Abstract
Objectives
Volumetry is standard method for evaluating the volumes of the right liver (RL), left liver (LL), left lateral segments (LLS), total liver (TL) and future liver remnant (FLR). The aim of this study was to report a simple technique based on measurements of liver angles (angulometry) that can be used to predict liver ratios.
Methods
Fifty computed tomography (CT) scans obtained in subjects with normal liver were studied. Four CT scan levels were preselected: level 1 passed by the upper part of the hepatic veins; level 2 passed by the left portal vein branch division; level 3 passed by the right portal vein branch division, and level 4 passed by the gallbladder bed. Left and right tangent lines passing the liver edges were drawn and joined to the centre of the vertebra defining the TL angle. Two lines through, respectively, the plane of the middle hepatic vein and the left portal branches determined the angles of the RL, LL and LLS. Volumetric and angulometric data obtained on levels 2 and 3 in 50 different subjects were compared.
Results
Level 2 CT scans represented the most accurate way of obtaining angulometric measurements. The mean ± standard deviation (SD) angles of the TL and LL were 134 ± 12 ° and 55 ± 12 °, respectively. The mean ± SD percentages of the TL represented by the LL in angulometry and volumetry were 38 ± 7% and 36 ± 6%, respectively (non-significant difference). The mean ± SD percentages of the TL represented by the LLS in angulometry and volumetry were 25 ± 4% and 20 ± 3%, respectively (P < 0.05). The mean ± SD overestimation of the percentage of the TL represented by the LLS in angulometry was 2.7 ± 7.0%.
Conclusions
Angulometry is a simple and accurate technique that can be used to estimate the ratio of the FLR to TL volume on one or two CT (or magnetic resonance imaging) slices. It can be helpful for clinicians, especially before right or extended right hepatectomy and after right portal vein occlusion techniques.
Introduction
Modern hepatic surgery, including living donor liver transplantation, split-liver transplantation and extensive one- or two-step surgery in conjunction with preoperative portal vein embolization (PVE) or portal vein ligation (PVL), requires preoperative knowledge of the volume of the future liver remnant (FLR).1,2 Over the past decade, the standard method of estimating both FLR and total liver volume (TLV) has been represented by three-dimensional software-assisted computed tomography (CT) (SACT) volumetry.2–4 In parallel, multiple formulae accurately estimate the ‘standard’ TLV. Both SACT and formulae are useful in clinical practice. Software-assisted CT is usually performed by radiologists after i.v. contrast-enhanced CT, whereas formulae such as those of Tongyoo et al.,2 Yoshizumi et al.,5 Vauthey et al.6 and Hashimoto et al.7 estimate the ‘standard’ TLV based on body weight (BW) or body surface area (BSA).
The estimation of functional TLV is quite different from that of volumetric TLV as it may in part relate to the presence of tumour in the liver. Although these volumes can be manually removed from TLV using SACT, factors such as age, the presence of liver steatosis, cholestasis and cirrhotic liver changes may affect the volume and functionality of the liver. Thus, even if formulae have the ability to standardize the liver volume calculation, they cannot always estimate the ratio of FLR to TLV, knowledge of which is required before extensive liver resection.2,3,6,8
Multimodal treatments such as target therapies (cetuximab, bevacizumab), in addition to chemotherapies (5-fluorouracil, oxaliplatin, irinotecan), are becoming increasingly efficient in reducing the size and number of liver colorectal metastases.9–14 Therefore, surgeons are more often confronted with the need to define complex multimodal strategies (one- or two-step surgery) including PVE or PVL with the concomitant use of radiofrequency ablation and resection of the primary tumour at the initial consultation and during multidisciplinary discussions.15–27 This requires a preoperative appreciation of the volume and quality of the FLR. In this regard, SACT is mandatory before surgery to estimate not only the amount of liver to remove, but also, and principally, to estimate the amount and quality of liver that will remain. However, in most patients estimations of liver volumes are not available at the first consultation and may not be at the time of multidisciplinary discussions. This is also true for patients with hepatocellular carcinoma (HCC), who may require anatomical major hepatectomy especially after downsizing by transarterial chemoembolization (TACE) and/or PVE.28–30 Furthermore, longterm chemotherapies and multiple sequences of TACE or intra-arterial chemotherapy may affect the nature of the non-tumorous liver parenchyma.30–38
The aim of this study was to report an easy and reproducible technique designated ‘liver angulometry’, which is based on the calculation of liver angles in two liver CT [or magnetic resonance imaging (MRI)] slices and can be used to assess the volumes of the total liver (TL), right liver (RL) and left liver (LL).
Materials and methods
Study design
The study was designed in three parts. The first part was intended to define which of four CT liver levels provided the most accurate estimate of the ratios between the RL, LL and TL in 50 consecutive subjects. In the second part, angulometric data were compared with volumetric data obtained by SACT in another 50 subjects using the most accurate CT scan level(s) identified in the first part of the study. The last part of the study was intended to report an example of angulometry estimating the FLR hypertrophy ratio after PVE or PVL.
Subjects
Intravenous contrast-enhanced CT scans obtained in 100 adults (> 15 years of age) without liver tumours (primary, secondary including liver cysts or other benign liver disease), steatosis, cholestasis, biliary or vascular disease were analysed in two French university hospitals (Louis Mourier University Hospital, Paris, and Reims University Hospital, Reims). Patients with previous hepatectomy, PVE or PVL were excluded from the two first parts of the study. Patients with previous uncomplicated cholecystectomies were included in this study.
Methods
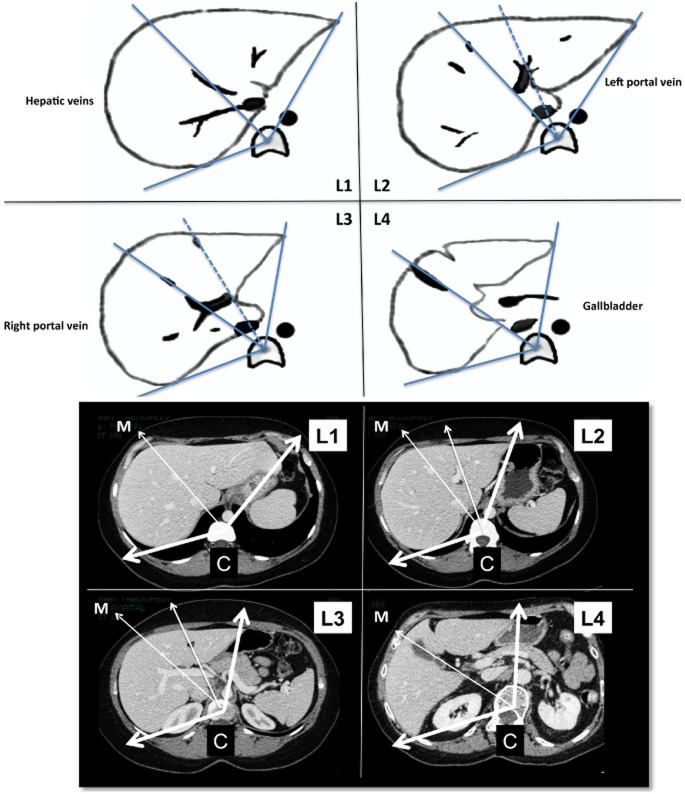
The first 50 CT scans of the liver were classified according to four levels (Fig. 1). Level 1 corresponded to the upper part of the LL along the plane of Couinaud's segment VIII (sVIII), segment IV (sIV) and segment II (sII) so that the three hepatic veins were visible inside the liver parenchyma. Level 2 corresponded to the plane of the left portal vein and branches. Level 3 corresponded to the plane of the right portal vein and branches. Level 4 corresponded to the lowest liver level at the plane of the gallbladder or gallbladder bed in cholecystectomized patients.
Figure 1.
Four levels of angulometry. Level 1 corresponds to the upper part of the liver on the plane of Couinard segments II, IV and VIII, where the three hepatic veins are visible inside the liver parenchyma. Level 2 corresponds to the plane of the left branch of the portal vein. Level 3 corresponds to the plane of the right portal vein and sectorial branches. Level 4 corresponds to the lower level of the plane of the gallbladder. C, central point on the vertebral body; M, middle line
After preliminary adjustments, the centre of the vertebral body was considered as the ‘C’ point. From this point, the TL angles were calculated according to two exterior lines passing tangentially by the edges of the left lateral segments (LLS) and RL (Fig. 1). Then, a middle ‘M’ line in the plane of the middle hepatic vein determined the limits between the RL and LL. This provided four levels of liver (L1 to L4) in which TL, RL and LL angles and the ratios between them could be calculated.
In the second part of the study, the two liver levels that provided the best estimates of RL and LL ratios were selected. Software-assisted CT volumetric data obtained using the OsiriX application (Version 3.9.4, 32 bits; Pixmeo SARL, Geneva, Switzerland)39,40 in 50 different subjects were compared with angulometric data obtained on levels 2 and 3, and reviewed by an independent radiologist who validated volumetric and angulometric data for final analyses.
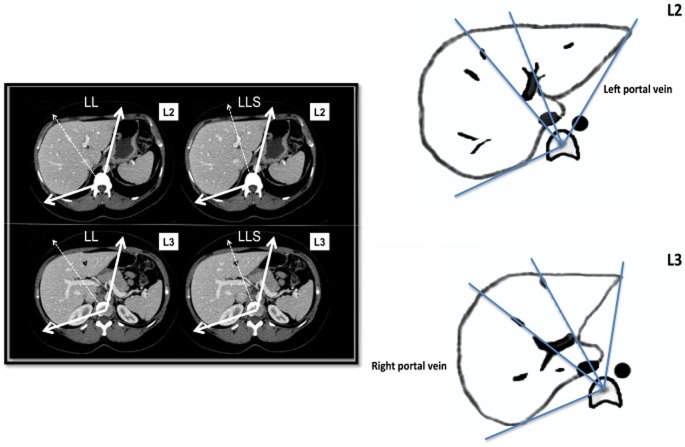
The last part of the study reports an example of an angulometric estimation of the FLR : TLV ratio before and after portal vein occlusion. Indeed, in most patients, the FLR corresponds to the LL or LLS (segments II and III). To calculate the LLS angle corresponding to the FLR, a line passing the central part of the left portal branches (close to the sIII and sIV portal branches on level 2 and passing through the round ligament on level 3) was defined (Fig. 2). To estimate the TLV, a simple formula close to the formulae reported previously was used on the basis that liver weight corresponds to 2.0–2.2% in Theoritical TLV (in litres) = 2% of BW (kg). Therefore, LLS volume (in litres) = (LLS: TLV angle ratio) × TLV (in litres).
Figure 2.
The angle of the left lateral segment (LLS) corresponds to the angle delimited by a line passing by the central part of the left portal branches close to Couinard segments II, III and IV on level 2 (L2) and by the round ligament on level 3 (L3)
Statistical analyses
Descriptive data were expressed as the median and range, or as the mean ± standard deviation (SD). Quantitative data were compared using non-parametric tests. Qualitative data were compared using the chi-squared test. 95% confidence intervals (CIs) were calculated. A P-value of < 0.05 was considered to indicate statistical significance. Regression lines between angulometric and volumetric values were generated and a two-tailed paired t-test was used to compare values.
Results
The characteristics of subjects used in the first part of the study (n = 50) are presented in Table 1. Angulometric data for the LL, RL and TL, obtained on four different liver levels, are represented in Table 2. Levels 2 and 3 were the most accurate levels at which to determine TL, RL and LL angles and ratios. Mean ± SD angles on level 2 were 134 ± 12 ° for the TL and 55 ± 12 ° for the LL. The mean LL : TL ratio on level 2 was 0.38 (range: 0.18–0.54). The comparison between ratios showed that the LL : TL ratio was overestimated at level 1, whereas the posterior part of the RL was overestimated at level 4. Morphometric data showed that 35% of patients in this series had an ‘L < R volume predominant liver’ meaning that the LL had a > 40 ° development to the LL edge on level 1, whereas 29% of patients had a ‘deep liver’, in which an RL angle of > 10 ° developed posteriorly from a horizontal line passing by the ‘C’ point on level 4 (Fig. 3).
Table 1.
Characteristics of subjects assessed in the first part of the study (n = 50)
| Sex, male/female | 17/33 |
| Age, years, mean ± SD | 45.5 ± 15.0 |
| Body weight, kg, mean ± SD | 67 ± 11 |
| Height, cm, mean ± SD | 167 ± 7 |
| BMI, kg/m2, mean ± SD | 23.9 ± 3.0 |
| Patients with previous cholecystectomy, n | 9 |
SD, standard deviation; BMI, body mass index.
Table 2.
Left liver and right liver angulometric data obtained on four levels of computed tomography liver scans in 50 subjects
| Left liver angles, ° | Right liver angles, ° | |||
|---|---|---|---|---|
| Median (range) | Mean ± SD | Median (range) | Mean ± SD | |
| Level 1 | 58 (41–101) | 63 ± 13 | 75 (15–107) | 72 ± 15 |
| Level 2 | 56 (30–82) | 55 ± 12 | 79 (58–132) | 78 ± 12 |
| Level 3 | 56 (23–95) | 55 ± 13 | 77 (50–104) | 77 ± 9 |
| Level 4 | 51 (23–87) | 51 ± 14 | 73 (56–87) | 70 ± 7 |
SD, standard deviation.
Figure 3.
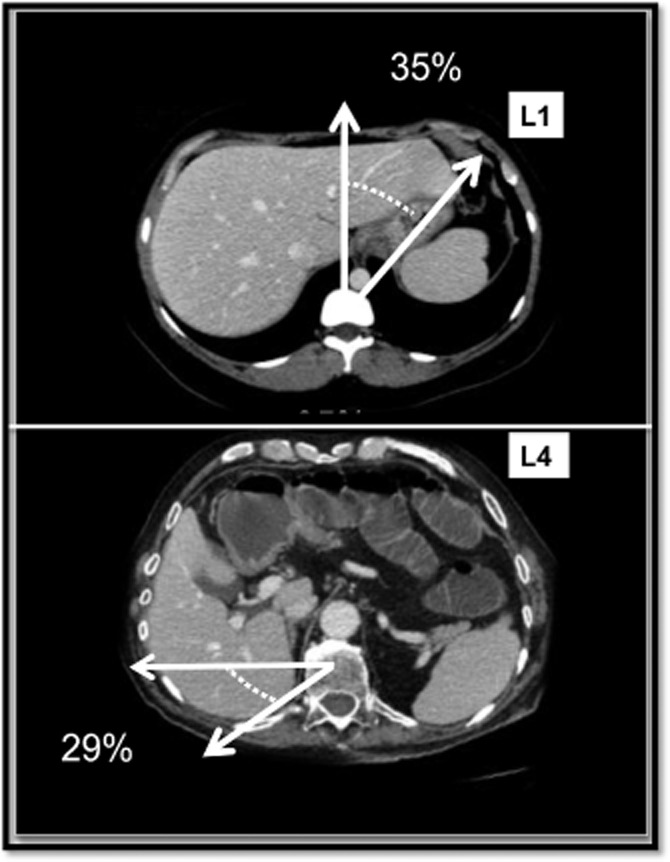
Morphometric data showed that 35% of patients had ‘L> R predominant liver’ meaning that the left liver had a > 40 ° development to the left liver edge on level 1 (L1), and 29% of patients had a ‘deep liver’, with a right liver angle of > 10 ° developed posteriorly from a horizontal line passing point C on level 4 (L4)
The comparisons between volumetric data obtained by SACT and angulometric data (observed on levels 2 and 3) in the remaining 50 patients showed no statistical difference between ratios of RL : TL and LL : TL. Level 2 was the most accurate level for estimating LL ratios. On this level, mean ± SD angulometric versus volumetric LL : TL ratios were 38 ± 7% (95% CI 18–54) versus 36 ± 6% (95% CI 15–52), respectively (non-significant). Contrary to the LL : TL ratio, the mean LLS : TL ratio differed significantly according to whether data were obtained by angulometry or volumetry at 25 ± 4% (95% CI 20–31) versus 20 ± 3% (95% CI 13–25), respectively (P < 0.05) (Fig. 4). However, the mean ± SD percentage of this overestimation of the LLS : TL ratio in angulometry versus volumetry was 2.7 ± 7%.
Figure 4.
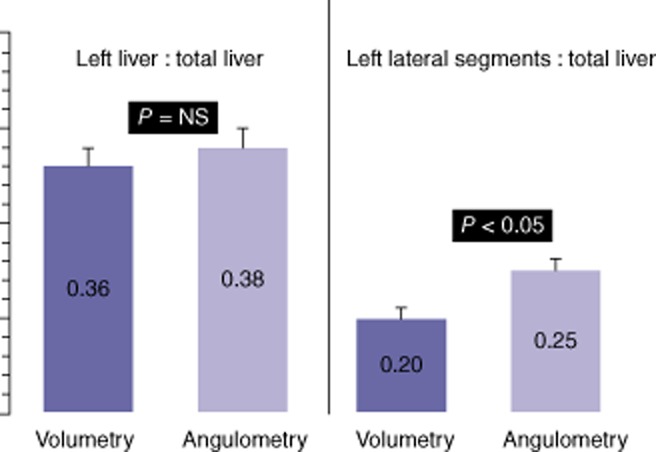
On level 2, mean ± standard deviation (SD) left liver : total liver ratios obtained in angulometry and volumetry, respectively, were 38 ± 7% (range: 18–54%) and 36 ± 6% (range: 15–52%), respectively (P = not significant). Mean ± SD left lateral segments : total liver (LLS : TL) ratios obtained in angulometry and volumetry differed significantly at 25 ± 4% (range: 20–31%) and 20 ± 3% (range: 13–25%), respectively (P < 0.05). The mean ± SD percentage of angulometric overestimation of the LLS : TL ratio was 2.7 ± 7.0%
Formulae might lead to a standard definition of TLV based on BW or BSA. Angulometric data might estimate LL : TL or LLS : TL ratios that correspond to the FLR in most patients who require right or extended right hepatectomy. Consequently, as a first-line tool one or two CT (or MRI) slices can be used to determine easily whether there is a need to perform PVE or PVL before right or extended right hepatectomies. In addition, preliminary results showed that the comparison between the LL and LLS angles before and after right PVE (or PVL) may by itself suggest whether there is (or not) a significant degree of LL hypertrophy (Fig. 5).
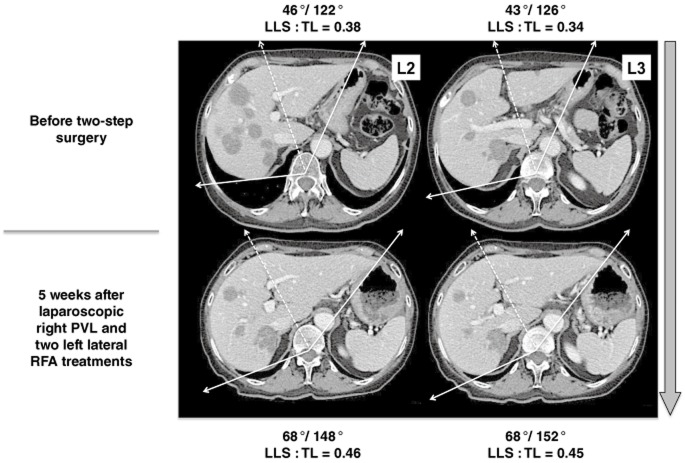
Figure 5.
This patient initially presented with multiple bilobar colorectal liver metastases (type III) that became resectable after 12 cycles of chemotherapy (5-fluorouracil, oxaliplatin and irinotecan). The hypertrophy progression rates after the laparoscopic first step, including radiofrequency ablation (RFA) of the left lateral segment (LLS) nodules, plus right portal vein ligation (PVL) showed significant changes in LLS : total liver (LLS : TL) ratios measured on levels 2 and 3 (L2 and L3). After right PVL, LLS angles progressed from 46° to 68° on L2 and from 43° to 68° on L3. The progression LLS: TL angulometric ratios were from 38% to 46% and from 34% to 45% on L2 and L3 respectively
Discussion
The results of this study showed that angulometry is a simple technique that can help clinicians to accurately calculate the LL : TL and LLS : TL ratios based on one or two CT (or MRI) slices obtained on the plane of the left or right portal veins (levels 2 and 3). The technique can be used to easily predict the FLR : TLV ratio and provide orientation for strategies including right PVE or two-step surgery with right PVL, especially in patients who have undergone a long period of systemic chemotherapy and who may present with an injured liver parenchyma.21–23,25,32,41 The technique can also be applied retrospectively with ease, if necessary.
Many formulae based exclusively on BSA (or BW) are available and can accurately estimate TLV without taking into account volumes that may relate to liver tumours or vascular elements.2 However, although these formulae give an accurate estimation of standard liver volume (weight), they cannot estimate the volume of the RL or LL, nor that of the FLR, and should therefore be combined with SACT volumetry in clinical practice. Indeed, to avoid postoperative liver failure caused by a remaining liver volume that is small for size, the FLR is required to amount to at least 25–30% of the TLV in patients with strictly normal liver parenchyma or in liver transplantation, and 40% in patients with an injured liver parenchyma.3 Injured liver parenchyma includes fibrotic and cirrhotic liver, and organs with chronic liver disease. More and more patients with colorectal liver metastases receive prolonged chemotherapy (or targeted therapies) that may affect the nature of the liver parenchyma.32,33,42–45 Thus, knowledge of the lower limit of the FLR : TLV ratio is crucial if postoperative liver failure and infection are to be avoided. Schindl et al.46 studied 104 patients subjected to liver resection and reported a statistical correlation between the percentage of a smaller FLR and postoperative liver failure and postoperative infectious complications. In this series, an FLR of < 26.6% of TLV was identified as critically related to the occurrence of severe hepatic dysfunction (P < 0.0001) in patients subjected to liver resection for colorectal metastases. Consequently, lower limits for the FLR equivalent to 30% of TLV in patients with normal liver and > 40% in patients with underlying injured liver parenchyma are recommended.3,6
Even if SACT remains the reference standard technique for the evaluation of liver volumes and ratios, several minor critical points should be reconsidered. Firstly, it is vital to ensure correct liver edging and thickness slicing, especially at the juncture between the RL and LL, and before and after PVE, particularly on the plane of the middle hepatic vein. Secondly, it should be noted that some circumstances may cause some variation in liver volumes such as those related to tumour volume. These circumstances may refer to the administration of systemic or intra-arterial treatments for liver metastases, as well as for liver primaries, such as HCC. Thirdly, the inclusion (or exclusion) of the volume of major vessels, including the porta hepatis, inferior vena cava, origin of the hepatic veins and round ligament, in the TLV should be considered. Finally, liver volume may vary according to the nature of the underlying disease or chemo-induced liver diseases, including hepatosteatosis, steatohepatitis, sinusoidal obstructive syndrome (SOS), nodular hyperplastic lesions (regenerative nodular hyperplasia), cholestasis, fibrosis and liver cirrhosis. These issues may in part explain the variations observed between volumetric data and those published after partial graft weighting in living donor transplantation.47 In addition, when SACT is not available, the present authors believe that a simple first-line tool such as angulometry could present a valid alternative method for estimating the FLR : TLV ratio. Indeed, the importance of estimating the volume of the FLR has been shown in the past. Ribero et al.3 reported that estimation of the FLR : TLV ratio could help to anticipate indications for right PVE or PVL and increase the safety of RL resections. Abdalla et al.48 measured liver volumes in 102 Western patients without underlying liver disease. They found that, on average, the LL represented around 33% of the TLV. The LLS represented approximately half of the volume of the LL or 15–16% of TLV. However, a large degree of inter-patient variability in liver volume was observed, with the contributions of the RL and LL to the TLV ranging from 49% to 82%, and 17% to 49%, respectively. Notably, the LLS accounted for 5–27% of TLV, representing 20% of the TLV in more than 75% of patients.48 Angulometric findings in the present series concord with previously reported volumetric data. However, it is important to note that levels 1 and 4 do not provide accurate estimations of RL : TL or LL : TL ratios. Moreover, levels 2 and 3 were found to be the most appropriate for estimating liver volumes and ratios between volumes. However, in the present study, a tendency to overestimate LL : TL and LLS : TL ratios was observed on both levels 2 and 3. Thus, the mean percentage of this overestimation between angulometry and volumetry data was 2–5%. This overestimation is probably related to the presence of variations in the left lobe in the thickness of the anterosuperior part of the LLS and to the edging technique, which requires that a line be drawn tangentially from the edge of the LLS to the ‘C’ point.
Clinicians, and especially liver surgeons working in the setting of tertiary cancers, are confronted with the need to give opinions to patients who present with multiple liver metastases and/or complex liver tumours. Not all patients routinely undergo SACT. Therefore, the angulometric estimation of the LLS : TL ratio using level 2 CT represents an interesting tool with which to predict the necessity of complex strategies. In this setting, the role of liver angulometry is not to replace SACT, but to serve as a first-line tool that will assist in making decisions on whether or not to perform PVE or PVL, especially in patients who require a right or extended right hepatectomy.
In addition, when angulometric LL : TL and LLS : TL ratios are measured, the FLR can be calculated according to the BW of the patient. For example, an adult with a BW of 100 kg will have an estimated standard TLV of 2000 ml. This patient may need an extended right hepatectomy or right hepatectomy with radiofrequency ablation on the LL for chemosensitive colorectal liver metastases. If, in this patient, the ratios of LLS : TL (FLR) obtained on levels 2 and 3 are 20% and 22%, respectively, his FLR volume might be estimated as 400–440 ml (20–22% of 2000 ml). It appears that this patient should benefit from techniques such as PVE or two-step surgery with PVL in order to increase the safety and tolerance of the extended right hepatectomy, especially if he received more than five cycles of chemotherapy.32,41 Furthermore, if at 3–5 weeks after right PVE, the same patient does not have any hypertrophy in the LLS : TL ratio on angulometry, or even if exclusively LLS angle measurements (FLR) remain unchanged, PVE can be considered to have failed and the indications for extended right hepatectomy should be reconsidered as this strategy now appears to be highly risky.46,49 It is the present authors' opinion that this issue is particularly interesting in patients who require right or extended right hepatectomy and who present with liver parenchyma injured by either fibrosis or longterm chemotherapy. For example, patients with RL large HCC on cirrhotic liver might benefit from TACE and PVE before right hepatectomy. If the FLR angles do not increase after 4 weeks, indications for right hepatectomy should be reconsidered. In addition, in cirrhotic patients with enlarged or shrunken livers, volumetric TLV assessment may be inaccurate if it is measured according to functional tests, such as indocyanine green retention at 15 min. In these instances, the only way to judge the capacity of the FLR to regenerate is to measure, by either standard volumetry or angulometry, the degree of hypertrophy of the LL following PVE and/or TACE.50,51
In conclusion, angulometry is not intended to replace SACT, which remains the reference standard method for estimating FLR and TL volumes, but should be regarded as an easy-to-use and cost-effective way of estimating liver volumes and ratios. It can be useful in patients with multiple liver metastases and in those with primary liver tumours with underlying liver disease.
Conflicts of interest
None declared.
References
- 1.Abdalla EK. Portal vein embolization (prior to major hepatectomy) effects on regeneration, resectability, and outcome. J Surg Oncol. 2010;102:960–967. doi: 10.1002/jso.21654. [DOI] [PubMed] [Google Scholar]
- 2.Tongyoo A, Pomfret EA, Pomposelli JJ. Accurate estimation of living donor right hemi-liver volume from portal vein diameter measurement and standard liver volume calculation. Am J Transplant. 2012;12:1229–1239. doi: 10.1111/j.1600-6143.2011.03909.x. [DOI] [PubMed] [Google Scholar]
- 3.Ribero D, Chun YS, Vauthey JN. Standardized liver volumetry for portal vein embolization. Semin Intervent Radiol. 2008;25:104–109. doi: 10.1055/s-2008-1076681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama Y, Li Q, Katsuragawa S, Ikeda R, Hiai Y, Awai K, et al. Automated hepatic volumetry for living related liver transplantation at multisection CT. Radiology. 2006;240:743–748. doi: 10.1148/radiol.2403050850. [DOI] [PubMed] [Google Scholar]
- 5.Yoshizumi T, Taketomi A, Kayashima H, Yonemura Y, Harada N, Ijichi H, et al. Estimation of standard liver volume for Japanese adults. Transplant Proc. 2008;40:1456–1460. doi: 10.1016/j.transproceed.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 6.Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto T, Sugawara Y, Tamura S, Hasegawa K, Kishi Y, Kokudo N, et al. Estimation of standard liver volume in Japanese living liver donors. J Gastroenterol Hepatol. 2006;21:1710–1713. doi: 10.1111/j.1440-1746.2006.04433.x. [DOI] [PubMed] [Google Scholar]
- 8.Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann K, Rickenbacher A, Weber A, Pestalozzi BC, Clavien PA. Chemotherapy before liver resection of colorectal metastases: friend or foe? Ann Surg. 2012;255:237–247. doi: 10.1097/SLA.0b013e3182356236. [DOI] [PubMed] [Google Scholar]
- 10.Nordlinger B, Adam R, Arnold D, Zalcberg JR, Gruenberger T. The role of biological agents in the resection of colorectal liver metastases. Clin Oncol (R Coll Radiol) 2012;24:432–442. doi: 10.1016/j.clon.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Foncillas J, Diaz-Rubio E. Progress in metastatic colorectal cancer: growing role of cetuximab to optimize clinical outcome. Clin Transl Oncol. 2010;12:533–542. doi: 10.1007/s12094-010-0551-3. [DOI] [PubMed] [Google Scholar]
- 12.Uehara K, Ishiguro S, Hiramatsu K, Nishio H, Takeuchi E, Takahari D, et al. Conversion chemotherapy using cetuximab plus FOLFIRI followed by bevacizumab plus mFOLFOX6 in patients with unresectable liver metastases from colorectal cancer. Jpn J Clin Oncol. 2011;41:1229–1232. doi: 10.1093/jjco/hyr115. [DOI] [PubMed] [Google Scholar]
- 13.Robinson SM, Wilson CH, Burt AD, Manas DM, White SA. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:4287–4299. doi: 10.1245/s10434-012-2438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neeff HP, Drognitz O, Klock A, Illerhaus G, Opitz OG, Hopt UT, et al. Impact of preoperative targeted therapy on postoperative complications after resection of colorectal liver metastases. Int J Colorectal Dis. 2012;27:635–645. doi: 10.1007/s00384-011-1360-z. [DOI] [PubMed] [Google Scholar]
- 15.Machado MA, Makdissi FF, Surjan RC, Kappaz GT, Yamaguchi N. Two-stage laparoscopic liver resection for bilateral colorectal liver metastasis. Surg Endosc. 2010;24:2044–2047. doi: 10.1007/s00464-009-0859-7. [DOI] [PubMed] [Google Scholar]
- 16.de Baere T, Robinson JM, Deschamps F, Rao P, Teriitheau C, Goere D, et al. Preoperative portal vein embolization tailored to prepare the liver for complex resections: initial experience. Cardiovasc Intervent Radiol. 2010;33:976–982. doi: 10.1007/s00270-009-9785-2. [DOI] [PubMed] [Google Scholar]
- 17.Abdalla EK. Resection of colorectal liver metastases. J Gastrointest Surg. 2011;15:416–419. doi: 10.1007/s11605-011-1429-6. [DOI] [PubMed] [Google Scholar]
- 18.Narita M, Oussoultzoglou E, Jaeck D, Fuchschuber P, Rosso E, Pessaux P, et al. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br J Surg. 2011;98:1463–1475. doi: 10.1002/bjs.7580. [DOI] [PubMed] [Google Scholar]
- 19.Robles R, Marin C, Lopez-Conesa A, Capel A, Perez-Flores D, Parrilla P. Comparative study of right portal vein ligation versus embolization for induction of hypertrophy in two-stage hepatectomy for multiple bilateral colorectal liver metastases. Eur J Surg Oncol. 2012;38:586–593. doi: 10.1016/j.ejso.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Pinto Marques H, Barroso E, de Jong MC, Choti MA, Ribeiro V, Nobre AM, et al. Perioperative chemotherapy for resectable colorectal liver metastasis: does timing of systemic therapy matter? J Surg Oncol. 2012;105:511–519. doi: 10.1002/jso.22133. [DOI] [PubMed] [Google Scholar]
- 21.Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling two-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 22.Kianmanesh R, Sauvanet A, Hentic O, Couvelard A, Levy P, Vilgrain V, et al. Two-step surgery for synchronous bilobar liver metastases from digestive endocrine tumours: a safe approach for radical resection. Ann Surg. 2008;247:659–665. doi: 10.1097/SLA.0b013e31816a7061. [DOI] [PubMed] [Google Scholar]
- 23.Aussilhou B, Lesurtel M, Sauvanet A, Farges O, Dokmak S, Goasguen N, et al. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg. 2008;12:297–303. doi: 10.1007/s11605-007-0410-x. [DOI] [PubMed] [Google Scholar]
- 24.Homayounfar K, Liersch T, Schuetze G, Niessner M, Goralczyk A, Meller J, et al. Two-stage hepatectomy (R0) with portal vein ligation – towards curing patients with extended bilobular colorectal liver metastases. Int J Colorectal Dis. 2009;24:409–418. doi: 10.1007/s00384-008-0620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karoui M, Vigano L, Goyer P, Ferrero A, Luciani A, Aglietta M, et al. Combined first-stage hepatectomy and colorectal resection in a two-stage hepatectomy strategy for bilobar synchronous liver metastases. Br J Surg. 2010;97:1354–1362. doi: 10.1002/bjs.7128. [DOI] [PubMed] [Google Scholar]
- 26.Brouquet A, Overman MJ, Kopetz S, Maru DM, Loyer EM, Andreou A, et al. Is resection of colorectal liver metastases after a second-line chemotherapy regimen justified? Cancer. 2011;117:4484–4492. doi: 10.1002/cncr.26036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poston GJ, Adam R, Alberts S, Curley S, Figueras J, Haller D, et al. OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23:7125–7134. doi: 10.1200/JCO.2005.08.722. [DOI] [PubMed] [Google Scholar]
- 28.Vilgrain V, Sibert A, Zappa M, Belghiti J. Sequential arterial and portal vein embolization in patients with cirrhosis and hepatocellular carcinoma: the Hospital Beaujon experience. Semin Intervent Radiol. 2008;25:155–161. doi: 10.1055/s-2008-1076689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng PD, Hyder O, Bloomston M, Marques H, Corona-Villalobos C, Dixon E, et al. Sequential intra-arterial therapy and portal vein embolization is feasible and safe in patients with advanced hepatic malignancies. HPB. 2012;14:523–531. doi: 10.1111/j.1477-2574.2012.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okabe K, Beppu T, Masuda T, Hayashi H, Okabe H, Komori H, et al. Portal vein embolization can prevent intrahepatic metastases to non-embolized liver. Hepatogastroenterology. 2012;59:538–541. doi: 10.5754/hge09764. [DOI] [PubMed] [Google Scholar]
- 31.Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2009;16:137–144. doi: 10.1007/s00534-008-0016-z. [DOI] [PubMed] [Google Scholar]
- 32.Pessaux P, Chenard MP, Bachellier P, Jaeck D. Consequences of chemotherapy on resection of colorectal liver metastases. J Visc Surg. 2010;147:193–201. doi: 10.1016/j.jviscsurg.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Rubbia-Brandt L, Lauwers GY, Wang H, Majno PE, Tanabe K, Zhu AX, et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430–439. doi: 10.1111/j.1365-2559.2010.03511.x. [DOI] [PubMed] [Google Scholar]
- 34.Soubrane O, Brouquet A, Zalinski S, Terris B, Brezault C, Mallet V, et al. Predicting high-grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg. 2010;251:454–460. doi: 10.1097/SLA.0b013e3181c79403. [DOI] [PubMed] [Google Scholar]
- 35.Narita M, Oussoultzoglou E, Chenard MP, Fuchshuber P, Rather M, Rosso E, et al. Liver injury due to chemotherapy-induced sinusoidal obstruction syndrome is associated with sinusoidal capillarization. Ann Surg Oncol. 2012;19:2230–2237. doi: 10.1245/s10434-011-2112-6. [DOI] [PubMed] [Google Scholar]
- 36.Kemeny NE, Melendez FD, Capanu M, Paty PB, Fong Y, Schwartz LH, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27:3465–3471. doi: 10.1200/JCO.2008.20.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091–1098. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 38.Imamura H, Seyama Y, Makuuchi M, Kokudo N. Sequential transcatheter arterial chemoembolization and portal vein embolization for hepatocellular carcinoma: the University of Tokyo experience. Semin Intervent Radiol. 2008;25:146–154. doi: 10.1055/s-2008-1076683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Vorst JR, van Dam RM, van Stiphout RS, van den Broek MA, Hollander IH, Kessels AG, et al. Virtual liver resection and volumetric analysis of the future liver remnant using open-source image processing software. World J Surg. 2010;34:2426–2433. doi: 10.1007/s00268-010-0663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dello SA, Stoot JH, van Stiphout RS, Bloemen JG, Wigmore SJ, Dejong CH, et al. Prospective volumetric assessment of the liver on a personal computer by non-radiologists prior to partial hepatectomy. World J Surg. 2011;35:386–392. doi: 10.1007/s00268-010-0877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 43.Abdalla EK, Vauthey JN. Chemotherapy prior to hepatic resection for colorectal liver metastases: helpful until harmful? Dig Surg. 2008;25:421–429. doi: 10.1159/000184733. [DOI] [PubMed] [Google Scholar]
- 44.Chun YS, Laurent A, Maru D, Vauthey JN. Management of chemotherapy-associated hepatotoxicity in colorectal liver metastases. Lancet Oncol. 2009;10:278–286. doi: 10.1016/S1470-2045(09)70064-6. [DOI] [PubMed] [Google Scholar]
- 45.Choti MA. Chemotherapy-associated hepatotoxicity: do we need to be concerned? Ann Surg Oncol. 2009;16:2391–2394. doi: 10.1245/s10434-009-0512-7. [DOI] [PubMed] [Google Scholar]
- 46.Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim KW, Lee J, Lee H, Jeong WK, Won HJ, Shin YM, et al. Right lobe estimated blood-free weight for living donor liver transplantation: accuracy of automated blood-free CT volumetry – preliminary results. Radiology. 2010;256:433–440. doi: 10.1148/radiol.10091897. [DOI] [PubMed] [Google Scholar]
- 48.Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404–410. doi: 10.1016/j.surg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 49.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kianmanesh R, Regimbeau JM, Belghiti J. Selective approach to major hepatic resection for hepatocellular carcinoma in chronic liver disease. Surg Oncol Clin N Am. 2003;12:51–63. doi: 10.1016/s1055-3207(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 51.Pawlik TM, Poon RT, Abdalla EK, Ikai I, Nagorney DM, Belghiti J, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicentre study. Surgery. 2005;137:403–410. doi: 10.1016/j.surg.2004.12.012. [DOI] [PubMed] [Google Scholar]