Abstract
Transfer RNA’s role in decoding the genome is critical to the accuracy and efficiency of protein synthesis. Though modified nucleosides were identified in RNA 50 years ago, only recently has their importance to tRNA’s ability to decode cognate and wobble codons become apparent. RNA modifications are ubiquitous. To date, some 100 different posttranslational modifications have been identified. Modifications of tRNA are the most extensively investigated; however, many other RNAs have modified nucleosides. The modifications that occur at the first, or wobble position, of tRNA’s anticodon and those 3′-adjacent to the anticodon are of particular interest. The tRNAs most affected by individual and combinations of modifications respond to codons in mixed codon boxes where distinction of the third codon base is important for discriminating between the correct cognate or wobble codons and the incorrect near-cognate codons (e.g. AAA/G for lysine versus AAU/C asparagine). In contrast, other modifications expand wobble codon recognition, such as U·U base pairing, for tRNAs that respond to multiple codons of a 4-fold degenerate codon box (e.g. GUU/A/C/G for valine). Whether restricting codon recognition, expanding wobble, enabling translocation, or maintaining the messenger RNA, reading frame modifications appear to reduce anticodon loop dynamics to that accepted by the ribosome. Therefore, we suggest that anticodon stem and loop domain nucleoside modifications allow a limited number of tRNAs to accurately and efficiently decode the 61 amino acid codons by selectively restricting some anticodon–codon interactions and expanding others.
INTRODUCTION
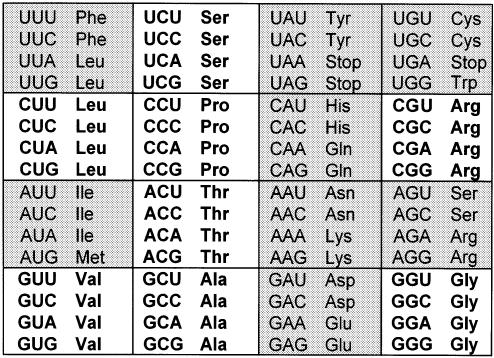
Very often, our understanding of biology is derived from a testable hypothesis and the experimental results that follow. So it was in understanding how genetic information encoded in DNA was transformed by the cell into a unique sequence of a protein’s amino acids. With the first mention of the adaptor molecule hypothesis came a search for and the testing of sRNA’s (tRNA’s) ability to translate genetic information (1). A set of coding triplets or three letter codes composed of the nucleosides adenosine (A), guanosine (G), cytidine (C) and uridine (U) was envisioned to account for the 20 amino acids (2). The three letter code was supported by experiments in vivo including some of the earliest applications of translational frameshifting (3). In vitro, specific coding triplets were associated with individual amino acids through ribosome-mediated aminoacyl-tRNA binding to either homopolymers, enzymatically synthesized heterotriplets (4,5) or chemically synthesized polynucleotides (6–9). The deciphering of the triplets resulted in what is now the historical presentation of the Genetic Code (Fig. 1). The first two letters of the code, A, G, C or U, create 16 possible combinations, each of which is displayed in a separate ‘codon box’. Each codon box is composed of four, three letter codes, 64 in all. Sixty-one codons are recognized by tRNAs for the incorporation of amino acids, and three codons signal the termination of protein synthesis. Eight of the codon boxes each code for only a single amino acid and therefore, are 4-fold degenerate (Fig. 1). The remaining 12 amino acids have codons in 2-fold degenerate codon boxes (e.g. asparagine and lysine, or tyrosine and stop), are 3-fold degenerate (isoleucine), or have only one codon (e.g. methionine and tryptophan).
Figure 1.
The Genetic Code. The 61 codons for the 20 amino acids and three codons for the translational stop signals are shown in the historical coding chart. Codon boxes with white backgrounds contain four codes for one amino acid and are therefore 4-fold degenerate. Codon boxes with shaded backgrounds contain codons for more than one amino acid, or an amino acid plus stop codons and therefore are 2-fold and 3-fold degenerate codons.
The evident degeneracy of the genetic code required that amino acid specific tRNAs respond to multiple coding triplets that differed only in the third letter and gave rise to the ‘Wobble Hypothesis’ (10) and a considerable number of questions, not the least of which has to do with the importance of posttranscriptional modification to the decoding process. This review focuses on the functional contributions of tRNA’s modifications to the decoding of genomic information. In particular, decoding that requires modification of tRNA’s anticodon stem and loop domain (ASL) for accurate and effective protein elongation will be emphasized. With this information, the codes (Fig. 1) are presented in a mechanistic light for their being productively recognized by tRNA for protein synthesis.
The establishment of the Genetic Code (11), the first sequencing of a tRNA, yeast tRNAAla (12,13), the presence of a modified nucleoside, inosine (I34), at what was thought to be the first position of the anticodon, position 34 (14) (Fig. 1), and Francis Crick’s Wobble Hypothesis (10) and its confirmation (15) focused attention on the detailed principles of anticodon–codon interaction. As early as the 1966 Cold Spring Harbor Symposium on Quantitative Biology, modifications within tRNA’s anticodon stem and loop domain (ASL) were thought to play pivotal roles in codon recognition and binding, and/or in aminoacyl-tRNA synthetase recognition of cognate tRNA (16). However, I34 was far from the first modified nucleoside identified in RNA. Probably, the ubiquitous pseudouridine, Ψ, has that distinction, being discovered in 1951 (17) and identified in 1959 (18,19). Over the next two decades, some 35 modified nucleosides were identified in RNAs (20). With improvements in the site-selected introduction of modified nucleosides through automated chemical synthesis (21), the Wobble Hypothesis could be tailored to include uridine modifications (22). Advances in analytical instrumentation and methodology (23) greatly accelerated the discovery and identification of modified nucleosides. In particular, tRNAs from hyperthermophile organisms were found to provide a rich source of new compounds and structures (24). Today the list of modified nucleosides identified in RNA has grown to about 100, and is still growing by several nucleotides a year.
In general, the chemical properties that modifications contribute to nucleosides are similar to those of the amino acid side chains, hydrophilic (polar and charged), or hydrophobic (aromatic or aliphatic) (25). Some of the modifications are as simple as methylations, whereas others involve multiple step additions of aromatic rings, amino acid derivatives and sugars. Modified nucleoside identification, RNA of origin, biosyntheses, organism differences and possible functions are catalogued in databases and a number of books (26–31). Some modified nucleosides that are so highly conserved in type and location in tRNA (dihydrouridine or D, ribothymidine or T, and pseudouridine or Ψ) have become part of the nomenclature associated with tRNA structure (Fig. 2). These and other modifications that are highly common in type and location in RNA sequences appear to be important to RNA folding (33,34). The availability of many whole genome sequences, and the assignment of many unidentified open reading frames or genetic markers to tRNA modifying enzymes (35) has given new impetus to the investigation of the enzymes and mechanisms of RNA modification (36). However, connecting modified nucleoside chemistry and structure to the decoding process has been challenging (25,31,37).
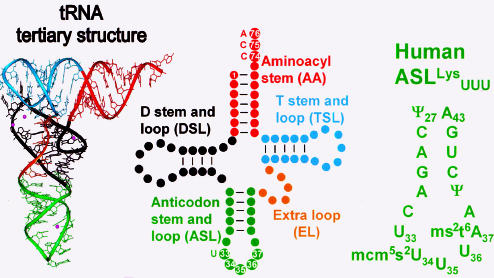
Figure 2.
The structure and domains of tRNA. The three-dimensional structure of tRNA is represented on the left by the crystallographic structure of yeast tRNAPhe (32). The cloverleaf secondary structure in the center is color coded to identify the structural domains of the crystal structure: amino acid accepting stem, or aminoacyl-stem (AA) is in red; dihydrouridine stem and loop domain (DSL) in black; anticodon stem and loop domain (ASL) in green; extra loop (EL) in gold; and the ribothymidine, or TΨC, stem and loop (TSL) in light blue. The positions of the invariant U33 and the amino acid accepting 3′-terminus (C74, C75 and A76) are shown. The sequence and secondary structure of the ASL from human tRNALys3 is shown on the right. The nucleosides are numbered according to the accepted protocol for tRNAs, with the anticodon nucleosides as 34, 35 and 36. The modified nucleosides in ASLLys3 are: pseudouridine, Ψ27,39; 5-methoxycarbonylmethyl-2-thiouridine, mcm5s2U34; and 2-methylthio-N6-threonylcarbamoyl-adenosine, ms2t6A37.
The term decoding, once limited to the actual event in translation, has been appropriated to genomics to identify the products of coding genes, and to systems biology for the monitoring of pathways and timing in responses to stimuli. Recoding has been used to refer to the natural changes in a specific mRNA such that genetic readout is a programmed translational mRNA frameshift, or a redefinition of codons (38,39), as well as the man-made manipulations to insert non-natural amino acids (40–43). Translational bypassing has been used to describe a ribosome’s skipping over a number of mRNA nucleosides and resulting in the joining of two non-contiguous, open reading frames (44). Non-standard decoding continues to refer to an organism’s or organelle’s use of a code for a different amino acid than that traditionally ascribed to it, or for using a stop code for an amino acid insertion (45–50), other than suppression (51). In general, decoding in the cytoplasm is not altered from that established some 40 years ago. However, a number of yeasts of the genus Candida have a reassigned cytoplasmic decoding of the leucine codon CUG to serine (Fig. 1). The codon is read by a novel tRNASer in which the invariant U33 (Fig. 2) is replaced by a G33 (52,53). This tRNASer has the modified nucleoside N1-methylguanosine (m1G37), as do the leucine isoacceptors, and accepts leucine in vitro and in vivo indicating the only known ambiguous use of the codon CUG. tRNAs are edited by many eukaryotes and sometimes serve the purpose of expanding the ability to read codons. The first example of thiolation of the invariant U33 was demonstrated in an edited mitochondrial and cytosolic tRNATrp (54). An understanding of why the universal 64 codes exist for the 20 amino acids and three stop codons has evolved for decades and many exceptions have proved the rule. In contrast, the evolution of thought on modified nucleoside function in decoding is still developing.
SURVEY AND DISCUSSION
The decoding process
The RNA and protein components of the ribosome are active participants in protein synthesis. The RNA of the large subunit catalyzes the peptidyl-transferase reaction (55). Decoding of mRNA on the small subunit is a multistep process involving the small subunit RNA and one or two ribosomal proteins. In protein elongation, decoding of mRNA codons begins with a ternary complex of aminoacyl-tRNA, elongation factor and GTP entering the ribosome’s aminoacyl-, or A-site, to bind either cognate or wobble codons. A productive placement of tRNA in the A-site requires that the anticodon–codon interaction chronologically precedes formation of any significant interactions of the ternary complex with the ribosome. Results from cryo-EM of the ribosome place the entering aminoacyl-tRNA initially at a location different from its final position in the A-site where it is in preparation for the peptidyl-transferase reaction (56). The establishment of a correct anticodon–codon base pairing heralds the hydrolysis of GTP and release of elongation factor. Thus, it is the A-site at which the correct anticodon–codon interaction must be assessed and verified, and incorrect associations discarded. A 20-year-old hypothesis (57) envisioned that the decoding site ribosomal components recognized the architectural correctness of the anticodon–codon interaction. In fact, fidelity of codon recognition by tRNA’s anticodon involves small ribosomal RNA dynamics (58,59). tRNA decoding of the cognate codon results in the small ribosomal subunit transitioning between open and closed forms (60). Crystal structures of the small 30S ribosome subunit characterize a dynamic in which correct codon recognition by tRNA results in a closure of small subunit domains around the decoding site, whereas in the large 50S subunit of the ribosome, elements involved in intersubunit contacts or in substrate binding are flexible, but overall there is a greater order to the crystal structure (61). Structures of Escherichia coli wild-type and hyper-accurate ribosomes at resolutions of 10 and 9 Å, respectively, indicated that mRNA decoding is coupled primarily to movement within the small subunit body (62). The functional contributions of tRNA’s anticodon domain modifications to small subunit recognition of a stereochemically correct anticodon–codon interaction could be crucial to the accuracy and stability of the interactions, and the rate of protein synthesis.
Decoding accuracy. Ribosome mediated decoding is far more accurate than that expected from simple anticodon base pairing with codon (63). In addition, Watson–Crick and wobble codon binding by tRNA does not account energetically, structurally or mechanistically for the A-site selection of tRNAs by the ribosome. Thus, the ribosome contributes significantly to the selection of the correct cognate and wobble codon interactions and to the affinity and dissociation of codon with the correct anticodon (64,65). Several incorrect aminoacyl-tRNA ternary complexes may be tried and dissociated at the A-site until the correct one is found and a closed 30S subunit conformation is stabilized concluding A-site tRNA selection (60). Mechanistically, the anticodon domain of A-site tRNA interacts with ribosomal components, as well as with codon. Correct cognate and wobble codon–anticodon binding enables universally conserved, A-site, 16S rRNA nucleosides G530, A1492 and A1493 to stabilize anticodon–codon base pairing by forming hydrogen bonds with the tRNA anticodon domain backbone and with the mRNA backbone (66). tRNA’s 2′-hydroxyl groups at anticodon positions 35 and 36 interact with the 16S rRNA nucleosides A1492 and A1493, respectively. A1492 and A1493 must flip out from the rRNA’s helix 44 to be able to enter the minor groove of a correctly formed anticodon–codon mini-helix where hydrogen bonding to and stabilization of the first and second base pairs takes place. The glycosidic bond of G530 is syn, but rotates to anti to bond to and stabilize the anticodon–codon interaction at the third base pair. With near-cognate codon, anticodon–codon interaction deviates sufficiently from Watson–Crick geometry that 16S rRNA nucleosides folding into the anticodon–codon minor groove do not stabilize the interaction. Thus, the transition to a small subunit closed form is unfavorable for near-cognate, anticodon–codon pairs.
The 2′-hydroxyl groups of tRNA’s universally conserved U33, and of anticodon nucleosides 35 and 36 are important for tRNA’s translocation from the ribosome’s aminoacyl-site to the peptidyl-site, A- to P-site (67). Methylation of the 2′OH of wobble position 34 occurs in a number of tRNAs, for instance Gm34 in yeast phenylalanine tRNA (25,68). However, modifications of the 2′OH of anticodon positions 35 and 36 have not been found in native tRNAs. Though 2′-O-methylation of a C at position 34 enhanced recognition of the cognate codon ending in G, chemically introduced 2′O-methyls at positions 35 and 36 significantly decreased the tRNA’s decoding ability (69). Therefore, at the very minimum, the 2′OH of anticodon nucleosides 35 and 36 are part of a universally conserved mechanism of ribosomal translocation. In addition, lysines in particular of small subunit protein S12, and possibly S13, appear to provide counter charges to the anionic tRNA in the A-site (66,70). Thus, the ribosomes stabilizing components are able to discern structurally correct anticodon interactions with cognate and wobble codons, and discriminate against stereochemically incorrect interactions with similar, near-cognate and dissimilar, non-cognate codons.
Rates and free energy considerations. At the A-site of the ribosome, the tRNA with the correct anticodon is competing for codon binding with other tRNAs. Yet, the different aminoacyl-tRNA ternary complexes and their interactions with the ribosome are presumably identical. Escherichia coli has 41 tRNAs and some will have anticodon sequences that are similar to the correct sequence. The ribosome’s ability to select the correct tRNA is exacerbated by having to distinguish a correct three base pair cognate or wobble interaction from a near-cognate interaction differing in only one base pair. This hydrogen bonding is truly a very small part of what must be multiple contacts between the ternary complex and the ribosome (71). Could the all important distinction between correct and incorrect anticodon–codon pairing be based solely on the small energy differences in cognate/wobble base pairing versus near-cognate base pairing, and in the architectural correctness of the pairing? The high accuracy and speed exhibited in the process of anticodon–codon pairing could be achieved in a two-step process in which accuracy occurs at the decoding recognition step followed rapidly by an A-site accommodation of the tRNA (71). The free energy difference between cognate and near-cognate base pairing is small leading one to predict an error rate of 1 in a 100 amino acids. Yet, in vivo the ribosome incorporates the wrong amino acid approximately once for every 10 000 peptide bonds formed under normal growth conditions (72). Independent of ribosomes, the binding of tRNA to a coding triplet or the binding of one tRNA to another with a complementary anticodon exhibit dissociation constants and dissociation rate constants that are 10- to 100-fold higher in solution than on the ribosome. The kinetics of tRNA binding to the ribosome have been thoroughly examined (73). In addition, there is a 70- to 100-fold difference in rate constants for accommodating cognate versus near-cognate codon–tRNA interactions on the ribosome (74). Thus, the selectivity and lower dissociation constants of anticodon–codon interactions on the ribosome may be achieved kinetically and mechanistically by participation of ribosomal components in the stabilization of the cognate and wobble codon–anticodon interactions (66,75). The decoding process includes discrimination between correct and incorrect tRNA anticodons on the basis of the stabilities of anticodon–codon interaction. Rejection rates are due to differences in stabilities between a correct and incorrect anticodon–codon pairing (74). Correct and therefore, stable pairing is followed by a conformational rearrangement of the small subunit that invokes the enzyme analogy of an induced-fit model (74). Could the contributions of tRNA’s anticodon domain modifications generate the differential stability?
Modified nucleoside contributions to decoding
Wobble, degeneracy and modified nucleoside-dependent codon binding. The degeneracy of the codes for the 20 amino acids results in some amino acids being coded for by as many as six codons (leucine, serine), whereas others as few as one (methionine, tryptophan). To account for this, the original Wobble Hypothesis envisioned a decoding mechanism that included a broad spectrum of wobble for position 34 of tRNA. tRNA’s anticodon position 34 (Fig. 2) would be responsible for wobble to the third base of the codon. Uridine at tRNA’s position 34 would recognize A and wobble to G, whereas the modified nucleoside inosine would recognize C and wobble to A or U (10). In 1991, this was altered to include the directed wobble of the many modified nucleosides found at position 34, especially the 2-thionyl modified uridines, s2U (22). Ten years later, wobble position decoding rules were amended with regard to the codon binding by various derivatives of 5-substituted uridines (5-methyl-2-thiouridine, xm5s2U34 and methoxy-5-uridine, xmo5U34) (76). Thus, uridine would bind A, and wobble to all four bases, xm5s2U would bind A and wobble to G, and xmo5U would bind A and wobble to both G and U.
With only four exceptions, the wobble position of tRNAs contains U, C, G or I, the latter derived from adenosine. I and not A appears at position 34 because I binds C and has a wobble capacity to A and U exceeding that of A, and A in the wobble position of the P-site tRNA could destabilize the A-site anticodon–codon duplex (77). The four tRNAs found with an unmodified wobble position 34 adenosine include two mitochondrial arginine tRNAs (78,79), a Mycoplasma capricolum threonine tRNA (80), and a mutant of Salmonella typhimurium in which the wobble nucleoside G34 had been replaced by an unmodified A in tRNAProGGG (81). The binding of the two mitochondrial tRNAArgACG, tRNAProAGG and tRNAThrAGU to codons CCC, CGC and ACC, respectively, would presumably require a wobble position A+34·C base pair.
A theoretical analysis of the effect of tRNA modification on wobble base pair formation found that a productive wobble base pairing would require compensation for loss of hydrogen bonds or polar atom–ion bonds (77). Thus, modifications of U34 would restrict decoding at the wobble position to purines (with the exception of 5-oxy derivatives of uridine, xo5U, that would decode A, G and U). The 2-thio modification of uridine, s2U34, would be expected to weaken the wobble base pairing with G because one of the two hydrogen bonds of the guanosine NH2 group would be deformed. The model predicted that modifications of the first anticodon residue in the P-site tRNA would affect the stability of the A-site duplex (82). The possibility that the xo5- and xmethyl5s2-modified uridines would change the decoding rules was first envisioned over 30 years ago (83). NMR analyses of modified nucleosides has found that the 2-thio group restricts uridine dynamics to the anti, 3′-endo, gauche+ conformation and thereby promotes binding to adenosine (84,85) (Table 1). The s2U also influences 3′-adjacent nucleosides to take a similar conformation (99,100). In contrast, xo5U takes the C2′-endo form, as well as the C3′-endo form, possibly enabling wobble to guanosine and uridine, as well as binding to adenosine as the third letter of the codon (85). However, a number of mechanistic questions remain. For those amino acids with many codons (4-fold degenerate, Fig. 1), but far fewer isoaccepting tRNA species, do modifications expand wobble and contribute to a discernibly correct anticodon–codon architecture on the ribosome? For those amino acids with one or two codons (mixed codon boxes, Fig. 1), do ribosomes distinguish productive cognate and wobble anticodon–codon interactions from incorrect near-cognate interactions with the aid of tRNA modifications? A surprising number of modification-deficient tRNAs and ASLs fail at ribosome mediated codon binding, translocation and mRNA reading frame maintenance.
Table 1. tRNA decoding and reading frame maintenance of codons in mixed codon boxesa.
| tRNA/ASL-anticodon | Relative codon bindingb | Modification(s) present | Restored codon bindingb | Translocationc | Frameshiftingd | |||
|---|---|---|---|---|---|---|---|---|
| Unmodified | Modified | |||||||
| Lys-UUU | AAA | AAG | AAA | AAG | AAA | AAA | s2U34 or mnm5U34: | |
| – | – | s2U34 | ++++ | +++ | – | ++ | AAG or AAA | |
| mnm5U34 | +++ | – | – | |||||
| t6A37 | +++ | – | – | |||||
| Ψ39 | + | – | – | U39, AAA: None | ||||
| mnm5U34 and t6A37 | ++++ | ++ | ++++ | |||||
| Lys-CUU | AAA | AAG | t6A37 | AAA | AAG | |||
| – | ++++ | – | ++++ | |||||
| Arg-UCU | AGA | AGG | Mcm5U34 | AGA | AGG | |||
| – | ND | ++ | – | |||||
| Glu-UUC | GAA | GAG | GAA | GAG | ||||
| ND | s2U34 | +++ | + | |||||
| mnm5U34 | ++ | +++ | ||||||
| s2U34 | ++++ | +/– | ||||||
| mnm5s2U34 | +++ | ++ | ||||||
| Gln-UUG | CAA | CAG | CAA | CAG | mnm5U34: | |||
| – | ND | ND | ND | CAA | ||||
| Ser-GCU | AGC | AGU | ||||||
| + | ++ | |||||||
| Cys-GCA | UGC | UGU | ||||||
| – | ND | |||||||
| Trp-CCA | UGG | UGG | ||||||
| – | ND | |||||||
| Phe-GAA | UUU | UUC | Cm32; Gm34; yW37 | UUU | UUC | Gm34:UUU | A37 or i6A37: None | |
| +++ | ND | ms2i(o)6A37; Ψ39; m5C40 | ++++ | +++ | ++++ | U39: None | ||
| Tyr-GUA | UAU | UAC | Q34; ms2io6A37; Ψ39 | UAU | UAC | G34: UAU | ||
| ND | ND | ND | ND | A37 or i6A37: UAU/C | ||||
| Ile-GAU | AUC | AUU | t6A37 | AUC | AUU | |||
| ++ | ND | ++++ | ND | |||||
ND, not determined.
aAminoacyl- (A-) site and peptidyl- (P-) site ribosome binding by anticodon stem and loop domains (ASL) not previously reported were accomplished according to published methods (92–94). Translocation of ASLs from the A- to the P-sites was accomplished with published methods (67). Published results are from 86–98.
bRelative codon binding relates binding affinity to that of fully modified tRNA or ASL: ++++ equals tRNA or fully modified ASL (Kd ∼ 100 nM); –, no binding (Kd > 2000 nM).
cTranslocation for ASLLys normalized to ASLLysUUU with mnm5U34 and t6A37; for ASLPheGAA normalized to ASLPheGAA with Gm34; for ASLVal normalized to ASLVal with cmo5U34.
dFrameshifting by hypomodified tRNAs having the noted modifications and on the specified codons in mutant cells relative to fully modified tRNAs in wild-type cells.
Decoding of mixed codon boxes with modification-deficient tRNA. We (92–94) and others (96) have found that a considerable number of unmodified tRNA transcripts and ASLs (Fig. 2) will not bind codon in the A- or P-sites of the ribosome’s small subunit (30S) (Table 1). ASLs composed of five base-paired stems and seven nucleoside loops bind cognate codons on the 30S subunit with an affinity similar to that of the entire tRNA (101). However, if a particular unmodified ASL binds its cognate or wobble codon poorly, the full transcript of the tRNA, lacking modifications, is also found to bind poorly (92). Lack of modifications also enhances translational frameshifting in vivo (102) (Table 1). Of immediate interest are the many anticodon–codon interactions that decode 2-fold degenerate codons in mixed codon boxes (Fig. 1) and that are also affected by a lack of modifications. For instance, codons for glutamine, lysine, glutamic acid and arginine occur in mixed codon boxes requiring their respective tRNAs to distinguish cognate and wobble codons from near-cognate codons of histidine, asparagine, aspartic acid and serine, respectively (Fig. 1). In addition, these tRNAs have pyrimidine-rich anticodon loops composed of a pyrimidine at position 32 and the invariant U33. Lysine and glutamic acid tRNAs have all-pyrimidine anticodons and glutamine’s anticodon has a G at position 36. The anticodons have either a modified U34, or a C34 (Fig. 2). Unmodified ASLs of glutamine, lysine, glutamic acid, arginine (UCU anticodon) and cysteine did not bind their cognate codons (Table 1). Obviously ASLs, such as ASLLysUUU, that do not bind codon in the A-site will not translocate. In contrast, the unmodified ASLPheGAA with a purine-rich anticodon loop binds UUU in the A-site quite effectively, and translocates (Table 1). tRNAs for glutamine, lysine and glutamic acid all have a derivative of s2U at wobble position 34. The anticodon of E.coli tRNAGluUUC is complementary to that of tRNAPheGAA. The effect of the naturally occurring s2U34 on complementary anticodon–anticodon association of tRNAGluUUC with tRNAPheGAA demonstrated that replacement of the tRNAGluUUC thio group (s2U34) with a keto group (U34) destabilized complex formation and its maintenance (88). The mnm5- modification facilitated tRNAGluUUC recognition of wobble codon GAG while reducing recognition of cognate codon GAA, whereas s2- increased tRNAGluUUC recognition of GAA (95). tRNASerGCU responds to codons (AGU/C) in a mixed codon box also containing the arginine codons (AGA/G). ASLSerGCU bound cognate codon poorly (Table 1). Unmodified ASLArgUCU would not bind the cognate codon AGA (Table 1). In the case of isoleucine, there are three codons. Unmodified ASLIleGAU bound its cognate codon AUC, but binding to its wobble codon AUU has yet to be assessed (Table 1). The single tryptophan codon also occurs in a mixed codon box. The unmodified ASLTrp bound poorly to its codon. The tRNATrp anticodon loop is also pyrimidine-rich and usually has a C32 followed by U33C34C35A36 (68). Lack of, or inefficient codon binding by unmodified tRNAs with pyrimidine-rich anticodon loops was predicted by the ‘Modified Wobble Hypothesis’. The hypothesis describes these types of tRNAs as requiring modification to correctly conform the entire anticodon loop structure for proper codon recognition and binding (22). In fact, pyrimidine-rich ASLs have precluded structure determination of the unmodified loop due to conformational heterogeneity, whereas the addition of a single modification was enough to stabilize the loop (92,103).
Some tRNAs that respond to codons in mixed codon boxes do not have pyrimidine-rich anticodon loops. Wobble position 34 of tRNAs for aspartic acid, asparagine, histidine and tyrosine, all with codons in mixed codon boxes (Fig. 1), are conventionally found to have G modified to various deazaguanosines, derivatives of queuosine Q34 (25), that are synthesized de novo by bacteria, but provided to vertebrates by their digestive tract organisms. The presence of Q34 clearly affected the in vivo choice of codon by two histidine isoacceptors from Drosophila (104). tRNAHisGUG without the modification preferred CAC to the codon CAU. In contrast, tRNAHisQUG had little preference for the codon CAU. An E.coli strain lacking queuosine in its tRNAs was readily out-grown by an E.coli strain with queuosine (105). Similar to the empirical results with tRNAHisGUG, a very stable association was formed in models of the unmodified (guanosine-containing) tRNAAspGUC binding to GAC, but was much less stable in complex with a GAU (106). Also similar to the experimental results with tRNAHisQUG, the modeled tRNAAspQUC with Q34 exhibited no bias for either codons GAC or GAU and had a lower binding energy to the GAU codon than that of the guanosine-containing tRNA (106). Thus, it appears from the experimental (104,105) and modeling (106) results that the presence of Q34 is restrictive in that it seems to be involved in codon choice. Therefore, when unmodified, many tRNAs that respond to codons in mixed codon boxes and have pyrimidine-rich anticodon loops will not bind cognate codons, and some without pyrimidine-rich loops will have their codon selection affected.
Modifications restore decoding accuracy and maintain the reading frame. Correct cognate and wobble codon binding is restored with the incorporation of modifications at wobble position 34 and/or position 37, 3′-adjacent to the anticodon (Fig. 2). The one methionine codon shares a codon box with the three isoleucine codons (Fig. 1). A clear example of a modification that restricts codon recognition and binding involves codon discrimination by tRNAIleCAU. AUG is decoded for methionine in the initiation of protein synthesis in the ribosomal P-site and during protein elongation in the A-site. The gene for a minor isoleucine tRNA that responds to the codon AUA, was found to have a C in the wobble position and thus, a methionine anticodon CAU (107). Modification of the 2 position of C34 with lysine, creating the modification lysidine, k2C34, altered both the aminoacylation and codon recognition from that of methionine to isoleucine. All of the other sequenced bacterial, animal and plant cytoplasmic tRNAIle species have N6-threonylcarbamoyladenosine (t6A37) and either G34 or I34. tRNAIle isolated from E.coli cells grown on a suboptimal concentration of threonine was found to contain an average of 50% less t6A37 than tRNA isolated from cells grown under optimal conditions. Ribosome binding of the codon AUC indicated that t6A was required for tRNAIle to have an accurate anticodon–codon interaction (87). Therefore, purine 37 modifications adjacent to the anticodon, as well as wobble position 34 modifications within the anticodon, are important for correct cognate and wobble codon recognition.
The incorporation of the s2U34 or mnm5U34 modifications (92,93) or t6A37 (94) into otherwise unmodified ASLs for lysine substantially restored cognate codon AAA binding at the ribosome’s A- and P-sites (Table 1). The s2U34 modification, but not the mnm5U34 or t6A37, restored wobble codon binding to AAG, and some A- to P-site translocation for ASLLysUUU (Table 1). A combination of mnm5U34 and t6A37 restored wobble codon binding and translocation (94; Phelps,S.S., Malkiewicz,A., Agris,P.F. and Joseph,S., submitted). A difference in the decoding preference by the mammalian isoaccepting species tRNALysUUU for AAA and tRNALys1,2CUU for AAG and a tendency for only the former species to wobble was observed as early as 1981 (89). Fully modified mammalian tRNALysCUU with t6A37 decoded AAG faster than the hypomodified tRNA, but poorly decoded AAA, which is coded better by the hypomodified tRNA (90). We found that t6A37 did not alter the high affinity binding of human ASLLysCUU to AAG, and did not produce binding to AAA (Table 1). Therefore, tRNALysCUU apparently does not wobble to AAA, and the presence of t6A37 appears to ensure this. The modification t6A is also found in E.coli tRNAArgUCU which decodes AGA/G in the mixed codon box with serine. The modification had a small, but significant stabilization on polynucleotide-directed binding of tRNAArgUCU on the ribosome, ribosome-free trinucleotide binding to codon, and complementary anticodon–anticodon binding (86). Thus, modifications at either wobble position-34 or purine-37 can restore and/or influence specific codon recognition.
In evaluating tRNAPhe function in translation in vitro, individual rate constants for the elongation process showed that modifications increased the accuracy of translation by decreasing the rate of dipeptide synthesis and by increasing the rate of rejection with non-cognate codons (101). The modification ms2i6A37 stabilized anticodon–codon interaction (Table 1), thus preventing misreading of the genetic code (108). Though experiments with suppressors lacking the 2-methylthio- (ms2) group of 2-methylthio-(cis-hydroxy)-isopentenyladenosine (ms2io6A37), also known as 2-methylthio cis-ribozeatin (109), indicated that ms2- is important to the decoding efficiency of tRNA, the major contribution apparently comes from the io6- group alone (110). The anticodon stem modification m5C40 of yeast tRNAPhe actually negated ribosome binding of the ASL, yet significantly increased its thermal stability (111,112). Addition of 1-methylguanosine, m1G37, to the m5C40-modified ASL increased affinity for codon 10-fold, but also dramatically decreased thermal stability. Thus, modifications in the anticodon loop at wobble position 34 and position 37 appear to restructure the loop for correct decoding, and may do so by sacrificing overall thermal stability.
In decoding 2-fold degenerate codons, tRNA modifications aid in reading frame maintenance. Not surprisingly, some of the same unmodified tRNAs or ASLs that are ineffective at cognate or wobble codon binding in vitro are also prone to translational frameshifting in vivo when site-specifically unmodified or hypomodified. Messenger RNA reading frame shifting errors occur during translation. Although some frameshifting is enabled by certain viral RNA and mRNA sequences, frameshift errors in the normal course of translation with fully modified tRNAs are less than 5 × 10–5 per codon (113). The ability of anticodon modifications to diminish the frameshifting effects of 5′- and 3′-neighboring codons, i.e. codon context frameshifting, has been explored extensively. It has been proposed that the affinity of the P-site tRNA for its codon is key to P-site frameshifting. The lower the anticodon affinity for codon, the more likely frameshifting will occur (114,115). In comparison to codon binding in vitro, frameshifting analyses are accomplished in vivo. Relative to wild-type tRNALysUUU with 5-methylaminomethyl-2-thiouridine (mnm5s2U34), hypomodified tRNALysUUU with either s2U34 or mnm5U34 exhibited significant +1 frameshifting in response to the wobble codon AAG, and less in response to the cognate codon AAA (Table 1) (102,116). Modifications at other nucleosides of mutant tRNALys would be expected to be equivalent to that of wild-type, in particular those of the anticodon domain, t6A37 and Ψ39. Excessive frameshifting was observed for tRNAGlnUUG that has the same wobble position uridine modification as tRNALysUUU, but a considerably different modified A37, 2-methyladenosine (m2A37). When the s2U34 modification was absent and CAA was being decoded, tRNAGlnUUG frameshifted more often than fully modified tRNAGlnUUG in wild-type cells (Table 1). Presumably, excessive frameshifting would be observed for tRNAGluUUC of the same mutant because that tRNA also has the mnm5s2U34 modifications. In contrast to +1 frameshifting, the same two modification deficiencies had considerably little effect on –1 frameshifting (117).
As early as 1969, it was observed that in E.coli, one of three tyrosine tRNA isoacceptors lacking a modification 3′-adjacent to the anticodon at position 37 did not support protein synthesis and did not bind the appropriately programmed ribosome (118). The modification ms2io6A37 appears in eubacterial tRNAs for phenylalanine and tyrosine. Cells deficient in modifications of A37 exhibited increased +1 frameshifts for tRNAPhes and tRNATyrs deficient in either ms2- or ms2io6- (102). Although Ψ at position 39 has been shown to stabilize anticodon domain structure (119,120) with little effect on an otherwise unmodified ASLLysUUU to bind cognate codon (92,119), Ψ at positions 38 or 39 enhances suppressor efficiency for read through of stop codons and promotes +1 frameshifting in at least one tRNA (121), but its deficiency did not enhance either +1 or –1 frameshifting with tRNAs for asparagine, lysine, phenylalanine and leucine (116). Two other tRNAs that respond to codons in mixed codon boxes, tyrosine and histidine, differ in their frameshifting when unmodified at wobble position 34 though they have the same hypermodification, Q34 (Table 1). In comparison to the fully modified tRNATyrQUA in wild-type cells, tRNATyrGUA frameshifted in response to the codon UAU in Q34 deficient cells (102,116). However, tRNAHisGUG exhibited little to no frameshifting (Table 1). Thus, anticodon loop modifications, particularly those of purine-37 are important for reading frame maintenance by tRNAs responding to codons in mixed codon boxes.
Limited decoding of 4-fold degenerate codons by modification-deficient tRNA. The decoding of codons from completely degenerate codon boxes could be accomplished by four isoaccepting tRNA species responding to their corresponding cognate codons. Since A has only been found in four tRNAs at wobble position 34 (78–81), and in all others A is modified to I, minimally two isoaccepting tRNAs could respond to four codons. However, only 12% of tRNA genes for tRNA species that respond to 4-fold degenerate codon boxes are encoded with wobble position adenosines (68). The percentage of wobble position adenosines is dramatically higher (37%) in eukaryotic tRNAs. Significantly, 47% of all tRNAs (31.2% in eukaryotic tRNAs) responding to completely degenerate codons are encoded with a wobble position U. The remaining tRNAs are encoded with wobble positions Cs and Gs in a proportion close to the expected random appearance of 25% (22 and 19%, respectively). Thus, one could conclude that wobble position Us, that are almost always modified (68), are responsible for the majority of wobble codon recognitions by these tRNAs (22,122). An unmodified anticodon stem and loop of tRNAProUGG binds to its cognate codon (CCA) with high affinity (Table 2). However, ASLProUGG would not bind to its wobble codon (CCG) (Table 2). ASLValUAC bound its cognate codon (GUA) and translocated from the A- to P- sites, but bound all three wobble codons (GUG, GUC and GUU) very poorly. Unmodified ASLAlaUGC bound neither cognate (GCA) nor wobble (GCG) codons (Table 2). An ASLSerUGA was found to bind cognate codon (UCA) moderately well, but did not bind the wobble codon (UCG) (Table 2). An unmodified form of E.coli tRNASer1UGA, which normally has the cmo5U34 modification and recognizes the UCU/A/G codons, recognized the UCA codon (125). However, the UCU codon was recognized with low efficiency, and the UCC and UCG codons were not recognized at all. tRNAArg species with the anticodons CCG and ACG have the modification 2-thiocytidine-32 (s2C32), but differ in wobble position and position 37 modifications. tRNAArgCCG is not modified at position 34 (68) and has an m1G37, whereas tRNAArgACG is modified to I34 and has an m2A37. We found that the unmodified ASLArg constructs with either anticodon, CCG and ACG, would not bind their cognate codons (Table 2). Thus, some unmodified ASLs responding to 4-fold degenerate codons will bind cognate codon and translocate from the A- to P-site, but will not bind wobble codon, whereas other unmodified ASLs even bind cognate codon poorly and do not translocate.
Table 2. tRNA decoding and reading frame maintenance for 4-fold degenerate codonsa.
| tRNA/ASL and anticodon | Relative codon bindingb | Modification(s) present | Restored codon bindingb | Translocationc | Frameshiftingd | ||
|---|---|---|---|---|---|---|---|
| Unmodified | Modified | ||||||
| Arg CCG | CGG | CGA | s2C32; m1G37 | CGG/A | G37: CGG | ||
| – | ND | ND | |||||
| Arg ACG | CGU | CGC | |||||
| – | ND | ||||||
| Pro UGG | CCA | CCG | Um32; cmo5U34; | CCA/G/U/C | G37: CCA/G and | ||
| ++++ | – | m1G37; Ψ40 | ND | CCU/C | |||
| Ser UGA | UCA | UCU | |||||
| +++ | + | ||||||
| UCC/G– | |||||||
| Val UAC | GUA | GUG | cmo5U34 | GUA | GUG/U | cmo5U34: | |
| +++ | + | +++ | – | GUA/G/U | |||
| GUU | GUC | GUG ++ | ++++ | ||||
| – | ND | GUU ++ | |||||
| Ala UGC | GCA– | GCG– | |||||
| Leu | CUU/C | CUA/G | m1G37; Ψ38–39 | CUU/C/A/G | G37: CUU/A/G | ||
| ND | ND | ND | (CUC in P-site) | ||||
| U38–39: CUU, | |||||||
| CUA/G | |||||||
ND, not determined.
aAminoacyl- (A-) site and peptidyl- (P-) site ribosome binding by anticodon stem and loop domains (ASL) not previously reported were accomplished according to published methods (92–94). Translocation of ASLs from the A- to the P-sites was accomplished with published methods (67). Published results are from references 94,96,123–125.
bRelative codon binding relates binding affinity to that of fully modified tRNA or ASL: ++++ equals tRNA or fully modified ASL (Kd ∼ 100 nM); –, no binding (Kd > 2000 nM).
cTranslocation for ASLVal normalized to ASLVaUAC with cmo5U34.
dFrameshifting by hypomodified tRNA having the noted modifications and on the specified codons in mutant cells relative to fully modified tRNA in wild-type cells.
tRNA modifications maintain the reading frame in decoding 4-fold degenerate codons. The same tRNAs when unmodified or hypomodified at specific positions by mutation of modification enzymes were more likely to frameshift in vivo than their fully modified, wild-type counterparts (Table 2). In the absence of the m1G37-tRNA methyltransferase activity, tRNAPro, tRNALeu and tRNAArg frameshifted considerably more often than the corresponding fully modified tRNAs in wild-type cells (102,126). The lack of m1G37 in the m1G37-tRNA methyltransferase mutant may actually slow decoding by tRNAs normally containing the modification (127). tRNALeu minus m1G37 frameshifted in response to three of its four codons (Table 2). With mutation of a pseudouridine-tRNA synthase for the very common modifications Ψ38 and Ψ39, tRNALeu, with m1G37 but lacking the Ψ, frameshifted in response to the same three codons. tRNAPro minus m1G37 also frameshifted more than the wild-type tRNA in response to all four of its codons, but lacking Ψ40 in the middle of its anticodon stem (Fig. 2) had little effect on codon reading (128). Ψ residues, depending on their locations in tRNA, rRNA and even snRNA, may contribute differently to various RNA functions (129). Thus, for tRNA responding to 4-fold degenerate codon boxed, m1G37, and Ψ38 and Ψ39 are important to the maintenance of the translational reading frame.
Modifications expand tRNAs decoding of 4-fold degenerate codes. Four-fold degenerate coding boxes encompass half of the 64 codons and represent only eight of the 20 amino acids (Fig. 1). Using a MS2 RNA programmed protein synthesizing system in vitro, tRNAValUAC with the wobble position modification cmo5U34 read the codon GUU quite efficiently and tRNAValIAC (with I34) was just as effective in reading the codon GUG (123). We found that the modification cmo5U34 restored wobble codon binding of ASLValUAC (Table 2). Of particular interest, cmo5U34 restored binding to GUU. A cmo5U34·U base pairing occurred in a translocation assay requiring A-site codon binding prior to translocation from the A- to P-sites (Phelps,S.S., Malkiewicz,A., Agris,P.F. and Joseph,S., submitted). Thus, the cmo5U34 modification enables tRNAValUAC to read three of the four valine codes and translocate (Phelps,S.S., Malkiewicz,A., Agris,P.F. and Joseph,S., submitted). A variant of the tRNAVal modification, 5-methoxyuridine, found in tRNASerUGA enhanced the wobble reading of UCU and UCG codons (124). Therefore, the family of xo5U34 modifications expands the wobble recognition of tRNAs responding to 4-fold degenerate codons.
Mitochondrial and chloroplast decoding by tRNAs
The organellar tRNAs are discussed separately because there are far fewer tRNAs encoded than the nuclear encoded, cytoplasmic tRNAs, there appears to be more extensive wobble codon recognition in the mitochondrion, and modifications are both negative and positive determinants for importation of cytoplasmic tRNAs into organelles. A few of the modifications that appear in the anticodon stem and loop domains of eubacteria and eukaryotic cytoplasmic tRNAs also appear in mitochondrial and chloroplast tRNAs. Modifications of nuclear encoded tRNAs appear to both restrict decoding occurring in mixed codon boxes, and enhance wobble for 4-fold degenerate codons. Even though some mitochondria import nuclear encoded tRNAs, the total number of tRNAs operating in the organelle is less than in the cytoplasm. Some nuclear encoded tRNAs appear to require specific modifications for importation (130–132), or a particular modification, such as s2U34, would serve as a negative determinant for the tRNA’s importation (133,134). The chloroplast and mitochondrion of green plants translate the codes on the ribosome with a mechanism similar to that of cytoplasmic protein synthesis. However, chloroplasts use only 31 anticodons in translating the codes. Ten CNN anticodons have been eliminated. Green plant mitochondria augment their tRNA population by importing nuclear encoded tRNAs from the cytoplasm. Mitochondria from other organisms have evolved with recoded codons, such as AUA for methionine, AG(U/C) for serine and stop, AAA for asparagine, CU(A/C/G/U) for threonine, UGA for tryptophan, and may use as few as 22 anticodons for translation. One modification, Ψ35, at the center of the echinoderm mitochondrial tRNAAsn anticodon appears to serve to decode the unusual asparagine codon AAA, resulting in the alteration of the genetic code in echinoderm mitochondria (135).
Therefore, in order for all codons to be effective and efficiently decoded, mitochondrial translation relies on modification enhanced wobble and recoding of codons. Whether the reduced number of tRNAs results from natural pressure for a small mitochondrial genome, or from some other stressors, altered amino acid acceptance by some tRNAs, loss of or changes in release factors, anticodon modifications, or disappearance of codons from coding sequences, is debatable (136,137). The nucleotide sequences of all 29 Mycoplasma capricolum tRNA species indicate that this organism’s tRNAs have similarities to that of mitochondrial tRNAs (80). There is a single tRNA for each of 4-fold degenerate codon boxes of alanine, glycine, leucine, proline, serine and threonine and these tRNAs have unmodified uridines in wobble position 34. The two threonine tRNA species with anticodons UGU and AGU are unmodified at position 34. Of the metazoan mitochondrial tRNAs sequenced and responding to codons in mixed codon boxes, all, but one, lack modification of the position 34 nucleoside. In contrast, the modification t6A37 appears to be important for mitochondrial tRNALysCUU to respond to its cognate codon AAG in a mixed codon box (135). We had found that cytoplasmic tRNALysCUU, whether or not modified with t6A37, bound cognate codon AAG and not its wobble codon AAA (94). Mitochondrial modification deficiencies have been associated with human disease. Mutant mitochondrial tRNALysUUU from the pathogenic disease myoclonic epilepsy with ragged red fibers (MERRF), a mitochondrial encephalopathy, lack the wobble position 2-thiouridine derivative. In contrast to wild-type human mitochondrial tRNALys, the mutant tRNA did not bind to mRNA programmed ribosomes, but could be aminoacylated (138). Thus, the defect in anticodon modification negatively affects anticodon–codon pairing that is crucial to mitochondrial translation. Obviously anticodon domain modifications selectively contribute chemistry and/or structure to decoding and frameshift maintenance in cytoplasmic and organellar protein synthesis.
Anticodon domain contributions to structure and dynamics of decoding. Do modifications enable decoding for many tRNAs by creating the canonical anticodon structure of yeast tRNAPhe? Or do modifications enable decoding by destabilizing the anticodon loop to be later conformed by the ribosome in an induced-fit model to codon reading? Uridine modifications at position 34 seem to fall into two categories with regard to codon binding: s2U34 and its derivatives tend to bind codons ending in A better than those ending in G, though ASLLys with s2U34 bound its wobble codon quite well (Table 1). In comparison, xm5U34 anticodons tend to bind G ending codons better than A ending codons (Table 1). It would be simple if only the restricted anti C3′-endo, gauche+ conformation of s2U34 versus the less restricted sugar pucker of xnm5U were responsible for the difference in A and G binding. However, the 3′-adjacent nucleoside-35, and the presence or absence of a hypermodified nucleoside-37 probably influences the wobble base pairing (Table 1). In order to account for the xnm5U34 preferred binding to codons ending in G, a recent survey of the selective binding by anticodons with xnm5U34 suggests a non-conventional U·G pairing (122). Through protonation of the secondary amine of xnm5U34, it is proposed that the 2-thionyl becomes single bonded and negatively charged and the N-1 proton is lost. The G N1 imino proton is donated to either the S– or the N-1 of the uridine. The unique geometry of the suggested base pairing is intriguing even though the model may need to change in order to accommodate contributions of purine 37 modifications to anticodon architecture. The U·G base pair geometry should become apparent in a high resolution crystal structure determination of the appropriately modified ASL bound to a G-ending codon on the 30S ribosomal subunit. Recently, the crystal structure of ASLLysUUU with t6A37 bound to AAA on the 30S subunit was solved to 3 Å (Murphy,F., Agris,P.F., Malkiewicz,A. and Ramakrishnan,V., unpublished). The t6A37 is required for binding AAA and acted as a platform for the first codon–anticodon base pair (Cover Figure of ths issue). Binding of the wobble codon AAG could by achieved with an xm5U34 modification plus t6A37 (Table 1) and could reveal the exact base xm5U34·G pairing geometry. Thus, modified nucleoside conformation or geometry is partly the answer to enabling or enhancing codon binding, but not the full answer.
The importance of some anticodon conformational dynamics in codon binding on the ribosome has been suggested (139). Cryo-EM structure, kinetics and mutant tRNA suppressor data together appear to support the possibility of a deformed or waggling aminoacyl-tRNA transitional structure required for effective proofreading by the ribosome during decoding at the A site (140,141). Because of its fundamental importance to decoding, the structure and conformational dynamics of the anticodon have been the subject of numerous studies with sometimes contradictory results (142). Three distinct conformations of the yeast tRNAPhe anticodon domain were suggested by time and polarization resolved fluorescence measurements (143) and an NMR study found that anticodon domain modifications were particularly restricted in motion compared to other modifications (144) perhaps indicating slow exchange among a very small number of conformations. Completely unmodified yeast ASLPhe (111), a DNA analogue of the yeast ASLPhe (145) and E.coli ASLPhe (146) molecules exhibited two intra-loop base pairs extending the stem of the unmodified constructs. Whereas incorporation of the individual modifications m5C40 and Ψ39 in the stem increase thermal stability of the yeast tRNAPhe, incorporation of m1G37 in the yeast ASLPhe, i6A37 in the E.coli ASLPhe, and t6A37 in human ASLLys decrease overall thermal stability (103,112). We had demonstrated that incorporation of m1G37 contributed to codon recognition on the ribosome (112) for both the yeast ASLPhe and its DNA analogue (147). Ribose 2′-O methylations within the loop of the heptadecamer anticodon stem and loop of eucaryotic tRNAPhe, Cm32 and Gm34, also affect reading frame maintenance (148). All of the naturally occurring purine modifications at residue 37, including m1G, negate the possibility of intra-loop hydrogen bonding by interfering with the Watson–Crick base pairing ‘face’ of the nucleoside (25). In fact, 95% of all tRNA sequences have the potential for two anticodon intra-loop base pairs that are modified at position 37 (147). More recently we have found that the anticodon conformation of yeast ASLPhe is realized in solution by modifications altering the conformational space sampled by the anticodon loop (142). For instance, human tRNALys3UUU and possibly other tRNALysUUU require modifications at position 37, as well as at wobble position 34, to achieve the canonical U-turn structure (149,150) and function (93,94). The incorporation of m1G37, the precursor to the hypermodification wyosine, into an already triply modified (Cm32, Gm34, m5C40) anticodon stem and loop domain of yeast tRNAPhe directed the conformation dynamics of anticodon loop toward the structure found in the crystal (Fig. 3) (142). The i6A37 in E.coli tRNAPhe may enhance ribosome binding by negating intra-loop hydrogen bonding in the anticodon domain (146), as does modification of G37 in yeast tRNAPhe (147), thus enabling anticodon–codon helix formation. The predicted most stable conformation of i6A37 and its derivatives including ms2i6A37 has the isopentenyl substituent away from the adenosine imidazole ring and coplanar with the adenine ring (151). The same is true of t6A37 (103). In a study of the effects of modified adenosines, particularly the i6-derivatives, on duplex stability, most of the modifications destabilized the duplexes (152). The nature of the modification and the buffer composition significantly affected the thermodynamic stability of the RNA duplexes. However, the decreased thermal stability of duplex formation is exactly what is required to achieve an open anticodon loop that will be ordered for codon recognition and binding (103,142,147). Therefore, some conformational freedom of the anticodon loop with some order and boundaries to motion conveyed by modification, particularly at position 37, is also important for codon recognition.
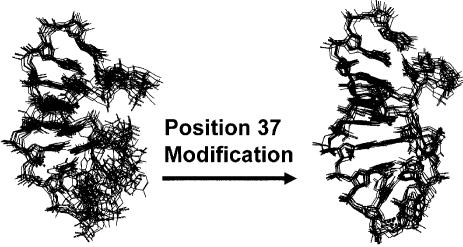
Figure 3.
Purine 37 modifications order the anticodon loop. The structure and dynamics of triply (left) and quadruply (right) modified ASLs for yeast tRNAPhe have been compared (142). The only difference in sequence between the two constructs is the incorporation of m1G37 versus G37. Each structure is represented by a family of the 10 lowest energy structures derived from NMR and restrained molecular dynamics.
In contrast to most other modifications, Ψ and the 2′O-methyl modified nucleosides are by far the most highly conserved in location and are the most ubiquitous modifications. Both appear in the ASL domains of tRNAs. Pseudouridine is most often found in the stem and adjacent to the anticodon loop at position 39, but also at 31, or adjacent to the stem at position 38. It is also found in the anticodon at position 35. The 2′-O-methylated nucleosides have been found at position 32 adjacent to the loop and in the anticodon. The thermal stabilization of structure contributed by Ψ38,39 immediately adjacent to, or in the anticodon stem does not appear to be through direct hydrogen bonding afforded by the additional imino proton at N1 (119,120). Hydrogen bonding through water has not been excluded. Though Ψ39 contributes thermal stability, it is incapable of restoring codon binding to ASLLysUUU which in the absence of other modifications will not bind cognate or wobble codons in the ribosomal A- or P-sites (92–94). By Ψ coordinating structural water via its free N1-H, and/or enhancing base stacking, it has a significant negative effect on RNA dynamics. This effect on structure and dynamics will impact RNA function and may be the reason that Ψ is so prevalent. Thus, it appears that anticodon domain modifications balance structure with dynamics in a direction that creates the correct anticodon conformation and mobility for ribosome binding.
CONCLUSIONS
Organisms have evolved different patterns of bias in codon usage of the degenerate Genetic Code (153). In general, tRNAs with the appropriate anticodons are expressed in proportion to the codons appearing in mRNAs. However, any particular tRNA species in a cell may be composed of molecules that are not equivalent in modification. Usually a small proportion of tRNAs are undermodified at particular positions. Because modification enzyme activities seem to be unable to keep pace with tRNA transcription and processing in rapidly dividing cells, the proportion of site-specifically undermodified tRNA molecules would be expected to be higher in those cells. Indeed, tRNAs undermodified at various positions, but particularly wobble position 34 and position 37, have been reported (27,28) to occur in rapidly dividing cells and in cloned overexpression of tRNAs (154–160). As discussed, undermodified species of some tRNAs may not bind codon, whereas others may. In some instances, a cell’s rarely used codons could be read by undermodified tRNA species, whereas the modified species would be more codon selective. Disparities have been reported for codon binding results in vitro in comparison to codon selection in vivo (161). In vitro codon binding experiments may indicate a requirement for a modification for cognate or wobble decoding, whereas in vivo a modification enzyme mutation indicates a tendency to frameshift or read near-cognate codons more or less effectively. Surprisingly, the hypomodified E.coli tRNALysUUU was reported to misread asparagine codons in vivo less than the fully modified tRNA (91). This result not only is at odds with the in vitro data, but needs to be clarified with respect to the frameshifting experiments in vivo in which selected hypomodification increased frameshifting (102,116). The s2U34 modification has been found to be quite restrictive in its recognition of complementary A versus G and that mnm5U34 is less so (124). The presence of the t6A37 modification in combination with the hypomodified U34 may have contributed to the undermodified tRNA’s restrictive decoding behavior in vivo.
We have found that anticodon loops modified at positions 34, s2U34, and 37, t6A37, carry a redundancy in their ability to effectively read cognate and wobble codons in vitro (Table 1). The disparity between results in vitro and in vivo may very well be due to the redundancy built into the system. There are many redundant systems in biology, and modification is not without them. For instance, some years ago we had found that antisuppressor mutations reduced the efficiency of nonsense suppressors in Schizosaccharomyces pombe (162). Mutation of the sin4 or sin3 genes led to loss of 5-(methoxycarbonylmethyl)-2-thiouridine (mcm5s2U34) from the first anticodon position of tRNAs. The major sulfur-carrying nucleoside in wild-type S.pombe tRNA is mcm5s2U34. It was reduced but not devoid in the mutant strains. Two other thiolated nucleosides were also present, s2U and a nucleoside of unknown structure, and neither was affected by the antisuppressor mutations. Independent from their effect on suppressors, the two mutations sin3 and sin4 reduced the growth rate of cells, and sin3 also increased cell length. In vivo decoding of the serine codon UCG by the UCA reading serine tRNA was not promoted by the two antisuppressor mutations (163). Where differences between results in vitro and in vivo have been found, investigations of double and triple mutants that negate all modifications at 34 and 37 may resolve the discrepancies between experiments in vitro and in vivo and yield a greater understanding of redundancy. In vitro data taken together would suggest that such multiple mutations could be lethal. Interpretation of results from codon binding by modification deficient tRNAs, thus necessitates consideration of the effects of remaining modifications.
Modifications and the enzymes responsible for their syntheses demonstrate biological redundancy and duality of function. For some tRNAs, anticodon stem and loop modifications are important determinants for aminoacylation (164–167), whereas others, such as methylations of the anticodon loop, enhance protein recognition but are not required (101,168,169). Some of the most ubiquitous modification enzymes such as those for Ψ (36) and 2′-O-methylation have multiple substrates, tRNA and rRNA, tRNA and snRNA. But this plurality of substrates is not readily predictable (170). When Ψ55 is missing from tRNA of the human pathogen Shigella flexneri, there is a decreased expression of several genes associated with virulence (171).
Other modifying enzymes appear to have functions other than modification of RNAs (172). When E.coli’s trmA gene for the enzyme m5U54-tRNA methyltransferase that synthesizes the highly conserved T54 of tRNA’s TΨC loop was mutated (173), wild-type cells had a slight advantage in culture but no dramatic physiological result was witnessed (174). However, insertions in the gene produced nonviable cells and the conclusion that the enzyme had multiple functions (175). Similar results of mutant, and missing trm2 genes for the same enzyme were observed in Saccharomyces cerevisiae (176). The truB gene product synthesis of Ψ55 may not be critical, but contributes to E.coli’s tolerance of thermal stress (177). This may be associated with Ψ55’s positive effect on the interaction of tRNA’s D-loop with its T-loop (33). The E.coli trmE gene product involved in synthesis of mnm5s2U34 also is a GTPase that can be essential under certain conditions (178).
The data on anticodon modified nucleosides, in particular uridines at tRNA’s wobble position 34, prompted the composition of a ‘Modified Wobble Hypothesis’, in which specific base modifications had evolved to select particular codons (22). At the extreme, this hypothesis is exemplified by a wobble position 34 modification restricting an isoleucine tRNA species to reading the isoleucine codon (AUA) and not reading the methionine codon AUG (107). In general, modifications of U34 for tRNAs responding to codons in mixed codon boxes restrict codon reading, s2U34, for instance (Fig. 4). We proposed that this is accomplished by 2-thio modified pyrimidines having well defined anti, C3′-endo, gauch+ conformations that may also influence 3′-neighboring nucleoside conformations (22). Modifications of tRNAs responding to 4-fold degenerate codes expand wobble (Fig. 4). The modification cmo5U34 enhances wobble by having a less well defined conformation and a tendency to be in the C2′-endo conformer rather than C3′-endo (85). Position 37 purine modifications, such as m1G37, t6A37 and (ms2)i(o)6A37, maintain the mRNA reading frame and counter frameshifting, as do some modifications at wobble position 34, and Ψ38,39 (Fig. 4) (102,116,121,179–181). The purine 37 modifications open the anticodon loop by negating intra-loop base pairing and order loop nucleosides, thereby potentiating a significant effect on the anticodon and other loop modifications.
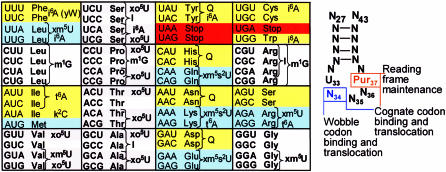
Figure 4.
The Genetic Code and its decoding by tRNA. The traditional representation of the codons (left) is accompanied by tRNA’s anticodon stem and loop domain modifications known to influence decoding, translocation and/or frameshifting (Tables 1 and 2). Cognate and wobble codons that are possibly read by a wobble position, modified uridine-34 responding to one of the 4-fold degenerate codon boxes have backgrounds of white and gray. Codons representing a single amino acid in each of the mixed codon boxes are shaded in yellow and light blue. The stop codons are shaded in red. Modifications are abbreviated to represent all derivatives of the modified nucleoside, such as derivatives of xm5s2U and i6A. The wobble position 34, and purine-37 modifications (ASL right) have been discussed. Briefly, wobble position uridine modifications that include 2-thiolation (xm5s2U) can be required for codon binding and are restrictive to binding A and wobbling to G. The 2-thiolation promotes translocation. Modifications of the 5-carbon of uridine-34, in the absence of 2-thiolation, bind the cognate codon ending in A and promote binding to the wobble codon ending in G (xm5U). Oxy-modifications expand wobble beyond G to U (xo5U). I34 binds C and wobbles to A and U. Q34 promotes binding to the cognate codon ending in C over that ending in U. The position 37 modification t6A and its derivative ms2t6A promote binding to cognate codon and with position 34 uridine modifications ensure wobble codon binding and translocation. A number of position 37 modifications (i(o)6A, m1G) maintain the translational reading frame.
OUTLOOK
With the completion of over 50 genomic sequences, tRNA gene sets are now available for study and comparison. It is no surprise that the number of tRNA gene copies correlate with the expression level of those tRNAs, a specific codon bias by the organism and its amino acid usage, and that particular tRNA gene sequences are conserved, particularly that of initiator methionine (122,182–184). However, it is too early to extract detailed information about modification-dependent decoding from direct comparisons of genomic information. One can conclude that the application of the general wobble rules will be altered when the wobble position 34 modifications of eukaryotic tRNAs are considered in comparison to those of prokaryotes (183). The rapidly increasing genomic information pool is attractive as a base for development of testable hypotheses (122,183). However, empirical evidence, such as that for purine 37 modifications having significant roles in decoding, translocation and reading frame maintenance in both bacterial and eukaryotic tRNAs, precludes developing codon recognition rules based solely on wobble position 34 nucleosides and their possible modifications. With an ever increasing understanding of the recognition determinants for the many enzymes involved in modification of anticodon domain nucleosides, we will be able to accurately predict the modified nucleosides that will be found in the products of these tRNA genes. Though many unmodified tRNAs and ASLs have been found devoid of cognate codon or wobble codon binding in vitro, site-specific modification-dependent restoration of cognate and wobble codon binding and translocation of most tRNA species awaits analyses. The effects of selectively incorporating modifications, individually and in combinations need to be assessed in vitro and in vivo. Anticodon domain modifications are just as likely to affect translation by contributing to the on and off rates of anticodon–codon interaction, as they are to affect the stereochemistry of a correct interaction on the ribosome. The tRNA modifications found in thermophilic and cryophilic organisms and their contributions to anticodon domain conformational dynamics may provide clues to codon selection and translational rates at temperature extremes. Finally, application of technical advances in oligonucleotide chemical synthesis, spectroscopy and crystallography will accelerate our understanding of the physiochemical contributions of tRNA’s modified nucleosides to decoding the genome.
Acknowledgments
ACKNOWLEDGEMENTS
The author thanks Dr Dieter Söll (Yale University) for advice on the manuscript and Dr Michele DeRider for the cover image from the crystallographic data. The work is supported by the Department of Health and Human Services and the National Science Foundation (PHS NIH Grant GM-23027 and MCB9986011 to P.F.A.).
REFERENCES
- 1.Crick F.H.C. (1957) In The Structure of Nucleic Acids and their Role in Protein Synthesis. Biochem. Soc. Symp. 14. Cambridge University Press, UK, pp. 25–26. [PubMed] [Google Scholar]
- 2.Crick F.H.C., Barnett,L., Brenner,S. and Watts-Tobin,R.J. (1961) General nature of the genetic code for proteins. Nature, 192, 1227–1232. [DOI] [PubMed] [Google Scholar]
- 3.Streisinger G., Okada,Y., Emrich,J., Newton,J., Tsugita,A., Terzaghi,E. and Inouye,M. (1966) Frameshift mutations and the genetic code. Cold Spring Harbor Symp. Quant. Biol., 31, 77–84. [DOI] [PubMed] [Google Scholar]
- 4.Leder P. and Nirenberg,M. (1964) RNA codewords and protein synthesis, II Nucleotide sequence of a valine RNA codeword. Proc. Natl Acad. Sci. USA, 52, 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nirenberg M., Caskey,T., Marshall,R., Brimacombe,R., Kellogg,D., Doctor,B., Hatfield,D., Levin,J., Rottman,F., Pestka,S., Wilcox,M. and Anderson,F. (1966) The RNA code and protein synthesis (1966) Cold Spring Harbor Symp. Quant. Biol., 31, 11–24. [DOI] [PubMed] [Google Scholar]
- 6.Khorana H.G. (1965) Polynucleotide synthesis and the genetic code. Fed. Proc., 24, 1473–1487. [PubMed] [Google Scholar]
- 7.Söll D., Ohtsuka,E., Jones,D.S., Lohrmann,R., Hayatsu,H., Nishimura,S. and Khorana,H.G. (1965) Studies on polynucleotides, XLIX. Stimulation of the binding of aminoacyl-sRNA’s to ribosomes by ribotrinucleotides and a survey of codon assignments for 20 amino acids. Proc. Natl Acad. Sci. USA, 54, 1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khorana H.G., Buchi,H., Ghosh,H., Gupta,N., Jacob,T.M., Kossel,H., Morgan,R., Narang,S.A., Ohtsuka,E. and Wells,R.D. (1966) Polynucleotide synthesis and the genetic code. Cold Spring Harbor Symp. Quant. Biol., 31, 39–49. [DOI] [PubMed] [Google Scholar]
- 9.Söll D., Cherayil,J., Jones,D.S., Faulkner,R.D., Hapel,A., Bock,R.M. and Khorana,H.G. (1966) sRNA specificity for codon recognition as studied by the ribosomal binding technique. Cold Spring Harbor Symp. Quant. Biol., 31, 51–61. [DOI] [PubMed] [Google Scholar]
- 10.Crick F.H.C. (1966) Codon–anticodon pairing: the wobble hypothesis. J. Mol. Biol., 19, 548–555. [DOI] [PubMed] [Google Scholar]
- 11.Crick F.H.C. (1966) The Genetic Code: Yesterday, today and tomorrow. Cold Spring Harbor Symp. Quant. Biol., 31, 3–9. [PubMed] [Google Scholar]
- 12.Holley R.W. (1965) Structure of an alanine transfer ribonucleic acid. JAMA, 194, 68–71. [PubMed] [Google Scholar]
- 13.Holley R.W., Apgar,J., Everett,G.A., Madison,J.T., Marquisee,M., Merrill,S.H., Penswick,J.R. and Zamir,A. (1965) Structure of ribonucleic acid. Science, 147, 1462–1465. [DOI] [PubMed] [Google Scholar]
- 14.Söll D. and RajBhandary,U.L. (1967) Studies on polynucleotides. LXXVI. Specificity of transfer RNA for codon recognition as studied by amino acid incorporation. J. Mol. Biol., 29, 113–124. [DOI] [PubMed] [Google Scholar]
- 15.Söll D., Jones,D.S., Ohtsuka,E., Faulkner,R.D., Lohrmann,R., Hayatsu,H. and Khorana,H.G. (1966) Specificity of sRNA for recognition of codons as studied by the ribosomal binding technique. J. Mol. Biol., 19, 556–573. [DOI] [PubMed] [Google Scholar]
- 16.Sueoka N., Kano-Sueoka,T. and Gartland,W.J. (1966) Modification of sRNA and regulation of protein synthesis. Cold Spring Harbor Symp. Quant. Biol., 31, 571–580. [DOI] [PubMed] [Google Scholar]
- 17.Cohn W.E. and Volkin,E. (1951) Nature, 167, 483–485. [Google Scholar]
- 18.Cohn W.E. (1959) 5-Ribosyl uracil, a carbon–carbon ribofuranosyl nucleoside in ribonucleic acids. Biochim. Biophys. Acta, 32, 569–571. [DOI] [PubMed] [Google Scholar]
- 19.Yu C.T. and Allen,F.W. (1959) Studies on an isomer of uridine isolated from ribonucleic acids. Biochim. Biophys. Acta, 32, 393–406. [DOI] [PubMed] [Google Scholar]
- 20.Hall R.H. (1971) The Modified Nucleosides in Nucleic Acids. Columbia University Press, NY. [Google Scholar]
- 21.Agris P.F., Malkiewicz,A., Brown,S., Kraszewski,A., Nawrot,B., Sochacka,E., Everett,K. and Guenther,G. (1995) Site-selected introduction of modified purine and pyrimidine ribonucleosides into RNA by automated phosphoramidite chemistry. Biochimie, 77, 125–134. [DOI] [PubMed] [Google Scholar]
- 22.Agris P.F. (1991) Wobble position modified nucleosides evolved to select transfer RNA codon recognition: a modified-wobble hypothesis. Biochimie, 73, 1345–1349. [DOI] [PubMed] [Google Scholar]
- 23.Crain P.F. and McCloskey,J.A. (1998) Applications of mass spectrometry to the characterization of oligonucleotides and nucleic acids. Curr. Opin. Biotechnol., 9, 25–34. [DOI] [PubMed] [Google Scholar]
- 24.McCloskey J.A., Graham,D.E., Zhou,S., Crain,P.F., Ibba,M., Konisky,J., Söll,D. and Olsen,G.J. (2001) Post-transcriptional modification in archaeal tRNAs: identities and phylogenetic relations of nucleotides from mesophilic and hyperthermophilic Methanococcales. Nucleic Acids Res., 29, 4699–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agris P.F. (1996) The importance of being modified: Roles of modified nucleosides and Mg2+ in RNA structure and function. In Cohn,W. and Moldave,K. (eds), Progress in Nucleic Acid Research and Molecular Biology, Vol. 53, pp. 79–129. [DOI] [PubMed] [Google Scholar]
- 26.Rozenski J., Crain,P.F. and McCloskey,J.A.(1999) The RNA Modification Database: 1999 update. Nucleic Acids Res., 27, 196–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agris P.F. (1980) The Modified Nucleosides of Transfer RNA. Alan R. Liss, Inc., NY. [Google Scholar]
- 28.Agris P.F. and Kopper,R.A. (1983) The Modified Nucleosides of Transfer RNA, II: A Laboratory Manual of Genetic Analyses, Identification and Sequence Determination. Alan R. Liss, Inc., NY. [Google Scholar]
- 29.Agris P.F., Hayden,J., Sierzputowska-Gracz,H., Ditson,S., Degres,J.A., Tempesta,M., Kuo,K.C. and Gehrke,C.W. (1990) Compendium on biological, biochemical, chemical, physical and spectroscopic properties of RNA and DNA nucleosides. In Chromatography and Modification of Nucleosides. Elsevier Publishing Co., Amsterdam, The Netherlands. [Google Scholar]
- 30.Gehrke C.W., Desgres,J.A., Gerhardt,K.O., Agris,P.F., Keith,G., Sierzputowska-Gracz,H., Tempesta,M.S. and Kuo,K.C. (1990) Structural elucidation of nucleosides in nucleic acids. In Chromatography and Modification of Nucleosides. Elsevier Publishing Co., Amsterdam, The Netherlands, pp. 159–223. [Google Scholar]
- 31.Grosjean H. and Benne,R. (1998) Modification and Editing of RNA. American Society for Microbiology Press, Washington, DC. [Google Scholar]
- 32.Kim S.H., Quigley,G.J., Suddath,F.L., McPherson,A., Sneden,D., Kim,J.J., Weinzierl,J. and Rich,A. (1973) Three-dimensional structure of yeast phenylalanine transfer RNA: folding of the polynucleotide chain. Science, 179, 285–288. [DOI] [PubMed] [Google Scholar]
- 33.Nobles K.N., Yarian,C.S., Liu,G., Guenther,R.H. and Agris,P.F. (2002) Highly conserved modified nucleosides influence Mg2+-dependent tRNA folding. Nucleic Acids Res., 30, 4751–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbonavicius J., Durand,J.M. and Björk,G.R. (2002) Three modifications in the D and T arms of tRNA influence translation in Escherichia coli and expression of virulence genes in Shigella flexneri. J. Bacteriol., 184, 5348–5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackman J.E., Montange,R.K., Malik,H.S. and Phizicky,E.M. (2003) Identification of the yeast gene encoding the tRNA m1G methyltransferase responsible for modification at position 9. RNA, 9, 574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferré-D’Amaré A.R. (2003) RNA-modifying enzymes. Curr. Opin. Struct. Biol., 13, 49–55. [DOI] [PubMed] [Google Scholar]
- 37.Agris P.F. (1996) Modified nucleosides in RNA structure and function. In Grant,D.M. and Harris,R.K. (eds), Encyclopedia of NMR (section editor Chan,S.), Wiley, UK, pp. 4151–4158. [Google Scholar]
- 38.Gesteland R.F. and Atkins,J.F. (1996) Recoding: dynamic reprogramming of translation. Annu. Rev. Biochem., 65, 741–768. [DOI] [PubMed] [Google Scholar]
- 39.Baranov P.V., Gesteland,R.F. and Atkins,J.F. (2002) Recoding: translational bifurcations in gene expression. Gene, 286, 187–201. [DOI] [PubMed] [Google Scholar]
- 40.Hohsaka T. and Sisido,M. (2002) Incorporation of non-natural amino acids into proteins. Curr. Opin. Chem. Biol., 6, 809–815. [DOI] [PubMed] [Google Scholar]
- 41.Hohsaka T., Ashizuka,Y., Taira,H., Murakami,H. and Sisido,M. (2001) Incorporation of non-natural amino acids into proteins by using various four-base codons in an Escherichia coli in vitro translation system. Biochemistry, 40, 11060–11064. [DOI] [PubMed] [Google Scholar]
- 42.Chin J.W., Cropp,T.A., Anderson,J.C., Mukherji,M., Zhang,Z. and Schultz,P. (2003) An expanded eukaryotic genetic code. Science, 301, 964–967. [DOI] [PubMed] [Google Scholar]
- 43.HyunBae J., Rubini,M., Jung,G., Wiegand,G., Seifert,M.H., Azim,M.K., Kim,J.S., Zumbusch,A., Holak,T.A., Moroder,L., Huber,R. and Budisa,N. (2003) Expansion of the genetic code enables design of a novel ‘gold’ class of green fluorescent proteins. J. Mol. Biol., 328, 1071–1081. [DOI] [PubMed] [Google Scholar]
- 44.Gallant J., Bonthuis,P. and Lindsley,D. (2003) Evidence that the bypassing ribosome travels through the coding gap. Proc. Natl Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jukes T.H. and Osawa,S. (1990) The genetic code in mitochondria and chloroplasts. Experientia, 46, 1117–1126. [DOI] [PubMed] [Google Scholar]
- 46.Osawa S., Jukes,T.H., Watanabe,K. and Muto,A. (1992) Recent evidence for evolution of the genetic code. Microbiol. Rev., 56, 229–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jukes T.H. (1993) The genetic code—function and evolution. Cell. Mol. Biol. Res., 39, 685–688. [PubMed] [Google Scholar]
- 48.Moura G., Miranda,I., Cheesman,C., Tuite,M.F. and Santos,M.A. (2003) Stop codon decoding in Candida albicans: from non-standard back to standard. Yeast, 19, 727–733. [DOI] [PubMed] [Google Scholar]
- 49.Santos M.A., Ueda,T., Watanabe,K. and Tuite,M.F. (1997) The non-standard genetic code of Candida spp.: an evolving genetic code or a novel mechanism for adaptation? Mol. Microbiol., 26, 423–431. [DOI] [PubMed] [Google Scholar]
- 50.Knight R.D., Landweber,L.F. and Yarus,M. (2001) How mitochondria redefine the code. J. Mol. Evol., 53, 299–313. [DOI] [PubMed] [Google Scholar]
- 51.Zerfass K. and Beier,H. (1992) Pseudouridine in the anticodon GΨA of plant cytoplasmic tRNA(Tyr) is required for UAG and UAA suppression in the TMV-specific context. Nucleic Acids Res., 20, 5911–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tuite M.F. and Santos,M.A. (1996) Codon reassignment in Candida species: an evolutionary conundrum. Biochimie, 78, 993–999. [DOI] [PubMed] [Google Scholar]
- 53.Santos M.A., Ueda,T., Watanabe,K. and Tuite,M.F. (1997) The non-standard genetic code of Candida spp.: an evolving genetic code or a novel mechanism for adaptation? Mol. Microbiol., 26, 423–431. [DOI] [PubMed] [Google Scholar]
- 54.Crain P.F., Alfonzo,J.D., Rozenski,J., Kapushoc,S.T., McCloskey,J.A. and Simpson,L. (2002) Modification of the universally unmodified uridine-33 in a mitochondria-imported edited tRNA and the role of the anticodon arm structure on editing efficiency. RNA, 8, 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore P.B. and Steitz,T.A. (2003) After the ribosome structures: how does peptidyl transferase work? RNA, 9, 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frank J. (2003) Electron microscopy of functional ribosome complexes. Biopolymers, 68, 223–233. [DOI] [PubMed] [Google Scholar]
- 57.Potapov A.P. (1982) A stereospecific mechanism for the aminoacyl-tRNA selection at the ribosome. FEBS Lett., 146, 5–8. [DOI] [PubMed] [Google Scholar]
- 58.vonAhsen U. (1998) Translational fidelity: error-prone versus hyper-accurate ribosomes. Chem. Biol., 5, R3–6. [DOI] [PubMed] [Google Scholar]
- 59.Woodson S.A. and Leontis,N.B. (1998) Structure and dynamics of ribosomal RNA. Curr. Opin. Struct. Biol., 8, 294–300. [DOI] [PubMed] [Google Scholar]
- 60.Ogle J.M., Murphy,F.V., Tarry,M.J. and Ramakrishnan,V. (2002) Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell, 111, 721–732. [DOI] [PubMed] [Google Scholar]
- 61.Yonath A. (2002) High-resolution structures of large ribosomal subunits from mesophilic eubacteria and halophilic archaea at various functional states. Curr. Protein Pept. Sci., 3, 67–78. [DOI] [PubMed] [Google Scholar]
- 62.Vila-Sanjurjo A., Ridgeway,W.K., Seymaner,V., Zhang,W., Santoso,S., Yu,K. and Cate,J.H. (2003) X-ray crystal structures of the WT and a hyper-accurate ribosome from Escherichia coli. Proc. Natl Acad. Sci. USA, 100, 8682–8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogle J.M., Carter,A.P. and Ramakrishnan,V. (2003) Insights into the decoding mechanism from recent ribosome structures. Trends Biochem. Sci., 28, 259–266. [DOI] [PubMed] [Google Scholar]
- 64.Rodnina M.V., Daviter,T., Gromadski,K. and Wintermeyer,W. (2002) Structural dynamics of ribosomal RNA during decoding on the ribosome. Biochimie, 84, 745–754. [DOI] [PubMed] [Google Scholar]
- 65.Ibba M. and Söll,D. (1999) Quality control mechanisms during translation. Science, 286, 1893–1897. [DOI] [PubMed] [Google Scholar]
- 66.Carter A.P., Clemons,W.M.,Jr, Brodersen,D.E., Morgan-Warren,R.J., Wimberly,B.T. and Ramakrishnan,V. (2000) Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature, 407, 340–348. [DOI] [PubMed] [Google Scholar]
- 67.Phelps S.S., Jerinic,O. and Joseph,S. (2002) Universally conserved interactions between the ribosome and the anticodon stem–loop of A site tRNA important for translocation. Mol. Cell., 10, 799–807. [DOI] [PubMed] [Google Scholar]
- 68.Sprinzl M. and Vassilenko,K.S. (2003) Compilation of tRNA sequences and sequences of tRNA genes August 2003 edition. http://www.uni-bayreuth.de/departments/biochemie/trna/. [DOI] [PMC free article] [PubMed]
- 69.Satoh A., Takai,K., Ouchi,R., Yokoyama,S. and Takaku,H. (2000) Effects of anticodon 2′-O-methylations on tRNA codon recognition in an Escherichia coli cell-free translation. RNA, 6, 680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stark H., Rodnina,M.V., Wieden,H.J., Zemlin,F., Wintermeyer,W. and van Heel,M. (2002) Ribosome interactions of aminoacyl-tRNA and elongation factor Tu in the codon-recognition complex. Nat. Struct. Biol., 9, 849–854. [DOI] [PubMed] [Google Scholar]
- 71.Wilson D.N. and Nierhaus,K.H. (2003) The ribosome through the looking glass. Angew. Chem. Int. Ed. Engl., 42, 3464–3486. [DOI] [PubMed] [Google Scholar]
- 72.Kurland C.G. (1992) Translational accuracy and the fitness of bacteria. Annu. Rev. Genet., 26, 29–50. [DOI] [PubMed] [Google Scholar]
- 73.Pape T., Wintermeyer,W. and Rodnina,M.V. (1998) Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E.coli ribosome. EMBO J., 17, 7490–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodnina M.V. and Wintermeyer,W. (2001) Fidelity of aminoacyl tRNA selection on the ribosome: kinetic and structural mechanisms. Annu. Rev. Biochem., 70, 415–435. [DOI] [PubMed] [Google Scholar]
- 75.Rodina M.V. and Wintermeyer,W. (2001) Ribosome fidelity: tRNA discrimination, proofreading and induced fit. Trends Biochem. Sci., 26, 124–130. [DOI] [PubMed] [Google Scholar]
- 76.Lim V.I. and Curran,J.F. (2001) Analysis of codon:anticodon interactions within the ribosome provides new insights into codon reading and the genetic code structure. RNA, 7, 942–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim V.I. (1994) Analysis of action of wobble nucleoside modifications on codon–anticodon pairing within the ribosome. J. Mol. Biol., 240, 8–19. [DOI] [PubMed] [Google Scholar]
- 78.Sibler A.P., Dirheimer,G. and Martin,R.P. (1986) Codon reading patterns in Saccharomyces cerevisiae mitochondria based on sequences of mitochondrial tRNAs. FEBS Lett., 194, 131–138. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe Y., Tsurui,H., Ueda,T., Furusihima-Shimogawara,R., Takamiya,S., Kita,K., Nishikawa,K. and Watanabe,K. (1997) Primary sequence of mitochondrial tRNA(Arg) of a nematode Ascaris suum: occurrence of unmodified adenosine at the first position of the anticodon. Biochim. Biophys. Acta, 1350, 119–122. [DOI] [PubMed] [Google Scholar]
- 80.Andachi Y., Yamao,F., Muto,A. and Osawa,S. (1989) Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J. Mol. Biol., 209, 37–54. [DOI] [PubMed] [Google Scholar]
- 81.Chen P., Qian,Q., Zhang,S., Isaksson,L.A. and Björk,G.R. (2002) A cytosolic tRNA with an unmodified adenosine in the wobble position reads a codon ending with the non-complementary nucleoside cytidine. J. Mol. Biol., 317, 481–492. [DOI] [PubMed] [Google Scholar]
- 82.Lim V.I. and Venclovas,C. (1992) Codon–anticodon pairing. A model for interacting codon–anticodon duplexes located at the ribosomal A- and P-sites. FEBS Lett., 313, 133–137. [DOI] [PubMed] [Google Scholar]
- 83.Nishimura S. (1972) Minor components in transfer RNA: their characterization, location and function. Prog. Nucleic Acid Res. Mol. Biol., 12, 49–85. [PubMed] [Google Scholar]
- 84.Sierzputowska-Gracz H., Sochacka,E., Malkiewicz,A., Kuo,K., Gehrke,C.W. and Agris,P.F. (1987) Chemistry and structure of modified uridines in the anticodon, wobble position of transfer RNA are determined by thiolation. J. Am. Chem. Soc., 109, 7171–7177. [Google Scholar]
- 85.Yokoyama S., Watanabe,T., Murao,K., Ishikura,H., Yamaizumi,Z., Nishimura,S. and Miyazawa,T. (1985) Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl Acad. Sci. USA, 82, 4905–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weissenbach J. and Grosjean,H. (1981) Effect of threonylcarbamoyl modification (t6A) in yeast tRNA Arg III on codon–anticodon and anticodon–anticodon interactions. A thermodynamic and kinetic evaluation. Eur. J. Biochem., 116, 207–213. [DOI] [PubMed] [Google Scholar]
- 87.Miller J.P., Hussain,Z. and Schweizer,M.P. (1976) The involvement of the anticodon adjacent modified nucleoside N-(9-(β-D-ribofuranosyl) purine-6-ylcarbamoyl)-threonine in the biological function of E.coli tRNAIle. Nucleic Acids Res., 3, 1185–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Houssier C., Degee,P., Nicoghosian,K. and Grosjean,H. (1988) Effect of uridine dethiolation in the anticodon triplet of tRNAGlu on its association with tRNAPhe. J. Biomol. Struct. Dyn., 5, 1259–1266. [DOI] [PubMed] [Google Scholar]
- 89.Smith D.W., McNamara,A.L., Rice,M. and Hatfield,D.L. (1981) The effects of a post-transcriptional modification on the function of tRNALys isoaccepting species in translation. J. Biol. Chem., 256, 10033–10036. [PubMed] [Google Scholar]
- 90.Smith D.W. and Hatfield,D.L. (1986) Effects of post-transcriptional base modifications on the site-specific function of transfer RNA in eukaryote translation. J. Mol. Biol. 189, 663–671. [DOI] [PubMed] [Google Scholar]
- 91.Hagervall T.G., Pomerantz,S.C. and McCloskey,J.A. (1998) Reduced misreading of asparagine codons by Escherichia coli tRNALys with hypomodified derivatives of 5-methylaminomethyl-2-thiouridine in the wobble position. J. Mol. Biol., 284, 33–42. [DOI] [PubMed] [Google Scholar]
- 92.Ashraf S.S., Sochacka,E., Cain,R., Guenther,R. Malkiewicz,A. and Agris,P.F. (1999) Single atom modification (O→S) of tRNA confers ribosome binding. RNA, 5, 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yarian C., Marszalek,M., Sochacka,E., Malkiewicz,A., Guenther,R., Miskiewicz,A. and Agris,P.F. (2000) Modified nucleoside dependent Watson–Crick and wobble codon binding by tRNALysUUU species. Biochemistry, 39, 13390–13395. [DOI] [PubMed] [Google Scholar]
- 94.Yarian C., Townsend,H., Czestkowski,W., Sochacka,E., Malkiewicz,A.J., Guenther,R., Miskiewicz,A. and Agris,P.F. (2002) Accurate translation of the genetic code depends on tRNA modified nucleosides. J. Biol. Chem., 277, 16391–16395. [DOI] [PubMed] [Google Scholar]
- 95.Kruger M.K., Pedersen,S., Hagervall,T.G. and Sorensen,M.A. (1998) The modification of the wobble base of tRNAGlu modulates the translation rate of glutamic acid codons in vivo. J. Mol. Biol., 284, 621–631. [DOI] [PubMed] [Google Scholar]
- 96.vonAhsen U., Green,R., Schroeder,R. and Noller,H.F. (1997) Identification of 2′-hydroxyl groups required for interaction of a tRNA anticodon stem–loop region with the ribosome. RNA, 3, 49–56. [PMC free article] [PubMed] [Google Scholar]
- 97.Sekiya T., Takeishi,K. and Ukita,T. (1969) Specificity of yeast glutamic acid transfer RNA for codon recognition. Biochim. Biophys. Acta, 182, 411–426. [DOI] [PubMed] [Google Scholar]
- 98.Weissenbach J. and Dirheimer,G. (1978) Pairing properties of the methylester of 5-carboxymethyl uridine in the wobble position of yeast tRNA3Arg. Biochim. Biophys. Acta, 518, 530–534. [DOI] [PubMed] [Google Scholar]
- 99.Agris P.F., Sierzputowska-Gracz,H., Smith,W., Malkiewicz,A., Sochacka,E. and Nawrot,B. (1992) Thiolation of uridine carbon-2 restricts the motional dynamics of the transfer RNA wobble position nucleoside. J. Am. Chem. Soc., 114, 2652–2656. [Google Scholar]
- 100.Smith W.S., Sierzputowska-Gracz,H., Sochacka,E., Malkiewicz,A. and Agris,P.F. (1992) Chemistry and structure of modified uridine dinucleosides are determined by thiolation. J. Am. Chem. Soc., 114, 7989–7997. [Google Scholar]
- 101.Harrington K.M., Nazarenko,I.A., Dix,D.B., Thompson,R.C. and Uhlenbeck,O.C. (1993) In vitro analysis of translational rate and accuracy with an unmodified tRNA. Biochemistry, 32, 7617–7622. [DOI] [PubMed] [Google Scholar]
- 102.Urbonavicius J., Qian,Q., Durand,J.M., Hagervall,T.G. and Björk,G.R. (2001) Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J., 20, 4863–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stuart J.W., Gdaniec,Z., Guenther,R., Marszalek,M., Sochacka,E., Malkiewicz,A. and Agris,P.F. (2000) Functional anticodon architecture of human tRNALys3 includes disruption of intra-loop hydrogen bonding by the naturally occurring amino acid modification, t6A. Biochemistry, 39, 13396–13404. [DOI] [PubMed] [Google Scholar]
- 104.Meier F., Suter,B., Grosjean,H., Keith,G. and Kubli,E. (1985) Queuosine modification of the wobble base in tRNAHis influences ‘in vivo’ decoding properties. EMBO J., 4, 823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dineshkumar T.K., Thanedar,S., Subbulakshmi,C. and Varshney,U. (2002) An unexpected absence of queuosine modification in the tRNAs of an Escherichia coli B strain. Microbiology, 148, 3779–3787. [DOI] [PubMed] [Google Scholar]
- 106.Morris R.C., Brown,K.G. and Elliott,M.S. (1999) The effect of queuosine on tRNA structure and function. J. Biomol. Struct. Dyn., 16, 757–774. [DOI] [PubMed] [Google Scholar]
- 107.Muramatsu T., Nishikawa,K., Nemoto,F., Kuchino,Y., Nishimura,S., Miyazawa,T. and Yokoyama,S. (1988) Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature, 336, 179–181. [DOI] [PubMed] [Google Scholar]
- 108.Wilson R.K. and Roe,B.A. (1989) Presence of the hypermodified nucleotide N6-(delta 2-isopentenyl)-2-methylthioadenosine prevents codon misreading by Escherichia coli phenylalanyl-transfer RNA. Proc. Natl Acad. Sci. USA, 86, 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buck M. and Ames,B.N. (1984) A modified nucleotide in tRNA as a possible regulator of aerobiosis: synthesis of cis-2-methyl-thioribosylzeatin in the tRNA of Salmonella. Cell, 36, 523–531. [DOI] [PubMed] [Google Scholar]
- 110.Esberg B. and Björk,G.R. (1995) The methylthio group (ms2) of N6-(4-hydroxyisopentenyl)-2-methylthioadenosine (ms2io6A) present next to the anticodon contributes to the decoding efficiency of the tRNA. J. Bacteriol., 177, 1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Y., Sierzputowska-Gracz,H., Guenther,R., Everett,K. and Agris,P.F. (1993) Methyl-5-cytidine is required for cooperative binding of Mg2+ and a conformational transition at the anticodon stem-loop of yeast phenylalanine tRNA. Biochemistry, 32, 10249–10253. [DOI] [PubMed] [Google Scholar]
- 112.Ashraf S.S., Guenther,R.H., Ansari,G., Malkiewicz,A., Sochacka,E. and Agris,P.F. (2000) Role of modified nucleosides of yeast tRNAPhe in ribosomal binding. Cell Biochem. Biophys., 33, 241–252. [DOI] [PubMed] [Google Scholar]
- 113.Stahl G., McCarty,G.P. and Farabaugh,P.J. (2002) Ribosome structure: revisiting the connection between translational accuracy and unconventional decoding. Trends Biochem. Sci., 27, 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Curran J.F. (1993) Analysis of effects of tRNA:message stability on frameshift frequency at the Escherichia coli RF2 programmed frameshift site. Nucleic Acids Res., 21, 1837–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hansen T.M., Baranov,P.V., Ivanov,I.P., Gesteland,R.F. and Atkins,J.F. (2003) Maintenance of the correct open reading frame by the ribosome. EMBO Rep., 4, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Urbonavicius J., Stahl,G., Durand,J.M., Ben Salem,S.N., Qian,Q., Farabaugh,P.J. and Björk,G.R. (2003) Transfer RNA modifications that alter +1 frameshifting in general fail to affect –1 frameshifting. RNA, 9, 760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brierley I., Meredith,M.R., Bloys,A.J. and Hagervall,T.G. (1997) Expression of a coronovirus frameshift signal in Escherichia coli: Influence of tRNA modification on frameshifting. J. Mol. Biol., 270, 360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gefter M.L. and Russell,R.L. (1969) Role modifications in tyrosine transfer RNA: a modified base affecting ribosome binding. J. Mol. Biol., 39, 145–157. [DOI] [PubMed] [Google Scholar]
- 119.Yarian C.S., Cain,R., Basti,M.M., Ansari,G., Guenther,R.H., Sochacka,E., Malkiewicz,A. and Agris,P.F. (1999) Structural and functional roles of the N1- and N3-protons of Ψ at tRNA’s position 39. Nucleic Acids Res., 27, 3543–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davis D.R., Veltri,A. and Nielsen,L. (1998) An RNA model system for investigation of pseudouridine stabilization of the codon–anticodon interaction in tRNALys, tRNAHis and tRNATyr. J. Biomol. Struct. Dyn., 15, 1121–1132. [DOI] [PubMed] [Google Scholar]
- 121.Lecointe F., Namy,O., Hatin,I., Simos,G., Rousset,J.P. and Grosjean,H. (2002) Lack of pseudouridine 38/39 in the anticodon arm of yeast cytoplasmic tRNA decreases in vivo recoding efficiency. J. Biol. Chem., 277, 30445–30453. [DOI] [PubMed] [Google Scholar]
- 122.Takai K. and Yokoyama,S. (2003) Roles of 5-substituents of tRNA wobble uridines in the recognition of purine-ending codons. Nucleic Acids Res., 31, 6383–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mitra S.K., Lustig,F., Akesson,B., Axberg,T., Elias,P. and Lagerkvist,U. (1979) Relative efficiency of anticodons in reading the valine codons during protein synthesis in vitro. J. Biol. Chem., 254, 6397–6401. [PubMed] [Google Scholar]
- 124.Takai K., Okumura,S., Hosono,K., Yokoyama,S. and Takaku,H. (1999) A single uridine modification at the wobble position of an artificial tRNA enhances wobbling in an Escherichia coli cell-free translation system. FEBS Lett., 447, 1–4. [DOI] [PubMed] [Google Scholar]
- 125.Takai K., Takaku,H. and Yokoyama,S. (1996) Codon-reading specificity of an unmodified form of Escherichia coli tRNA1Ser in cell-free protein synthesis. Nucleic Acids Res., 24, 2894–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Björk G.R., Wikstrom,P.M. and Bystrom,A.S. (1989) Prevention of translational frameshifting by the modified nucleoside 1-methylguanosine. Science, 244, 986–989. [DOI] [PubMed] [Google Scholar]
- 127.Björk G.R. and Nilsson,K. (2003) 1-methylguanosine-deficient tRNA of Salmonella enterica serovar Typhimurium affects thiamine metabolism. J. Bacteriol., 185, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li J., Esberg,B., Curran,J.F. and Björk,G.R. (1997) Three modified nucleosides present in the anticodon stem and loop influence the in vivo aa-tRNA selection in a tRNA-dependent manner. J. Mol. Biol., 271, 209–221. [DOI] [PubMed] [Google Scholar]
- 129.Charette M. and Gray,M.W. (2002) Pseudouridine in RNA: what, where, how and why. IUBMB Life, 49, 341–351. [DOI] [PubMed] [Google Scholar]
- 130.Marechal-Drouard L., Weil,J.H. and Guillemaut,P. (1988) Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res., 16, 4777–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marechal-Drouard L., Neuburger,M., Guillemaut,P., Douce,R., Weil,J.H. and Dietrich,A. (1990) A nuclear-encoded potato (Solanum tuberosum) mitochondrial tRNA(Leu) and its cytosolic counterpart have identical nucleotide sequences. FEBS Lett., 262, 170–172. [DOI] [PubMed] [Google Scholar]
- 132.Schneider A., McNally,K.P. and Agabian,N. (1994) Nuclear-encoded mitochondrial tRNAs of Trypanosoma brucei have a modified cytidine in the anticodon loop. Nucleic Acids Res., 22, 3699–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rusconi C.P. and Cech,T.R. (1996) The anticodon is the signal sequence for mitochondrial import of glutamine tRNA in Tetrahymena. Genes Dev., 10, 2870–2880. [DOI] [PubMed] [Google Scholar]
- 134.Kaneko T., Suzuki,T., Kapushoc,S.T., Rubio,M.A., Ghazvini,J., Watanabe,K., Simpson,L. and Suzuki,T. (2003) Wobble modification differences and subcellular localization of tRNAs in Leishmania tarentolae: implication for tRNA sorting mechanism. EMBO J., 22, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tomita K., Ueda,T., Ishiwa,S., Crain,P.F., McCloskey,J.A. and Watanabe,K. (1999) Codon reading patterns in Drosophila melanogaster mitochondria based on their tRNA sequences: a unique wobble rule in animal mitochondria. Nucleic Acids Res., 27, 4291–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jukes T.H. and Osawa,S. (1990) The genetic code in mitochondria and chloroplasts. Experientia, 46, 1117–1126. [DOI] [PubMed] [Google Scholar]
- 137.Knight R.D., Landweber,L.F. and Yarus,M. (2001) How mitochondria redefine the code. J. Mol. Evol., 53, 299–313. [DOI] [PubMed] [Google Scholar]
- 138.Yasukawa T., Suzuki,T., Ishii,N., Ohta,S. and Watanabe,K. (2001) Wobble modification defect in tRNA disturbs codon–anticodon interaction in a mitochondrial disease. EMBO J., 20, 4794–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yarus M. and Smith,D. (1995) In Söll,D. and Rajbhandary,U. (eds), tRNA: Structure, Biophysics and Function. American Society for Microbiology, Washington, DC, pp. 443–468. [Google Scholar]
- 140.Yarus M., Valle,M. and Frank,J. (2003) A twisted tRNA intermediate sets the threshold for decoding. RNA, 9, 384–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Valle M., Sengupta,J., Swami,N.K., Grassucci,R.A., Burkhardt,N., Nierhaus,K.H., Agrawal,R.K. and Frank,J. (2002) Cryo-EM reveals an active role for aminoacyl-tRNA in the accommodation process. EMBO J., 21, 3557–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stuart J.W., Koshlap,K.M., Guenther,R. and Agris,P.F. (2003) Naturally-occurring modification restricts the anticodon domain conformational space of tRNAPhe. J. Mol. Biol., 334, 901–918. [DOI] [PubMed] [Google Scholar]
- 143.Striker G., Labuda,D. and Vega-Martin,M.C. (1989) The three dimensional conformations of the anticodon loop of yeast tRNAPhe. J. Biomol. Struct. Dyn., 7, 235–255. [DOI] [PubMed] [Google Scholar]
- 144.Schmidt P.G., Sierzputowska-Gracz,H. and Agris,P.F. (1987) Internal motions in yeast phenylalanine transfer RNA from 13C NMR relaxation rates of modified base methyl groups: A model-free approach. Biochemistry, 26, 8529–8534. [DOI] [PubMed] [Google Scholar]
- 145.Basti M.M., Stuart,J.W., Lam,A.T., Guenther,R. and Agris,P.F. (1996) Design, biological activity and NMR solution structure of a DNA analog of yeast tRNAPhe anticodon domain. Nat. Struct. Biol., 3, 38–44. [DOI] [PubMed] [Google Scholar]
- 146.Cabello-Villegas J., Winkler,M.E. and Nikonowicz,E.P. (2000) Solution conformations of unmodified and A37N6-dimethylallyl modified anticodon stem–loops of Escherichia coli tRNAPhe. J. Mol. Biol., 319, 1015–1034. [DOI] [PubMed] [Google Scholar]
- 147.Dao V., Guenther,G., Malkiewicz,A., Nawrot,B., Sochacka,E., Kraszewski,A., Everett,K. and Agris,P.F. (1994) Ribosome binding of DNA analogs to tRNA requires base modifications and supports the ‘Extended Anticodon’. Proc. Natl Acad. Sci. USA, 91, 2125–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Carlson B.A., Mushinski,J.F., Henderson,D.W., Kwon,S.Y., Crain,P.F., Lee,B.J. and Hatfield,D.L. (2001) 1-Methylguanosine in place of Y base at position 37 in phenylalanine tRNA is responsible for its shiftiness in retroviral ribosomal frame shifting. Virology, 279, 130–135. [DOI] [PubMed] [Google Scholar]
- 149.Sundaram M., Durant,P.C. and Davis,D.R. (2000) Hypermodified nucleosides in the anticodon of tRNALys stabilize a canonical U-turn structure. Biochemistry, 39, 12575–12584. [DOI] [PubMed] [Google Scholar]
- 150.Benas P., Bec,G., Keith,G., Marquet,R., Ehresmann,C., Ehresmann,B. and Dumas,P. (2000) The crystal structure of HIV reverse-transcription primer tRNA(Lys,3) shows a canonical anticodon loop. RNA, 6, 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sonawane K.D., Sonavane,U.B. and Tewari,R. (2002) Conformational preferences of anticodon 3′-adjacent hypermodified nucleic acid base cis-or trans-zeatin and its 2-methylthio derivative, cis- or trans-ms(2)zeatin. J. Biomol. Struct. Dyn., 19, 637–648. [DOI] [PubMed] [Google Scholar]
- 152.Kierzek E. and Kierzek,R. (2003) The thermodynamic stability of RNA duplexes and hairpins containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. Nucleic Acids Res., 31, 4472–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Akashi H. (2001) Gene expression and molecular evolution. Curr. Opin. Genet. Dev., 11, 660–666. [DOI] [PubMed] [Google Scholar]
- 154.Roe B.A., Stankiewicz,A.F., Rizi,H.L., Weisz,C., DiLauro,M.N., Pike,D., Chen,C.Y. and Chen,E.Y. (1979) Comparison of rat liver and Walker 256 carcinosarcoma tRNAs. Nucleic Acids Res., 6, 673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Raba M., Limburg,K., Burghagen,M., Katze,J.R., Simsek,M., Heckman,J.E., Rajbhandary,U.L. and Gross,H.J. (1979) Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur. J. Biochem., 97, 305–318. [DOI] [PubMed] [Google Scholar]
- 156.Shindo-Okada N., Kuchino,Y., Harada,F., Okada,N. and Nishimura,S. (1981) Biological and structural differences between tRNAVal species isolated from rat ascites hepatoma cells and normal rat liver. J. Biochem. (Tokyo), 90, 535–544. [DOI] [PubMed] [Google Scholar]
- 157.Shindo-Okada N., Terada,M. and Nishimura,S. (1981) Changes in amount of hypo-modified tRNA having guanine in place of queuine during erythroid differentiation of murine erythroleukemia cells. Eur. J. Biochem., 115, 423–428. [DOI] [PubMed] [Google Scholar]
- 158.Kuchino Y., Shindo-Okada,N., Ando,N., Watanabe,S. and Nishimura,S. (1981) Nucleotide sequences of two aspartic acid tRNAs from rat liver and rat ascites hepatoma. J. Biol. Chem., 256, 9059–9062. [PubMed] [Google Scholar]
- 159.Grunberger D., Pergolizzi,R.G., Kuchino,Y., Mushinski,J.F. and Nishimura,S. (1983) Alterations in post-transcriptional modification of the Y base in phenylalanine tRNA from tumor cells. Recent Results Cancer Res., 84, 133–145. [DOI] [PubMed] [Google Scholar]
- 160.Morris R.C., Brooks,B.J., Hart,K.L. and Elliott,M.S. (1996) Modulation of queuine uptake and incorporation into tRNA by protein kinase C and protein phosphatase. Biochim. Biophys. Acta, 1311, 124–132. [DOI] [PubMed] [Google Scholar]
- 161.Munz P., Leopold,U., Agris,P. and Kohli,J. (1981) In vivo decoding rules in Schizosaccharomyces pombe are at variance with in vitro data. Nature, 294, 187–188. [DOI] [PubMed] [Google Scholar]
- 162.Heyer W.D., Thuriaux,P., Kohli,J., Ebert,P., Kersten,H., Gehrke,C., Kuo,K.C. and Agris,P.F. (1984) An antisuppressor mutation of S.pombe affects the posttranscriptional modification of the wobble base in the anticodon of tRNAs. J. Biol. Chem., 259, 2856–2862. [PubMed] [Google Scholar]
- 163.Grossenbacher A.-M., Stadelmann,B., Heyer,W.-D., Thuriaux,P., Kohli,J., Smith,C., Agris,P.F., Kuo,K.C. and Gehrke,C. (1986) Antisuppressor mutations and sulphur carrying nucleosides in transfer RNAs of Schizosaccharomyces pombe. J. Biol. Chem., 261, 16351–16355. [PubMed] [Google Scholar]
- 164.Sylvers L.A., Rogers,K.C., Shimizu,M., Ohtsuka,E. and Söll,D. (1993) A 2-thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl-tRNA synthetase. Biochemistry, 32, 3836–3841. [DOI] [PubMed] [Google Scholar]
- 165.Rogers K.C., Crescenzo,A.T. and Söll,D. (1995) Aminoacylation of transfer RNAs with 2-thiouridine derivatives in the wobble position of the anticodon. Biochimie, 77, 66–74. [DOI] [PubMed] [Google Scholar]
- 166.Madore E., Florentz,C., Giege,R., Sekine,S., Yokoyama,S. and Lapointe,J. (1999) Effect of modified nucleotides on Escherichia coli tRNAGlu structure and on its aminoacylation by glutamyl-tRNA synthetase. Predominant and distinct roles of the mnm5 and s2 modifications of U34. Eur. J. Biochem., 266, 1128–1135. [DOI] [PubMed] [Google Scholar]
- 167.Beuning P.J. and Musier-Forsyth,K. (1999) Transfer RNA recognition by aminoacyl-tRNA synthetases. Biopolymers, 52, 1–28. [DOI] [PubMed] [Google Scholar]
- 168.Nazarenko I.A., Harrington,K.M. and Uhlenbeck,O.C. (1994) Many of the conserved nucleotides of tRNAPhe are not essential for ternary complex formation and peptide elongation. EMBO J., 13, 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sampson J.R., Behlen,L.S., DiRenzo,A.B. and Uhlenbeck,O.C. (1992) Recognition of yeast tRNAPhe by its cognate yeast phenylalanyl-tRNA synthetase: An analysis of specificity. Biochemistry, 31, 4161–4167. [DOI] [PubMed] [Google Scholar]
- 170.Patton J.R. and Padgett,R.W. (2003) Caenorhabditis elegans pseudouridine synthase 1 activity in vivo: tRNA is a substrate, but not U2 small nuclear RNA. Biochem. J., 372, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Urbonavicius J., Durand,J.M. and Björk,G.R. (2002) Three modifications in the D and T arms of tRNA influence translation in Escherichia coli and expression of virulence genes in Shigella flexneri. J. Bacteriol., 184, 5348–5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Persson B.C. (1993) Modification of tRNA as a regulatory device. Mol. Microbiol., 8, 1011–1016. [DOI] [PubMed] [Google Scholar]
- 173.Björk G.R. and Isaksson,L.A. (1970) Isolation of mutants of Escherichia coli lacking 5-methyluracil in transfer ribonucleic acid or 1-methylguanine in ribosomal RNA. J. Mol. Biol., 51, 83–100. [DOI] [PubMed] [Google Scholar]
- 174.Björk G.R. and Neidhardt,F.C. (1975) Physiological and biochemical studies on the function of 5-methyluridine in the transfer ribonucleic acid of Escherichia coli. J. Bacteriol., 124, 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Persson B.C., Gustafsson,C., Berg,D.E. and Bjork,G.R. (1992) The gene for a tRNA modifying enzyme, m5U54-methyltransferase, is essential for viability in Escherichia coli. Proc. Natl Acad. Sci. USA, 89, 3995–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Johansson M.J. and Bystrom,A.S. (2002) Dual function of the RNA (m(5)U54) methyltransferase in tRNA maturation. RNA, 8, 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kinghorn S.M., O’Byrne,C.P., Booth,I.R. and Stansfield,I. (2002) Physiological analysis of the role of truB in Escherichia coli: a role for tRNA modification in extreme temperature resistance. Microbiology, 148, 3511–3520. [DOI] [PubMed] [Google Scholar]
- 178.Cabedo H., Macian,F., Villarroya,M., Escudero,J.C., Martinez-Vicente,M., Knecht,E. and Armengod,M.E. (1999) The Escherichia coli trmE (mnmE) gene, involved in tRNA modification, codes for an evolutionarily conserved GTPase with unusual biochemical properties. EMBO J., 18, 7063–7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Murgola E.J. (1990) Suppression and the code: beyond codons and anticodons. Experientia, 46, 1134–1141. [DOI] [PubMed] [Google Scholar]
- 180.Ericson J.U. and Björk,G.R. (1991) tRNA anticodons with the modified nucleoside 2-methylthio-N6-(4-hydroxyisopentenyl)adenosine distinguish between bases 3′ of the codon. J. Mol. Biol., 218, 509–516. [DOI] [PubMed] [Google Scholar]
- 181.Schwartz R. and Curran,J.F. (1997) Analyses of frameshifting at UUU-pyrimidine sites. Nucleic Acids Res., 25, 2005–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kanaya S., Yamada,Y., Kudo,Y. and Ikemura,T. (1999) Studies of codon usage and tRNA genes of 18 unicellular organisms and quantification of Bacillus subtilis tRNAs: gene expression level and species-specific diversity of codon usage based on multivariate analysis. Gene, 238, 143–156. [DOI] [PubMed] [Google Scholar]
- 183.Percudiani R. (2001) Restricted wobble rules for eukaryotic genomes. Trends Genet., 17, 133–135. [DOI] [PubMed] [Google Scholar]
- 184.Marck C. and Grosjean,H. (2002) tRNonmics analysis of tRNA genes from 50 genomes of Eukarya, Archaea and Bacteria reveals anticodon-sparing strategies and domain-specific features. RNA, 8, 1189–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]