Abstract
Objectives
Surgical excision is the standard strategy for managing liver metastases from colorectal carcinoma. The achievement of negative (R0) margins is a major determinant of disease-free survival in these patients. Current imaging techniques are of limited value in achieving this goal. A new approach to the intraoperative detection of colorectal liver metastatic tissue based on the emission of indocyanine green (ICG) fluorescence was evaluated.
Methods
A total of 25 consecutive patients with liver metastases from primary colorectal cancers who were eligible for liver resection received a bolus of ICG (0.5 mg/kg body weight) 24 h before surgery. During surgery, ICG fluorescence, which accumulates around lesions as a result of defective biliary clearance, was detected with a near-infrared camera system, the Photodynamic Eye (PDE). Numbers of lesions detected by, respectively, PDE + ICG, intraoperative ultrasound (IOUS) and preoperative computed tomography (CT) were recorded.
Results
The near-infrared camera plus ICG revealed a total of 77 metastatic liver nodules. Preoperative CT demonstrated 45 (58.4%) and IOUS showed 55 (71.4%). Preoperative CT and IOUS alone were inferior to the combined use of PDE + ICG and IOUS in the detection of lesions of ≤3 mm in size.
Conclusions
This experience suggests that PDE + ICG, combined with IOUS, may represent a safe and effective tool for ensuring the complete surgical eradication of liver metastases from colorectal cancer.
Introduction
Colorectal carcinoma (CRC) is one of the most commonly occurring tumours throughout the world.1 The prognosis in CRC has improved, mainly as a result of the integrated use of oncological and surgical therapies, but the development of liver metastases is still a frequent event in the natural history of the disease.2,3 In a significant percentage of patients, the liver is the sole site of seeding.3,4 The development and progression of hepatic metastases have been widely studied in the field of surgery.5 Left untreated, these lesions are associated with a median survival of about 5 months.3–5 In the 1980s, Adson and van Heerden demonstrated that liver metastases could be potentially cured by liver resection,6 and subsequently surgery became the reference-standard treatment for these lesions.7 However, although surgery did appear to offer the best chance of cure, only about 20% of patients with liver metastases were eligible for resection.8 The percentage is not much higher today, although recent studies show that resectability rates can be increased with the use of new technologies, drugs and surgical procedures.9–13 The contraindications for liver resection defined in the 1980s are no longer valid: patients with more than three metastatic nodules or nodules of >5 cm in diameter can now be safely treated with surgical resection. Recent studies indicate that liver surgery, including extensive resection, is associated with mortality rates of 1–2%.14 Survival rates have also improved and several large studies have shown disease-free survival rates of around 45%.15–22
There is widespread agreement that one single variable has a very significant impact on survival in this setting: the achievement of negative (R0) resection margins.23–26 This requires the complete removal of all tumour so that microscopic examination of the margins shows no neoplastic cells. The presence of residual foci of tumour cells, whether they are macroscopic or microscopic, is associated with tumour recurrence and lower survival rates.24 The importance of radical tumour removal is undeniable, although the optimal dimensions of the tumour-free margin are still a topic of debate.24
This is a complicated problem because small nodules can be located at varying distances from larger metastatic lesions, and the resolution offered by current imaging techniques may not be sufficient to detect them. Computed tomography (CT), magnetic resonance imaging and ultrasonography (US) are the mainstays of liver tumour detection and staging.27,28 All have a high degree of accuracy and the preoperative use of these imaging techniques is useful in planning a liver resection. However, they can easily miss lesions with diameters of ≤ 3 mm and they provide still pictures only, which are of limited value as a navigation tool.
Intraoperative US (IOUS) is currently regarded as the reference-standard method of guiding liver resection. It offers the undeniable advantage of real-time visualization, but it also has several shortcomings: (i) it cannot detect lesions of ≤ 3 mm in size; (ii) its accuracy is highly dependent on the skill and experience of the operator; (iii) the images it provides are two- rather than three-dimensional, and (iv) there is a superficial blind area about 1 cm under the liver surface, which is especially problematic in colorectal liver metastases as these are mostly located on the surface of the liver parenchyma. The introduction of acoustic contrast enhancement is an appealing evolution, but the contrast effect is too short-lived to be of real value as a navigation tool during standard liver resection, which can take 2–6 h.
Recently, a new approach based on the emission of indocyanine green (ICG) fluorescence has been used to improve the intraoperative detection of neoplastic tissue.29,30 The aim of the present study was to test the hypothesis that this system could be used synergistically with IOUS to detect metastatic liver lesions in patients with CRC. Previous studies have shown that the combined use of both techniques can overcome the limitations of each and identify a substantial number of lesions that are not revealed by IOUS alone.29
Materials and methods
Patient characteristics
From August 2011 to June 2012, 25 patients (Table 1) with liver metastases from primary CRC were enrolled at the IRCCS (Istituto di Recovero e Cura a Carattere Scientifico) San Matteo General Hospital in Pavia. All had been previously evaluated by the hospital's multidisciplinary liver team and deemed eligible for resection with curative intent. The decisions were based on imaging findings (multidetector-row CT and contrast-enhanced US) and histological examination of US-guided percutaneous biopsies of a sample liver lesion. Patients were excluded if enrolment interviews revealed evidence of allergies to iodine or ICG. Written informed consent was obtained from each study participant.
Table 1.
Characteristics of patients included in the study (n = 25)
| Patient characteristics | ||
|---|---|---|
| Age, years, median (range) | 61.5 (42–87) | |
| Gender, n | Male | 17 |
| Female | 8 | |
| Preoperative chemotherapy, n | Yes | 13 |
| No | 12 | |
| Lesions (n = 78) | ||
| Synchronous | 38 | |
| Metachronous | 40 | |
Administration of ICG and fluorescence imaging
At 24 h prior to surgery, each patient was given an i.v. dose of ICG (0.5 mg/kg body weight). After 24 h, normal liver parenchyma will have washed out most of the ICG. The dye is retained as a fluorescent rim that is found in stromal tissue in the transition area between tumour and normal liver tissue in all liver metastases. During surgery, such fluorescence was detected with a near-infrared (NIR) camera system, the Photodynamic Eye (PDE) (Hamamatsu Photonics KK, Hamamatsu, Japan), which activates the ICG fluorescence with a series of diodes that emit light at a wavelength of 760 nm while cutting off emissions below 820 nm. The emissions are detected with a charge-coupled device. For intraoperative use, the unit was wrapped in a disposable, sterile drape, and the real-time video output of the system was displayed on a monitor next to that of the US scanner.
Surgical procedure and intraoperative lesion detection
After standard transverse laparotomy, the liver was freed from the surrounding tissues by dissection of the ligaments and vena cava. The liver hilum was dissected and circled with a tape for an eventual Pringle manoeuvre. The surgeon then performed a standard IOUS examination and PDE assessment of ICG fluorescence (PDE + ICG). The surgical procedure was then performed under dual US and PDE guidance. The parenchyma was dissected using a Cavitron ultrasonic surgical aspirator and the Kelly clamp crush technique. The resection planes were examined with IOUS and PDE + ICG during and after resection to ensure that there was no residual neoplastic tissue. Blood vessels and bile ducts of >5 mm in diameter were ligated; smaller vessels were sealed with a harmonic scalpel.
Evaluation of imaging
In each case, preoperative and intraoperative imaging data were subjected to post hoc review (Fig. 1). For each method used (CT, IOUS and PDE + ICG fluorescence), the total number of nodules detected was recorded, as were the numbers of nodules that were >3 mm or ≤ 3 mm in diameter (a diameter of 3 mm is commonly regarded as a reasonable detection threshold in both CT and US).
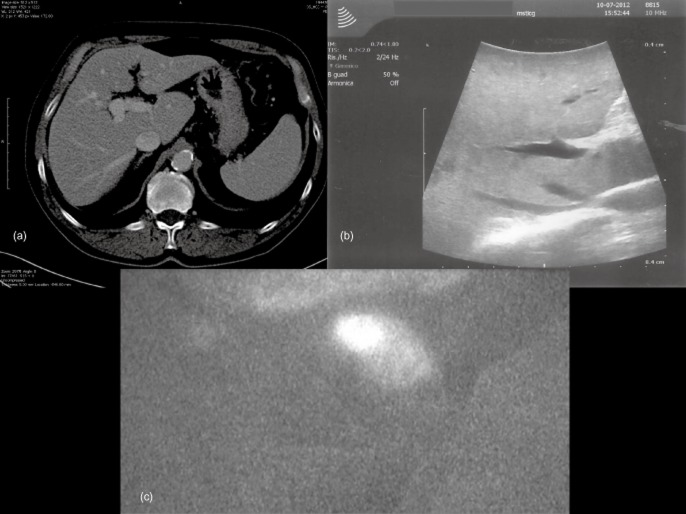
Figure 1.
In computed tomography (a) and intraoperative ultrasound (b), findings appear to be unremarkable. (c) Near-infrared imaging using the Photodynamic Eye demonstrates a nodule
Statistical analysis
Data were analysed using GraphPad Prism Version 6.00 for OsX (GraphPad Software, Inc., La Jolla, CA, USA). Differences were assessed with Kruskal–Wallis non-parametric analysis of variance (anova) and Dunn's multiple comparisons test. A P-value of <0.05 was considered to indicate statistical significance.
Results
Outcome of surgery
All patients recovered satisfactorily from surgery and had uneventful postoperative courses. As expected, transient alterations were observed in liver function parameters, but in all cases values returned to the preoperative baseline range by postoperative day 3. Postoperative complications included pleural effusion, fever and a slight delay in the return of bowel function. Mean ± standard deviation intraoperative blood loss was 300 ± 200 ml. Mean hospital stay was 6 days.
Detection of metastatic liver nodules
Figure 2 reports the findings of this study. After the postoperative histopathological examination, one of the lesions identified only by PDE + ICG was considered benign. Only one malignant lesion showed positive microscopic resection margins.
Figure 2.
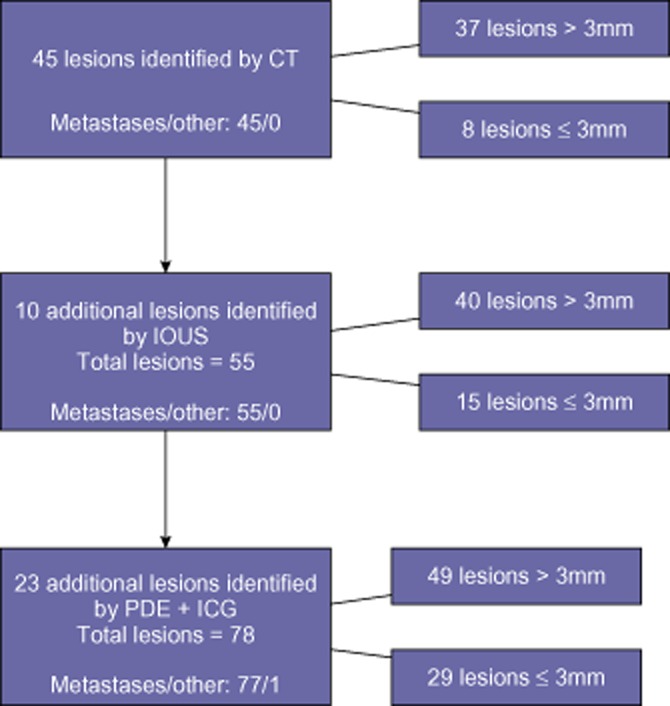
Stratification of findings among the cases. Only one event was deemed a false positive. CT, computed tomography; IOUS, intraoperative ultrasound; PDE, Photodynamic Eye; ICG, indocyanine green
The use of concomitant IOUS and PDE significantly improved the detection of liver metastases in comparison with preoperative CT (P = 0.0029). The use of IOUS alone, however, did not improve detection significantly in comparison with preoperative CT (P = 0.3905) (Fig. 3).
Figure 3.
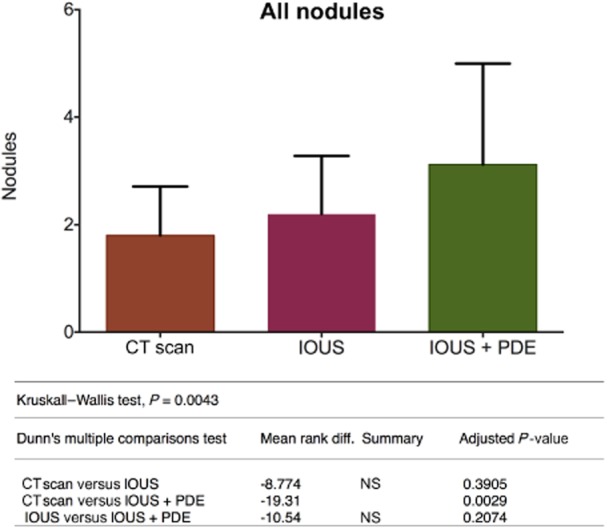
Overall evaluation of all observed nodules. CT, computed tomography; IOUS, intraoperative ultrasound; PDE, Photodynamic Eye; NS, not significant
The lesions identified were divided into two groups according to size, consisting of lesions of >3 mm and lesions of ≤3 mm in diameter.
No differences emerged between preoperative CT, IOUS and IOUS + PDE in the detection of lesions of >3 mm (P > 0.9999) (Fig. 4). Intraoperative US is limited to the detection of lesions of >3 mm in size.
Figure 4.
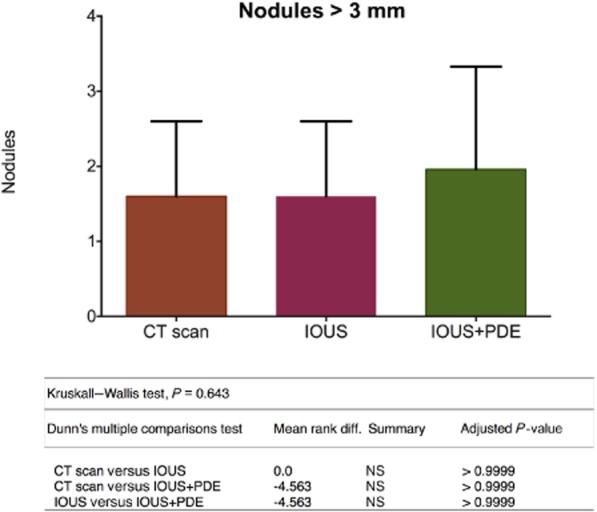
Stratification of the nodules by size shows no difference in findings of tumours of >3 mm. CT, computed tomography; IOUS, intraoperative ultrasound; PDE, Photodynamic Eye; NS, not significant
Differences among the three imaging methods emerged in the detection of lesions of ≤3 mm. The use of concomitant IOUS and PDE was most successful, detecting 29 lesions in comparison with 15 detected by IOUS alone (IOUS mean rank: 33.88; IOUS + PDE mean rank: 46.48; P = 0.0328) and nine detected by preoperative CT alone (CT mean rank: 22.00; IOUS + PDE mean rank: 46.48; P < 0.0001) There was no significant difference in numbers detected between CT and IOUS (CT mean rank: 22.00; IOUS mean rank: 33.88; P = 0.0708) (Fig. 5).
Figure 5.
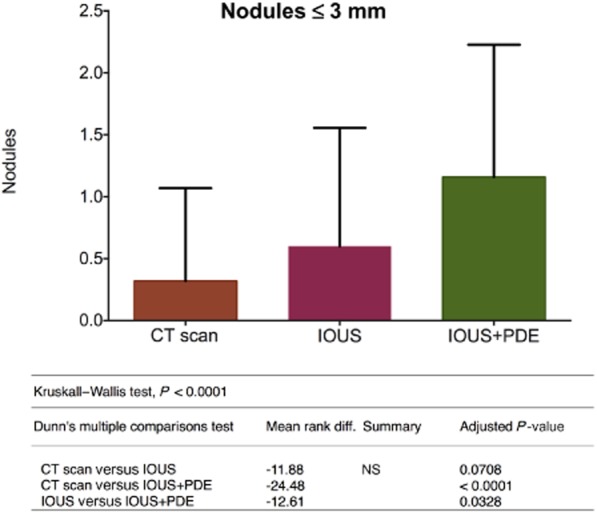
Stratification of the nodules by size shows that the combined use of intraoperative ultrasound and the Photodynamic Eye allows a significant advantage in the detection of nodules of ≤3 mm. CT, computed tomography; IOUS, intraoperative ultrasound; PDE, Photodynamic Eye; NS, not significant
Discussion
The present study provides preliminary clinical evidence indicating that the intraoperative detection of liver metastases, particularly of very small lesions, which are frequently missed with conventional imaging modalities, can be substantially improved with the use of ICG fluorescence detection (Fig. 6). The PDE + ICG system provides detailed, real-time video imaging that serves as an excellent guide throughout the liver resection procedure. This approach can be used alone or in combination with traditional IOUS, thereby exploiting the strong points of each method: US provides high sensitivity for the examination of inner regions of the liver and PDE + ICG provides a higher-resolution capture of the outermost regions. The detection of green fluorescence heralds the presence of neoplastic tissue.
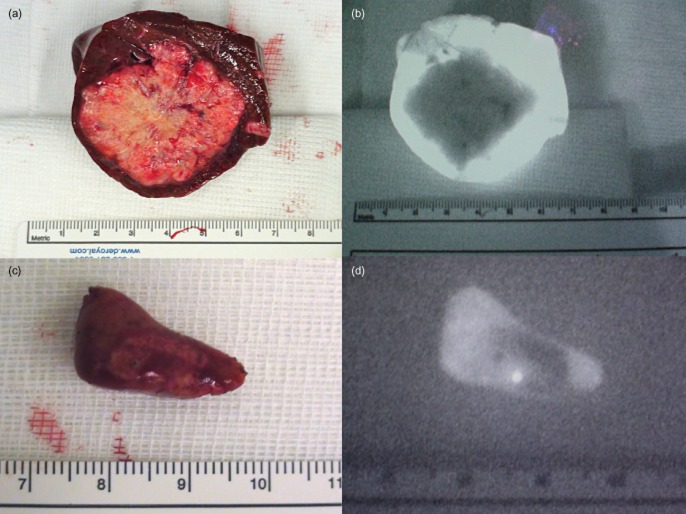
Figure 6.
The figure is a proof of concept demonstrated by resection specimens and associated images obtained using the Photodynamic Eye (PDE) with indocyanine green (ICG) staining. (a, b) When PDE + ICG is used to detect large nodules, it allows a safe resection in which the rim represents the limit of resection. A parenchyma-sparing resection is safer with this technique. (c, d) The PDE + ICG technique is particularly suited to demonstrating small nodules that may be missed by computed tomography and intraoperative ultrasound. The technique can be used to change the staging of the disease and possibly the therapeutic approach
The use of IOUS and PDE in combination can be very useful in achieving R0 surgery. Surgeons naturally make every attempt to identify and remove all macroscopically detectable neoplastic tissue. If there is macroscopic evidence of residual cancer at the end of surgery, it is generally agreed that the procedure should be regarded as a technical failure that can be expected to have a detrimental impact on both disease-free and overall survival. In most cases, however, this outcome is not the result of a medical error. Frequently, the disease manifestation is more advanced or complicated than it appeared on preoperative imaging findings. This reflects the intrinsic limits of currently available technology. The surgeon may find an unexpected area of infiltration or a major blood vessel or bile duct in close proximity to the lesion, which precludes wider dissection. Because of its infiltrating nature, the metastatic nodule will be irregularly shaped and thus the placement of each portion of the resection line potentially involves making a choice between the risk for immediate organ damage and the risks associated with a non-radical tumour resection. In some cases, it is simply impossible to eliminate all nests of viable cancer cells. In the present group's experience, NIR imaging was useful to define the surgical margin. Imaging by NIR was used not only before, but also after liver resection: in this way, all the metastatic tissue was removed, with an instant revision of the resection. In a single case (one of 77), pathology showed an R1 resection. Even in this resection, NIR imaging did not show any remaining fluorescence, but the section was close to a major vessel that could not be sacrificed.
The present findings suggest that the use of a combination of PDE + ICG and IOUS in these settings could increase the accuracy of intraoperative exploration and dramatically improve the chances of complete tumour eradication. Further studies will be necessary to confirm these conclusions in a larger population.
Conflicts of interest
None declared.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe BM, Donegan WL, Watson F, Spratt JS. Factors influencing survival in patients with untreated hepatic metastases. Surg Gynecol Obstet. 1968;127:1–11. [PubMed] [Google Scholar]
- 4.Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083–1090. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bengtsson G, Carlsson G, Hafström L, Jönsson PE. Natural history of patients with untreated liver metastases from colorectal cancer. Am J Surg. 1981;141:586–589. doi: 10.1016/0002-9610(81)90057-x. [DOI] [PubMed] [Google Scholar]
- 6.Adson MA, van Heerden JA. Major hepatic resections for metastatic colorectal cancer. Ann Surg. 1980;191:576–583. doi: 10.1097/00000658-198005000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdalla EK. Resection of colorectal liver metastases. J Gastrointest Surg. 2011;15:416–419. doi: 10.1007/s11605-011-1429-6. [DOI] [PubMed] [Google Scholar]
- 8.Folprecht G, Grothey A, Alberts S, Raab H, Köhne C. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 9.Adam R, Hoti E, Bredt LC. Oncosurgical strategies for metastatic liver cancer. Cir Esp. 2011;89:10–19. doi: 10.1016/j.ciresp.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomized phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 11.Homayounfar K, Liersch T, Schuetze G, Niessner M, Goralczyk A, Melleret J. Two-stage hepatectomy (R0) with portal vein ligation – towards curing patients with extended bilobular colorectal liver metastases. Int J Colorectal Dis. 2009;24:409–418. doi: 10.1007/s00384-008-0620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjövall A, Järv V, Blomqvist L, Singnomklao T, Cedermark B, Glimelius B, et al. The potential for improved outcome in patients with hepatic metastases from colon cancer: a population-based study. Eur J Surg Oncol. 2004;30:834–841. doi: 10.1016/j.ejso.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Evrard S, Becouarn Y, Fonck M, Brunet R, Mathoulin-Pelissier S, Picot V. Surgical treatment of liver metastases by radiofrequency ablation, resection, or in combination. Eur J Surg Oncol. 2004;30:399–406. doi: 10.1016/j.ejso.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Jonas S, Thelen A, Benckert C, Spinelli A, Sammain S, Neumann U, et al. Extended resections of liver metastases from colorectal cancer. World J Surg. 2007;31:511–521. doi: 10.1007/s00268-006-0140-3. [DOI] [PubMed] [Google Scholar]
- 15.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in longterm survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong MC, van Vledder MG, Ribero D, Hubert C, Gigot JF, Choti MA, et al. Therapeutic efficacy of combined intraoperative ablation and resection for colorectal liver metastases: an international, multi-institutional analysis. J Gastrointest Surg. 2011;15:336–344. doi: 10.1007/s11605-010-1391-8. [DOI] [PubMed] [Google Scholar]
- 17.Chua TC, Saxena A, Chu F, Zhao J, Morris DL. Predictors of cure after hepatic resection of colorectal liver metastases: an analysis of actual 5- and 10-year survivors. J Surg Oncol. 2011;103:796–800. doi: 10.1002/jso.21864. [DOI] [PubMed] [Google Scholar]
- 18.D'Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18:1096–1103. doi: 10.1245/s10434-010-1409-1. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Hsieh Y, Chen W, Hsu YN, Chau GY, Teng HW, et al. Adjuvant oxaliplatin- or irinotecan-containing chemotherapy improves overall survival following resection of metachronous colorectal liver metastases. Int J Colorectal Dis. 2010;25:1243–1249. doi: 10.1007/s00384-010-0996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez D, Sangha VK, Morris-Stiff G, Malik HZ, Guthrie AJ, Toogood GJ, et al. Outcomes of intensive surveillance after resection of hepatic colorectal metastases. Br J Surg. 2010;97:1552–1560. doi: 10.1002/bjs.7136. [DOI] [PubMed] [Google Scholar]
- 21.House MG, Ito H, Gonen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–752. doi: 10.1016/j.jamcollsurg.2009.12.040. 752–755. [DOI] [PubMed] [Google Scholar]
- 22.Welsh FKS, Tekkis PP, John TG, Rees M. Open liver resection for colorectal metastases: better short- and longterm outcomes in patients potentially suitable for laparoscopic liver resection. HPB. 2010;12:188–194. doi: 10.1111/j.1477-2574.2009.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsim N, Healey AJ, Frampton AE, Habib NA, Bansi DS, Wasan H, et al. Two-stage resection for bilobar colorectal liver metastases: R0 resection is the key. Ann Surg Oncol. 2011;18:1939–1946. doi: 10.1245/s10434-010-1533-y. [DOI] [PubMed] [Google Scholar]
- 24.Poultsides GA, Schulick RD, Pawlik TM. Hepatic resection for colorectal metastases: the impact of surgical margin status on outcome. HPB. 2010;12:43–49. doi: 10.1111/j.1477-2574.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias D, Cavalcanti A, Sabourin JC, Pignon JP, Ducreux M, Lasser P. Results of 136 curative hepatectomies with a safety margin of less than 10 mm for colorectal metastases. J Surg Oncol. 1998;69:88–93. doi: 10.1002/(sici)1096-9098(199810)69:2<88::aid-jso8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Robinson P. Indeterminate liver lesions in cancer. Cancer Imaging. 2002;2:130–132. [Google Scholar]
- 28.Albrecht T, Blomley MJK, Burns PN, Wilson S, Harvey CJ, Leen E, et al. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicentre study. Radiology. 2003;227:361–370. doi: 10.1148/radiol.2272011833. [DOI] [PubMed] [Google Scholar]
- 29.Uchiyama K, Ueno M, Ozawa S, Kiriyama S, Shigekawa Y, Yamaue H. Combined use of contrast-enhanced intraoperative ultrasonography and a fluorescence navigation system for identifying hepatic metastases. World J Surg. 2010;34:2953–2959. doi: 10.1007/s00268-010-0764-1. [DOI] [PubMed] [Google Scholar]
- 30.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]