Abstract
Objectives
The aim of this study was to identify predictors for longterm survival following pancreaticoduodenectomy (PD) for pancreatic and other periampullary adenocarcinomas.
Methods
Clinicopathological factors were compared between short-term (<5 years) and longterm (≥5 years) survival groups. Rates of actual 5-year and actuarial 10-year survival were determined.
Results
There were 109 (21.8%) longterm survivors among a sample of 501 patients. Patients with ampullary adenocarcinoma represented 76.1% of the longterm survivors. Favourable factors for longterm survival included female gender, lack of jaundice, lower blood loss, classical PD, absence of postoperative bleeding or intra-abdominal abscess, non-pancreatic primary cancer, earlier tumour stage, smaller tumour size (≤2 cm), curative resection, negative lymph node involvement, well-differentiated tumours, and absence of perineural invasion. Independent factors associated with longterm survival were diagnosis of primary tumour, jaundice, intra-abdominal abscess, tumour stage, tumour size, radicality, lymph node status and cell differentiation. The prognosis was best for ampullary adenocarcinoma, for which the rate of actual 5-year survival was 32.8%, and poorest for pancreatic head adenocarcinoma, for which actual 5-year survival was only 6.5%.
Conclusions
The majority of longterm survivors after PD for periampullary adenocarcinomas are patients with ampullary adenocarcinoma. The longterm prognosis in pancreatic head adenocarcinoma remains dismal.
Introduction
Pancreaticoduodenectomy (PD) is the treatment of choice for periampullary adenocarcinomas. An increase in experience and advances in perioperative care have reduced perioperative mortality following PD to <5% in high-volume centres.1–7 Only 10–15% of patients presenting with pancreatic adenocarcinoma are technically resectable8,9 and for those who do undergo resection, the prognosis remains poor, with median survival of 11–19 months.1,8–26 Even the addition of adjuvant therapies has resulted in only modest improvements in survival. Several studies have reported on actual longterm survival beyond 5 years in patients subjected to resection for pancreatic or other periampullary cancers.1,10–19,26–29 Clinical factors, such as lymph node metastasis, tumour size and resection margin, have been comprehensively studied with respect to determining short-term survival after PD for pancreatic and other periampullary cancers.11,13,19,27,30 However, prognostic factors that may predict longterm survival are poorly understood.
The purpose of this study was to identify the predictors of longterm survival (≥5 years) in patients with pancreatic and other periampullary adenocarcinomas after PD. In addition, actual 5-year and actuarial 10-year survival rates in all patients with periampullary adenocarcinomas after PD were determined.
Materials and methods
Data for patients with pancreatic or other periampullary adenocarcinomas who underwent PD between 1974 and 2006 were retrieved from a prospectively collected database for pancreatic surgery. Data for patients with other malignancies, such as intraductal papillary mucinous neoplasms, mucinous cystadenocarcinoma, acinar cell carcinoma, solid pseudopapillary neoplasms and neuroendocrine carcinoma, were excluded from the analysis.
The number of surgeons who performed at least one PD during the study period was 26. Five surgeons each performed ≥15 PDs and 65.3% of all PDs were performed by two high-volume surgeons, each of whom performed ≥15 PDs per year. A standard resection without extensive retroperitoneal lymph node dissection was performed. The decision on whether the procedure should consist of a classical PD or a pylorus-preserving resection was left to the discretion of the operating surgeon. A pancreaticogastrostomy or pancreaticojejunostomy was used for pancreaticoenteric reconstruction. Curative resection was defined as a PD without evidence of any residual cancer at the resection margins, including the pancreatic neck and distal common bile duct (CBD) cut-ends, retroperitoneal margin, and superior mesenteric and portal vein grooves. A palliative resection was defined as including either gross or microscopic evidence of cancer at the resection margin. The definition of primary tumour origin was mainly based on pathological findings; however, image studies and gross findings were also taken into consideration for primary tumour origin when pathological determination was unclear. Postoperatively, patients were followed at 6-month intervals for ≥5 years or until death. Patients with operative mortality were included in this study. Patient who died from causes other than cancer were also included, but their data were censored for survival analysis.
Based on survival time, patients were stratified into two groups consisting of, respectively, short-term (<5 years) and longterm (≥5 years) survivors. Patient demographics, the pathological characteristics of tumours, surgical factors and risk factors were compared between these two groups. Actual 5-year survival in all patients with pancreatic and other periampullary adenocarcinomas after PD was determined. For patients who survived 5 years, actuarial 10-year survival was calculated.
pasw Statistics Version 18 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. All continuous data were expressed as the mean ± standard deviation (SD) or as the median (range), as appropriate. Comparisons of data between the primary groups were performed using a Student's t-test or Wilcoxon rank sum test, as appropriate, for continuous variables. Categorical variables were compared using a chi-squared or Fisher's exact test. P-values were derived from two-tailed tests. Survival was calculated following PD. Using the binary logistic regression model for all factors with a P-value of <0.2 after univariate analysis, multivariate analysis was performed to identify independent predictors of longterm survival of ≥5 years after PD. Cumulative survival was estimated using the Kaplan–Meier method and differences between subgroups were determined using a log-rank test. A P-value of <0.05 was considered to indicate statistical significance.
Results
A total of 501 patients with periampullary adenocarcinomas underwent PD during the study period. Of these, 109 (21.8%) were longterm survivors (≥5 years) and 392 (78.2%) were short-term survivors (<5 years). Among the 109 longterm survivors, 83 (76.1%) were operated for ampullary adenocarcinoma, 11 (10.1%) for pancreatic head adenocarcinoma, 10 (9.2%) for distal CBD adenocarcinoma, and five (4.6%) for duodenal adenocarcinoma. Univariate analyses of various factors associated with survival status are shown in Tables 1–3. Following multivariate analysis with a binary logistic regression model, diagnosis of the primary tumour, jaundice, intra-abdominal abscess, tumour stage, tumour size, radicality, lymph node status and cell differentiation were found to be independent predictors of longterm survival after PD (Table 4).
Table 1.
Demographics of patients with periampullary adenocarcinomas submitted to pancreaticoduodenectomy
| Variable | Total | Survival <5 years | Survival ≥5 years | P-value |
|---|---|---|---|---|
| Patients, n (%) | 501 (100%) | 392 (78.2%) | 109 (21.8%) | |
| Gender, n (%) | <0.001 | |||
| Male | 368 (73.5%) | 303 (77.3%) | 65 (59.6%) | |
| Female | 133 (26.5%) | 89 (22.7%) | 44 (40.4%) | |
| Age, years | 0.201 | |||
| Median (range) | 67 (25–89) | 67 (25–89) | 65 (34–89) | |
| Mean ± SD | 64.6 ± 11.4 | 65.0 ± 11.4 | 63.4 ± 11.5 | |
| Diagnosis of primary tumour, n (%) | <0.001 | |||
| Pancreatic head | 169 | 158 (93.5%) | 11 (6.5%) | |
| Ampullary | 253 | 170 (67.2%) | 83 (32.8%) | |
| Distal CBD | 52 | 42 (80.8%) | 10 (19.2%) | |
| Duodenal | 27 | 22 (81.5%) | 5 (18.5%) | |
| Duration of symptoms, months, median (range) | 1 (0–120) | 1 (0–120) | 1 (0–24) | 0.514 |
| Symptoms, n (%) | ||||
| No symptoms | 9 (1.8%) | 7 (1.8%) | 2 (1.8%) | 1.000 |
| Jaundice | 393 (78.4%) | 320 (81.6%) | 73 (67.3%) | 0.002 |
| Epigastric pain | 219 (43.7%) | 165 (42.1%) | 54 (49.5%) | 0.190 |
| Body weight loss | 177 (35.3%) | 136 (34.7%) | 41 (37.6%) | 0.573 |
| Nausea/vomiting | 56 (11.2%) | 46 (11.2%) | 10 (9.3%) | 0.606 |
| Diabetes mellitus | 77 (8.3%) | 68 (17.3%) | 9 (8.3%) | 0.023 |
| Serum CA 19-9, U/ml, median (range) | 73 (0–18 545) | 92 (0–18 545) | 36 (2–12 000) | 0.207 |
| Serum CEA, ng/ml, median (range) | 4 (1–143) | 4 (1–143) | 4 (1–19) | 0.205 |
| Chemotherapy, n (%) | 0.204 | |||
| No | 275 (56.8%) | 219 (57.9%) | 56 (52.8%) | |
| Yes | 209 (43.2%) | 159 (42.1%) | 50 (47.2%) | |
| Radiotherapy, n (%) | 0.158 | |||
| No | 278 (57.6%) | 222 (58.9%) | 56 (52.8%) | |
| Yes | 205 (42.4%) | 155 (41.1%) | 50 (47.2%) | |
SD, standard deviation; CBD, common bile duct; CA 19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen.
Table 3.
Pathological parameters in patients with periampullary adenocarcinomas undergoing pancreaticoduodenectomy
| Total | Survival <5 years | Survival ≥5 years | P-value | |
|---|---|---|---|---|
| Stage, n (%) | 0.003 | |||
| 0 | 15 (3.0%) | 10 (2.6%) | 5 (4.6%) | |
| I | 84 (16.8%) | 56 (14.3%) | 28 (25.7%) | |
| II | 279 (55.7%) | 217 (55.4%) | 62 (56.9%) | |
| III | 92 (18.4%) | 83 (21.2%) | 9 (8.3%) | |
| IV | 31 (6.2%) | 26 (6.6%) | 5 (4.6%) | |
| Stage, n (%) | 0.001 | |||
| 0 + I + II | 387 (75.4%) | 283 (72.2%) | 95 (87.2%) | |
| III + IV | 123 (24.6%) | 109 (27.8%) | 14 (12.8%) | |
| Tumour size, n (%) | 0.006 | |||
| ≤2 cm | 218 (43.5%) | 158 (40.3%) | 60 (55.0%) | |
| >2 cm | 283 (56.5%) | 234 (59.7%) | 49 (45.0%) | |
| Tumour size, cm | 0.020 | |||
| Median (range) | 2.5 (0.4–15) | 2.5 (0.5–15) | 2.0 (0.4–8) | |
| Mean ± SD | 2.8 ± 1.6 | 2.9 ± 1.6 | 2.5 ± 1.4 | |
| Radicality, n (%) | <0.001 | |||
| Curative | 422 (84.2%) | 317 (80.9%) | 105 (96.3%) | |
| Palliative | 79 (15.8%) | 75 (19.1%) | 4 (3.7%) | |
| Lymph node involvement, n (%) | <0.001 | |||
| Negative | 302 (60.7%) | 211 (53.8%) | 93 (85.3%) | |
| Positive | 197 (39.3%) | 181 (46.2%) | 16 (14.7%) | |
| Cell differentiation, n (%) | 0.001 | |||
| Good | 61 (13.2%) | 40 (11.0%) | 21 (21.4%) | |
| Moderate | 330 (71.6%) | 259 (71.3%) | 71 (72.4%) | |
| Poor | 70 (15.2%) | 64 (17.6%) | 6 (6.1%) | |
| Perineural invasion, n (%) | <0.001 | |||
| Negative | 235 (62.0%) | 176 (57.5%) | 59 (80.8%) | |
| Positive | 144 (38.0%) | 130 (42.5%) | 14 (19.2%) | |
| Lymphovascular invasion, n (%) | 0.678 | |||
| Negative | 254 (67.0%) | 203 (66.3%) | 51 (69.9%) | |
| Positive | 125 (33.0%) | 103 (33.7%) | 22 (30.1%) | |
SD, standard deviation.
Table 4.
Independent predictors of longterm survival [≥5 years after pancreaticoduodenectomy (PD)] by multivariate analysis in a binary logistic regression model
| Odds ratio | 95% CI | P-value | |
|---|---|---|---|
| Gender | 1.015 | 0.348–2.965 | 0.798 |
| Diagnosis of primary tumour (pancreatic versus non-pancreatic) | 2.318 | 0.541–9.935 | 0.002 |
| Jaundice | 0.828 | 0.730–0.938 | 0.002 |
| Epigastric pain | 1.107 | 0.362–3.386 | 0.798 |
| Diabetes mellitus | 0.967 | 0.871–1.072 | 0.545 |
| Radiotherapy | 0.734 | 0.214–2.516 | 0.403 |
| Operation time (<7.5 h versus ≥7.5 h) | 1.039 | 0.266–4.053 | 0.312 |
| Blood loss (<800 ml versus ≥800 ml) | 0.522 | 0.180–1.511 | 0.136 |
| Pylorus-preserving PD | 1.673 | 0.435–6.438 | 0.415 |
| Post-PD bleeding | 1.200 | 0.178–8.106 | 0.907 |
| Intra-abdominal abscess | 1.257 | 0.869–1.817 | 0.003 |
| Stage (I + II versus III + IV) | 1.602 | 0.383–6.708 | 0.780 |
| Tumour size (≤2.0 cm versus >2.0 cm) | 0.384 | 0.127–1.162 | 0.006 |
| Radicality (curative versus palliative) | 0.500 | 0.072–3.496 | 0.015 |
| Lymph node involvement | 0.339 | 0.096–1.196 | 0.021 |
| Cell differentiation (good + moderate versus poor) | 0.000 | 0.000–1.265 | 0.028 |
| Perineural invasion | 1.177 | 0.817–1.695 | 0.085 |
95% CI, 95% confidence interval.
Table 2.
Surgical factors and risks in pancreaticoduodenectomy (PD)
| Total | Survival <5 years | Survival ≥5 years | P-value | |
|---|---|---|---|---|
| Operation time, h | 0.161 | |||
| Median (range) | 7.5 (3.5–16) | 7.5 (3.5–16) | 8.5 (4.0–14) | |
| Mean ± SD | 8.0 ± 2.3 | 7.8 ± 2.4 | 8.3 ± 2.0 | |
| Surgeon volume, n (%) | 0.294 | |||
| High (≥15 PD/year) | 406 (81.2%) | 315 (80.6%) | 91 (83.5%) | |
| Low (<15 PD/year) | 94 (8.8%) | 76 (19.4%) | 18 (16.5%) | |
| Blood loss, ml | 0.005 | |||
| Median (range) | 800 (50–5250) | 800 (50–5250) | 575 (100–2950) | |
| Mean ± SD | 903 ± 618 | 954 ± 639 | 709 ± 487 | |
| Pylorus-preserving PD, n (%) | 0.048 | |||
| No | 288 (57.5%) | 216 (55.1%) | 72 (66.1%) | |
| Yes | 213 (42.5%) | 176 (44.9%) | 37 (33.9%) | |
| Postoperative hospital stay, days, median (range) | 26.0 (1–383) | 26.0 (1–383) | 26.5 (5–87) | 0.531 |
| Surgical morbidity, overall, n (%) | 225 (44.9%) | 174 (44.4%) | 11 (10.1%) | 0.665 |
| Pancreatic leakage, n (%) | 55 (11.10%) | 44 (11.2%) | 11 (10.1%) | 0.863 |
| Gastric atonia, n (%) | 52 (10.3%) | 40 (10.2%) | 12 (11.0%) | 0.859 |
| Post-PD bleeding, n (%) | 53 (10.6%) | 49 (12.5%) | 4 (3.7%) | 0.007 |
| Intraluminal post-PD bleeding, n (%) | 31 (6.2%) | 30 (7.7%) | 1 (0.9%) | 0.006 |
| Extraluminal post-PD bleeding, n (%) | 32 (6.4%) | 28 (7.1%) | 4 (3.7%) | 0.267 |
| Intra-abdominal abscess, n (%) | 55 (11.0%) | 35 (8.9%) | 20 (18.3%) | 0.009 |
| Wound infection, n (%) | 54 (10.8%) | 39 (9.9%) | 15 (13.8%) | 0.294 |
SD, standard deviation.
Of the 392 (78.2%) patients who died within 5 years of PD, 378 (96.4%) died as a result of recurrent disease. Fourteen (3.6%) patients were lost to follow-up. Patients with ampullary adenocarcinoma (32.8%) showed the highest actual 5-year survival rate, followed by those with distal CBD adenocarcinoma (19.2%) and duodenal adenocarcinoma (18.5%). The lowest actual 5-year survival was found in patients with pancreatic head adenocarcinoma (6.5%). Overall actual 5-year survival in patients with periampullary adenocarcinomas was 21.8% (109 patients were alive). The median length of survival was 21.0 months across all patients, 13.7 months in patients with pancreatic head adenocarcinoma, 28.9 months in those with ampullary adenocarcinoma, 24.4 months in those with distal CBD adenocarcinoma, and 21.7 months in those with duodenal adenocarcinoma. Figure 1 shows actuarial survival curves for individual histological subtypes following PD.
Figure 1.
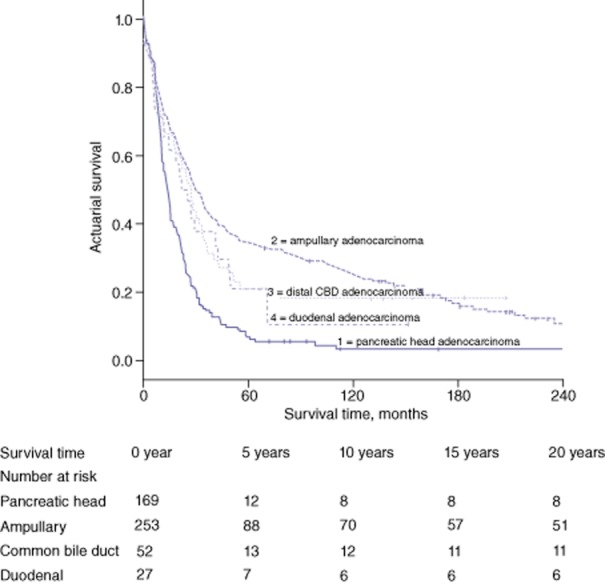
Actuarial survival following pancreaticoduodenectomy for periampullary adenocarcinomas in patients with (1) pancreatic head adenocarcinoma (n = 169), (2) ampullary adenocarcinoma (n = 253), (3) distal common bile duct (CBD) adenocarcinoma (n = 52) and (4) duodenal adenocarcinoma (n = 27). Subgroup comparisons showed: (1) versus (2), P = 0.000; (1) versus (3), P = 0.001; (1) versus (4), P = 0.028; (2) versus (3), P = 0.352; (2) versus (4), P = 0.202, and (3) versus (4), P = 0.779
Of the 109 actual 5-year survivors, 56 (51.4%) were alive at 10 years. A total of 24 (22.0%) 5-year survivors had died, 22 of them as a result of the disease process. Actuarial 10-year survival in actual 5-year longterm survivors according to histological subtype is shown in Table 5.
Table 5.
Data for 10-year survival in longterm survivors (survival ≥5 years) of periampullary adenocarcinomas after pancreaticoduodenectomy
| Survival, months, median (range) | Actuarial 10-year survival | Patients alive, n | |
|---|---|---|---|
| Periampullary adenocarcinomas (n = 109) | 137.3 (60.8–341.1) | 73.2% | 56 |
| Pancreatic head adenocarcinoma (n = 11) | 112.5 (60.8–293.7) | 49.1% | 2 |
| Ampullary adenocarcinoma (n = 83) | 143.9 (62.4–341.1) | 74.4% | 46 |
| Distal CBD adenocarcinoma (n = 10) | 137.1 (62.0–208.1) | 87.5% | 7 |
| Duodenal adenocarcinoma (n = 5) | 84.4 (60.8–341.1) | 50.0% | 1 |
CBD, common bile duct.
Discussion
Pancreaticoduodenectomy was previously abandoned by nihilistic surgeons confronted with patients diagnosed with periampullary adenocarcinomas because of the high surgical risk associated with the procedure and dismal survival outcomes, especially as pancreatic primary cancers were regarded as lethal.10,17,31 However, decreasing perioperative mortality and encouraging improvements in survival since the 1990s have allowed PD to become the standard of care for patients with resectable periampullary adenocarcinomas.8,10–17 Prognoses in periampullary adenocarcinomas vary and depend on the origin of the primary tumour. Patients with pancreatic head adenocarcinoma have the worst prognosis, with 5-year survival of 5–20% after resection.9,11,19–32 Prognosis is better in ampullary adenocarcinoma, in which 5-year survival is 30–40%,10,23,33–35 and duodenal adenocarcinoma, in which 5-year survival is 50–65%.10,23,36 Furthermore, 5-year survival in distal CBD adenocarcinoma is approximately 20–30%.10,33
Most of the 5-year survival rates reported in the literature are actuarial and represent estimates of 5-year survival after resection. In fact, few studies on periampullary adenocarcinomas report actual 5-year survival figures. It has been suggested that actuarial 5-year survival estimates may be higher than actual 5-year survival rates.13,24 The current study reports actual 5-year survival in a large group of patients with a variety of periampullary adenocarcinomas, which enables a subgroup analysis. This confirms that a worse prognosis is associated with pancreatic head adenocarcinoma compared with the other disease subgroups. Because the numbers of patients surviving to 10 years is low, it was not possible to compare 10-year actuarial survival among patients with pancreatic head, distal CBD and duodenal adenocarcinomas by histological subgroup. However, patients with ampullary adenocarcinoma who survived to 5 years were found to have a 74.4% probability of surviving to 10 years, with the most likely cause of death being the disease process itself.
The clinicopathological characteristics contributing to longterm survival (i.e. ≥5 years) remain controversial.12,13,29 Pathologic factors have been proposed as the most important prognostic indicators of longterm survival, and include non-pancreatic primary cancers, negative lymph node status, a low number of positive lymph nodes, smaller tumour size, curative resection, well-differentiated tumours, and no invasion of the extrapancreatic nerve plexus.10–16,19,26,27,29,30 Surgical factors, such as curative resection with negative margins, that lack postoperative complications are independent variables that are significantly associated with longterm survival.16,29 In the current study, many of these factors seemed to be important on univariate analysis. However, only primary tumour diagnosis, jaundice, intra-abdominal abscess, tumour stage, tumour size, radicality, lymph node status and cell differentiation remained as independent factors associated with longterm survival. Some investigators have reported that patients with more advanced stages of cancer (i.e. a large tumour size and/or lymph node metastasis) can be cured by surgical resection.12,13 This suggests that poor prognostic factors, such as large tumour size or lymph node metastasis, do not necessarily preclude an actual 5-year survival, and therefore active surgical resection with curative intent should be attempted in all patients with resectable disease.12,13 Chemotherapy is currently the standard of care for pancreatic cancer. However, only 43.2% of patients in the present study received chemotherapy. This may partially explain why actual survival in this study was found to be lower than actuarial survival reported in the literature.
In summary, of patients submitted to PD, most longterm survivors are those with ampullary adenocarcinoma. Actual 5-year survival in patients with pancreatic ductal adenocarcinoma is 6.5%. This would appear to be significantly lower than reported actuarial 5-year survival figures. Independent factors associated with longterm survival were primary tumour diagnosis, jaundice, intra-abdominal abscess, tumour stage, tumour size, radicality, lymph node status and cell differentiation.
Conflicts of interest
None declared.
References
- 1.Bottger TC, Junginger T. Factors influencing morbidity and mortality after pancreaticoduodenectomy: critical analysis of 221 resections. World J Surg. 1999;23:164–172. doi: 10.1007/pl00013170. [DOI] [PubMed] [Google Scholar]
- 2.Trede M, Schwall G, Saeger HD. Survival after pancreaticoduodenectomy: 118 consecutive resections without an operative mortality. Ann Surg. 1990;990:447–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430–438. doi: 10.1097/00000658-199305010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castillo CFD, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995;130:295–300. doi: 10.1001/archsurg.1995.01430030065013. [DOI] [PubMed] [Google Scholar]
- 5.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s, pathology, complications, and outcomes. Ann Surg. 1997;226:248–260. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson NB, Foulis AK, Oien KA, Going JJ, Glen P, Dickson EJ, et al. Positive mobilization margins alone do not influence survival following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2010;251:1003–1010. doi: 10.1097/SLA.0b013e3181d77369. [DOI] [PubMed] [Google Scholar]
- 8.Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–772. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, et al. Longterm survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–462. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 10.Adham M, Jaeck D, Le Borgne J, Oussoultzouglou E, Chenard-Neu MP, Mosnier JF, et al. Longterm survival (5–20 years) after pancreatectomy for pancreatic ductal adenocarcinoma: a series of 30 patients collected from three institutions. Pancreas. 2008;37:352–357. doi: 10.1097/MPA.0b013e31818166d2. [DOI] [PubMed] [Google Scholar]
- 11.Han SS, Jang JY, Kim SW, Kim WH, Lee KU, Park YH. Analysis of longterm survivors after surgical resection for pancreatic cancer. Pancreas. 2006;32:271–275. doi: 10.1097/01.mpa.0000202953.87740.93. [DOI] [PubMed] [Google Scholar]
- 12.Allison DC, Piantadosi S, Hruban RH, Dooley WC, Fishman EK, Yeo CJ, et al. DNA content and other factors associated with 10-year survival after resection of pancreatic carcinoma. J Surg Oncol. 1998;67:151–159. doi: 10.1002/(sici)1096-9098(199803)67:3<151::aid-jso2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, et al. Longterm survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard TJ, Krug JE, Yu J, Zyromski NJ, Schmidt CM, Jacobson LE, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to longterm survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338–1345. doi: 10.1016/j.gassur.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Longterm results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 16.Mosca F, Giulianotti PC, Balestracci T, Di Candio G, Pietrabissa A, Sbrana F, et al. Longterm survival in pancreatic cancer: pylorus-preserving versus Whipple pancreatoduodenectomy. Surgery. 1997;122:553–566. doi: 10.1016/s0039-6060(97)90128-8. [DOI] [PubMed] [Google Scholar]
- 17.Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–558. doi: 10.1016/j.ejca.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–73. doi: 10.1016/s0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 19.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends from 100,313 patients diagnosed from 1985–1995, using the national cancer database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 20.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients. Ann Surg. 1995;221:721–733. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma, analysis of 5-year survivors. Ann Surg. 1998;227:821–831. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreatic cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 23.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad NA, Lewis JD, Ginsberg GG, Haller DG, Morris JB, Williams NN, et al. Longterm survival after pancreatic resection for pancreatic adenocarcinoma. Am J Gastroenterol. 2001;96:2609–2615. doi: 10.1111/j.1572-0241.2001.04123.x. [DOI] [PubMed] [Google Scholar]
- 25.Zacharias T, Jaeck D, Oussoultzoglou E, Neuville A, Bachellier P. Impact of lymph node involvement on longterm survival after R0 pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas. J Gastrointest Surg. 2007;11:350–356. doi: 10.1007/s11605-007-0113-3. [DOI] [PubMed] [Google Scholar]
- 26.Schäfer M, Mullhaupt B, Clavien PA. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg. 2002;236:137–148. doi: 10.1097/00000658-200208000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kure S, Kaneko T, Takeda S, Inoue S, Nakao A. Analysis of longterm survivors after surgical resection for invasive pancreatic cancer. HPB Surg. 2005;7:129–134. doi: 10.1080/13651820510003744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Gudjonsson B. Cancer of the pancreas: 50 years of surgery. Cancer. 1987;60:2284–2303. doi: 10.1002/1097-0142(19871101)60:9<2284::aid-cncr2820600930>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.Edge SB, Schmeig RE, Rosenlof LK, Wilhelm MC. Pancreas cancer resection outcome in American university centres in 1989–1990. Cancer. 1993;71:3502–3507. doi: 10.1002/1097-0142(19930601)71:11<3502::aid-cncr2820711107>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Talamini MA, Moesinger RC, Pitt HA, Sohn TA, Hruban RH, Lillemoe KD, et al. Adenocarcinoma of the ampulla of Vater: a 28-year experience. Ann Surg. 1997;225:590–600. doi: 10.1097/00000658-199705000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monson JR, Donohue JH, McEntee GP, McIlrath DC, van Heerden JA, Shorter RG, et al. Radical resection for carcinoma of the ampulla of Vater. Arch Surg. 1991;126:353–357. doi: 10.1001/archsurg.1991.01410270099016. [DOI] [PubMed] [Google Scholar]
- 33.Matory YL, Gaynor J, Brennan M. Carcinoma of the ampulla of Vater. Surg Gynecol Obstet. 1993;177:366–370. [PubMed] [Google Scholar]
- 34.Sohn TA, Lillemoe KD, Cameron JL, Pitt HA, Kaufman HS, Hruban RH, et al. Adenocarcinoma of the duodenum: factors influencing longterm survival. J Gastrointest Surg. 1998;2:79–87. doi: 10.1016/s1091-255x(98)80107-8. [DOI] [PubMed] [Google Scholar]
- 35.Bakaeen FG, Murr MM, Sarr MG, Thompson GB, Farnell MB, Nagorney DM, et al. What prognostic factors are important in duodenal adenocarcinoma? Arch Surg. 2000;135:635–641. doi: 10.1001/archsurg.135.6.635. [DOI] [PubMed] [Google Scholar]
- 36.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumours. Ann Surg. 1996;224:463–475. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]


