C. 2009;113:20855–20860. doi: 10.1021/jp907216g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 38.Lauffer RB. Paramagnetic Metal-Complexes as Water Proton Relaxation Agents for Nmr Imaging - Theory and Design. Chem. Rev. 1987;87:901–927. [Google Scholar]
- 39.Helm L. Relaxivity in Paramagnetic Systems: Theory and Mechanisms. Prog. Nucl. Magn. Reson. Spectrosc. 2006;49:45–64. [Google Scholar]
- 40.Benetis N, Kowalewski J, Nordenskiold L, Wennerstrom H, Westlund PO. Nuclear-Spin Relaxation in Paramagnetic Systems - the Slow Motion Problem for Electron-Spin Relaxation. Mol. Phys. 1983;48:329–346. [Google Scholar]
- 41.Larsson T, Westlund PO, Kowalewski J, Koenig SH. Nuclear-Spin Relaxation in Paramagnetic-Complexes in the Slow-Motion Regime for the Electron-Spin - the Anisotropic Pseudorotation Model for S=1 and the Interpretation of Nuclear Magnetic- Relaxation Dispersion Results for a Low-Symmetry Ni(Ii) Complex. J. Chem. Phys. 1994;101:1116–1128. [Google Scholar]
- 42.Nilsson T, Kowalewski J. Slow-Motion Theory of Nuclear Spin Relaxation in Paramagnetic Low-Symmetry Complexes: A Generalization to High Electron Spin. J. Magn. Reson. 2000;146:345–358. doi: 10.1006/jmre.2000.2125. [DOI] [PubMed] [Google Scholar]
- 43.Rast S, Fries PH, Belorizky E, Borel A, Helm L, Merbach AE. A General Approach to the Electronic Spin Relaxation of Gd(III) Complexes in Solutions. Monte Carlo Simulations Beyond the Redfield Limit. J. Chem. Phys. 2001;115:7554–7563. [Google Scholar]
- 44.Bonnet CS, Fries PH, Gadelle A, Gambarelli S, Delangle P. A Rigorous Framework to Interpret Water Relaxivity. The Case Study of a Gd(III) Complex with an Alpha-Cyclodextrin Derivative. J. Am. Chem. Soc. 2008;130:10401–10413. doi: 10.1021/ja802347r. [DOI] [PubMed] [Google Scholar]
- 45.Bertini I, Galas O, Luchinat C, Parigi G. Computer-Program for the Calculation of Paramagnetic Enhancements Nuclear-Relaxation Rates in Slowly Rotating Systems. J. Magn. Reson. A. 1995;113:151–158. [Google Scholar]
- 46.Bertini I, Kowalewski J, Luchinat C, Nilsson T, Parigi G. Nuclear Spin Relaxation in Paramagnetic Complexes of S=1: Electron Spin Relaxation Effects. J. Chem. Phys. 1999;111:5795–5807. [Google Scholar]
- 47.Kruk D, Nilsson T, Kowalewski J. Nuclear Spin Relaxation in Paramagnetic Systems with Zero-Field Splitting and Arbitrary Electron Spin. Phys. Chem. Chem. Phys. 2001;3:4907–4917. [Google Scholar]
- 48.Song Y, Xu XY, MacRenaris KW, Zhang XQ, Mirkin CA, Meade TJ. Multimodal Gadolinium-Enriched DNA-Gold Nanoparticle Conjugates for Cellular Imaging. Angew. Chem. Int. Ed. 2009;48:9143–9147. doi: 10.1002/anie.200904666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SM, Song Y, Hong BJ, MacRenaris KW, Mastarone DJ, O'Halloran TV, Meade TJ, Nguyen ST. Modular Polymer-Caged Nanobins as a Theranostic Platform with Enhanced Magnetic Resonance Relaxivity and pH-Responsive Drug Release. Angew. Chem. Int. Ed. 2010;49:9960–9964. doi: 10.1002/anie.201004867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karfeld-Sulzer LS, Waters EA, Davis NE, Meade TJ, Barron AE. Multivalent Protein Polymer MRI Contrast Agents: Controlling Relaxivity Via Modulation of Amino Acid Sequence. Biomacromolecules. 2010;11:1429–1436. doi: 10.1021/bm901378a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Endres PJ, Paunesku T, Vogt S, Meade TJ, Woloschak GE. DNA-TiO2 Nanoconjugates Labeled with Magnetic Resonance Contrast Agents. J. Am. Chem. Soc. 2007;129:15760–15761. doi: 10.1021/ja0772389. [DOI] [PubMed] [Google Scholar]
- 52.Bull SR, Guler MO, Bras RE, Meade TJ, Stupp SI. Self-Assembled Peptide Amphiphile Nanofibers Conjugated to MRI Contrast Agents. Nano Lett. 2005;5:1–4. doi: 10.1021/nl0484898. [DOI] [PubMed] [Google Scholar]
- 53.Platas-Iglesias C, Vander Elst L, Zhou WZ, Muller RN, Geraldes CFGC, Maschmeyer T, Peters JA. Zeolite GdNaY Nanoparticles with Very High Relaxivity for Application as Contrast Agents in Magnetic Resonance Imaging. Chem. Eur. J. 2002;8:5121–5131. doi: 10.1002/1521-3765(20021115)8:22<5121::AID-CHEM5121>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 54.Ali MM, Woods M, Caravan P, Opina ACL, Spiller M, Fettinger JC, Sherry AD. Synthesis and Relaxometric Studies of a Dendrimer-Based pH-Responsive MRI Contrast Agent. Chem. Eur. J. 2008;14:7250–7258. doi: 10.1002/chem.200800402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gianolio E, Giovenzana GB, Longo D, Longo I, Menegotto I, Aime S. Relaxometric and Modelling Studies of the Binding of a Lipophilic Gd-AAZTA Complex to Fatted and Defatted Human Serum Albumin. Chem. Eur. J. 2007;13:5785–5797. doi: 10.1002/chem.200601277. [DOI] [PubMed] [Google Scholar]
- 56.Aime S, Frullano L, Crich SG. Compartmentalization of a Gadolinium Complex in the Apoferritin Cavity: A Route to Obtain High Relaxivity Contrast Agents for Magnetic Resonance Imaging. Angew. Chem. Int. Ed. 2002;41:1017–1019. doi: 10.1002/1521-3773(20020315)41:6<1017::aid-anie1017>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 57.Moriggi L, Cannizzo C, Dumas E, Mayer CR, Ulianov A, Helm L. Gold Nanoparticles Functionalized with Gadolinium Chelates as High-Relaxivity MRI Contrast Agents. J. Am. Chem. Soc. 2009;131:10828–10829. doi: 10.1021/ja904094t. [DOI] [PubMed] [Google Scholar]
- 58.Allen M, Bulte JWM, Liepold L, Basu G, Zywicke HA, Frank JA, Young M, Douglas T. Paramagnetic Viral Nanoparticles as Potential High-Relaxivity Magnetic Resonance Contrast Agents. Magn. Reson. Med. 2005;54:807–812. doi: 10.1002/mrm.20614. [DOI] [PubMed] [Google Scholar]
- 59.Caravan P, Farrar CT, Frullano L, Uppal R. Influence of Molecular Parameters and Increasing Magnetic Field Strength on Relaxivity of Gadolinium- and Manganese-Based T1 Contrast Agents. Contrast Media Mol. Imaging. 2009;4:89–100. doi: 10.1002/cmmi.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laus S, Sitharaman B, Toth E, Bolskar RD, Helm L, Wilson LJ, Merbach AE. Understanding Paramagnetic Relaxation Phenomena for Water-Soluble Gadofullerenes. J. Phys. Chem. C. 2007;111:5633–5639. [Google Scholar]
- 61.Laus S, Sitharaman B, Toth V, Bolskar RD, Helm L, Asokan S, Wong MS, Wilson LJ, Merbach AE. Destroying Gadofullerene Aggregates by Salt Addition in Aqueous Solution of Gd@C60(OH)(X) and Gd@C60[C(COOH2)](10) J. Am. Chem. Soc. 2005;127:9368–9369. doi: 10.1021/ja052388+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu J, Mei WN, Gao Y, Zeng XC, Jing MW, Li GP, Sabirianov R, Gao ZX, You LP, Xu J, Yu DP, Ye HQ. Structural and Electronic Properties of Gd@C60: All-Electron Relativistic Total-Energy Study. Chem. Phys. Lett. 2006;425:82–84. [Google Scholar]
- 63.Paratala BS, Jacobson BD, Kanakia S, Francis LD, Sitharaman B. Physicochemical Characterization, and Relaxometry Studies of Micro-Graphite Oxide, Graphene Nanoplatelets, and Nanoribbons. PLoS One. 2012:7. doi: 10.1371/journal.pone.0038185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dreyer DR, Park S, Bielawski CW, Ruoff RS. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010;39:228–240. doi: 10.1039/b917103g. [DOI] [PubMed] [Google Scholar]
- 65.Polyakova EY, Rim KT, Eom D, Douglass K, Opila RL, Heinz TF, Teplyakov AV, Flynn GW. Scanning Tunneling Microscopy and X-Ray Photoelectron Spectroscopy Studies of Graphene Films Prepared by Sonication-Assisted Dispersion. ACS Nano. 2011;5:6102–6108. doi: 10.1021/nn1009352. [DOI] [PubMed] [Google Scholar]
- 66.Moriya T, Takahashi Y. Itinerant Electron Magnetism. Annu. Rev. Mater. Sci. 1984;14:1–25. [Google Scholar]
- 67.Yazyev OV, Helm L. Defect-Induced Magnetism in Graphene. Phys. Rev. B. 2007;75:125408. [Google Scholar]
- 68.Hernandez Y, Nicolosi V, Lotya M, Blighe FM, Sun ZY, De S, McGovern IT, Holland B, Byrne M, Gun'ko YK, Boland JJ, Niraj P, Duesberg G, Krishnamurthy S, Goodhue R, Hutchison J, Scardaci V, Ferrari AC, Coleman JN. High-Yield Production of Graphene by Liquid-Phase Exfoliation of Graphite. Nat. Nanotechnol. 2008;3:563–568. doi: 10.1038/nnano.2008.215. [DOI] [PubMed] [Google Scholar]
- 69.Duch MC, Budinger GRS, Liang YT, Soberanes S, Urich D, Chiarella SE, Campochiaro LA, Gonzalez A, Chandel NS, Hersam MC, Mutlu GM. Minimizing Oxidation and Stable Nanoscale Dispersion Improves the Biocompatibility of Graphene in the Lung. Nano Lett. 2011;11:5201–5207. doi: 10.1021/nl202515a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kovtyukhova NI, Ollivier PJ, Martin BR, Mallouk TE, Chizhik SA, Buzaneva EV, Gorchinskiy AD. Layer-by-Layer Assembly of Ultrathin Composite Films from Micron-Sized Graphite Oxide Sheets and Polycations. Chem. Mater. 1999;11:771–778. [Google Scholar]
- 71.Liu Z, Robinson JT, Sun XM, Dai HJ. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008;130:10876–10877. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rourke JP, Pandey PA, Moore JJ, Bates M, Kinloch IA, Young RJ, Wilson NR. The Real Graphene Oxide Revealed: Stripping the Oxidative Debris from the Graphene-Like Sheets. Angew. Chem. Int. Ed. 2011;50:3173–3177. doi: 10.1002/anie.201007520. [DOI] [PubMed] [Google Scholar]
- 73.Fernandez-Merino MJ, Guardia L, Paredes JI, Villar-Rodil S, Solis-Fernandez P, Martinez-Alonso A, Tascon JMD. Vitamin C Is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C. 2010;114:6426–6432. [Google Scholar]
- 74.Wang B, Luo B, Liang M, Wang A, Wang J, Fang Y, Chang Y, Zhi L. Chemical Amination of Graphene Oxides and Their Extraordinary Properties in the Detection of Lead Ions. Nanoscale. 2011;3:5059–5066. doi: 10.1039/c1nr10901d. [DOI] [PubMed] [Google Scholar]
- 75.Wang S, Chia PJ, Chua LL, Zhao LH, Png RQ, Sivaramakrishnan S, Zhou M, Goh RGS, Friend RH, Wee ATS, Ho PKH. Band-Like Transport in Surface-Functionalized Highly Solution-Processable Graphene Nanosheets. Adv. Mater. 2008;20:3440–3446. [Google Scholar]
- 76.Bourlinos AB, Gournis D, Petridis D, Szabo T, Szeri A, Dekany I. Graphite Oxide: Chemical Reduction to Graphite and Surface Modification with Primary Aliphatic Amines and Amino Acids. Langmuir. 2003;19:6050–6055. [Google Scholar]
- 77.Compton OC, Dikin DA, Putz KW, Brinson LC, Nguyen ST. Electrically Conductive"Alkylated" Graphene Paper Via Chemical Reduction of Amine-Functionalized Graphene Oxide Paper. Adv. Mater. 2010;22:892–896. doi: 10.1002/adma.200902069. [DOI] [PubMed] [Google Scholar]
- 78.Djanashvili K, Peters JA. How to Determine the Number of Inner-Sphere Water Molecules in Lanthanide(III) Complexes by 17O NMR Spectroscopy. A Technical Note. Contrast Media Mol. Imaging. 2007;2:67–71. doi: 10.1002/cmmi.132. [DOI] [PubMed] [Google Scholar]
- 79.Supkowski RM, Horrocks WD. On the Determination of the Number of Water Molecules, q, Coordinated to Europium(III) Ions in Solution from Luminescence Decay Lifetimes. Inorg. Chim. Acta. 2002;340:44–48. [Google Scholar]
- 80.Kim J, Cote LJ, Kim F, Huang JX. Visualizing Graphene Based Sheets by Fluorescence Quenching Microscopy. J. Am. Chem. Soc. 2010;132:260–267. doi: 10.1021/ja906730d. [DOI] [PubMed] [Google Scholar]
- 81.Ashcroft NW, D MN. Solid State Physics. Belmont, CA: Brooks Cole; 1976. p. 675. [Google Scholar]
- 82.Aime S, Botta M, Fasano M, Paoletti S, Terreno E. Relaxometric Determination of the Exchange Rate of the Coordinated Water Protons in a Neutral Gd-III Chelate. Chem. Eur. J. 1997;3:1499–1504. [Google Scholar]
- 83.de la Torre JG, Huertas ML, Carrasco B. Hydronmr: Prediction of NMR Relaxation of Globular Proteins from Atomic-Level Structures and Hydrodynamic Calculations. J. Magn. Reson. 2000;147:138–146. doi: 10.1006/jmre.2000.2170. [DOI] [PubMed] [Google Scholar]
- 84.Lipari G, Szabo A. Model-Free Approach to the Interpretation of Nuclear Magnetic-Resonance Relaxation in Macromolecules .1. Theory and Range of Validity. J. Am. Chem. Soc. 1982;104:4546–4559. [Google Scholar]
- 85.Caravan P, Parigi G, Chasse JM, Cloutier NJ, Ellison JJ, Lauffer RB, Luchinat C, McDermid SA, Spiller M, McMurry TJ. Albumin Binding, Relaxivity, and Water Exchange Kinetics of the Diastereoisomers of MS-325, a Gadolinium(III)-Based Magnetic Resonance Angiography Contrast Agent. Inorg. Chem. 2007;46:6632–6639. doi: 10.1021/ic700686k. [DOI] [PubMed] [Google Scholar]
- 86.Torres S, Martins JA, Andre JP, Geraldes CFGC, Merbach AE, Toth E. Supramolecular Assembly of an Amphiphilic Gd-III Chelate: Tuning the Reorientational Correlation Time and the Water Exchange Rate. Chem. Eur. J. 2006;12:940–948. doi: 10.1002/chem.200500551. [DOI] [PubMed] [Google Scholar]
- 87.Kowall T, Foglia F, Helm L, Merbach AE. Molecular-Dynamics Simulation Study of Lanthanide Ions Ln(3+) in Aqueous-Solution - Analysis of the Structure of the First Hydration Shell and of the Origin of Symmetry Fluctuations. J. Phys. Chem. 1995;99:13078–13087. [Google Scholar]
- 88.Caravan P. Strategies for Increasing the Sensitivity of Gadolinium Based MRI Contrast Agents. Chem. Soc. Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.