Abstract
Objectives
This study was conducted to evaluate differences between 915-MHz and 2.45-GHz microwave ablation (MWA) systems in the ablation of hepatic tumours.
Methods
A retrospective analysis of patients undergoing hepatic tumour MWA utilizing two different systems over a 10-month period was carried out.
Results
Data for a total of 48 patients with a mean age of 58 ± 1.24 years were analysed. A total of 124 tumours were ablated; 72 tumours were ablated with a 915-MHz system and 52 with a 2.45-GHz system. Mean tumour diameters were 1.7 ± 0.1 cm in the 915-MHz group and 2.5 ± 0.2 cm in the 2.45-GHz group (P < 0.01). Mean ablation time per burn was 8.1 ± 0.3 min in the 915-MHz group and 4.0 ± 0.1 min in the 2.45-GHz group (P < 0.01). The mean number of burns per lesion was 2.0 ± 0.1 in the 915-MHz group and 1.7 ± 0.1 in the 2.45-GHz group (P < 0.05). The mean ablation time per lesion was 9.7 ± 0.7 min in the 915-MHz group, and 6.6 ± 0.6 min in the 2.45-GHz group (P < 0.01). The 2.45-GHz system demonstrated a better correlation between ablation time and tumour size (r2 = 0.6222) than the 915-MHz system; (r2 = 0.0696). Mean total energy applied per lesion, and energy applied per cm, were greater with the 915-MHz system (P < 0.05 and P < 0.01, respectively). Total energy applied per lesion was similarly correlated for the 2.45-GHz (r2 = 0.6263) and 915-MHz (r2 = 0.7012) systems. Mean total energy applied per cm/min was greater with the 2.45-GHz system (P < 0.05).
Conclusions
Both 915-MHz and 2.45-GHz MWA systems achieve reproducible hepatic tumour ablation. The 2.45-GHz system achieves equivalent, but more predictable and faster ablations using a single antenna system.
Introduction
Liver-directed locoregional therapy has significantly improved survival in patients presenting with hepatic tumours who are not candidates for resection, as well as in patients with specific primary liver cancers who do not undergo liver transplantation.1,2 Locoregional therapy has evolved and expanded significantly over the past two decades and now includes percutaneous ethanol or acetic acid injections, cryoablation, transarterial chemoembolization, transarterial radiotherapy, and thermal and non-thermal ablation.1,2 The popularity of thermal ablation approaches continues to grow, as is demonstrated by the frequent utilization of radiofrequency ablation (RFA) and the increasingly favoured microwave ablation (MWA). Indeed, the application of thermoablative modalities as an effective means of primary and secondary hepatic tumour control is widely supported in the literature.1,3–6
The application of a microwave field to tissue causes dipolar molecules (primarily water) to align and realign according to the variable electromagnetic field. Rapid changes in dipole rotation produce frictional heating,1 which results in tissue degeneration.7 The physics of MWA heating are complex and are attributed to highly interdependent antenna–tissue interactions in the ‘near field’ (the term derived from microwave physics and often used by surgeons to describe the ablation zone, which encompasses the near field).8 This is different from what happens in RFA, in which heating is primarily resistive as a result of the passage of current, and therefore much more susceptible to electric sinks such as blood vessels and conductive foreign bodies.2,9 As a result, a more focal ablation, which deposits greater energy in the target zone, can be applied to a tumour mass and surrounding tissue using MWA (compared to RFA), leading to increased maximal local ablation energy deposition and temperatures following ablation.9–11
Both 915-MHz and 2.45-GHz MWA systems are commercially available in the USA. The 915-MHz generator was introduced by Vivant Medical, Inc. (Mountain View, CA, USA) and approved by the Food and Drug Administration (FDA) in 2003. This system typically employs between one and three 13-gauge antennae, arranged in a customizable, multi-antenna configuration.1 Each antenna for this system connects to an independent, non-synchronized microwave generator and thus this arrangement should not be confused with an antenna array.12,13 An observed decrease in power-handling ability leads to notably restricted power delivery characteristics as a result of the impedance mismatch between the cable and the antenna.6,14,15 A 2.45-GHz system using a different single-antenna design was developed by Microsulis Medical Ltd (Denmead, UK) and approved by the FDA in 2006. In contrast to the 915-MHz system, the 2.45-GHz system uses a single antenna connected to a generator set to deliver 100 watts (W) of power.16 Although in essence both systems produce the desired tissue destruction, it is important to critically evaluate the technical differences between systems to gain a clear understanding of the amount of energy that is applied to a treated lesion.
The radiation efficiency of an antenna depends (largely) on how closely the frequency matches water molecule resonance. As such the 2.45-GHz system is more efficient than the corresponding 915-MHz antenna.17 For example, to achieve a 5-cm diameter ablation zone with the 915-MHz system, 45 W is delivered via each of three antennae and requires approximately 10 min of application.1,16 By contrast, the 2.45-GHz system, set to deliver 100 W via a single antenna, would require 4–6 min of application.1,16 Institutionally, both systems have been employed clinically in both open and laparoscopic approaches to treat primary and secondary hepatic tumours.
The aim of this study was to evaluate a single centre's clinical experience of using these systems and to directly compare the technical aspects of the two systems.
Materials and methods
Institutional review board approval was obtained at the Carolinas Medical Center (CMC), Charlotte, NC, USA. A prospectively collected database was utilized to retrospectively review data for all patients with liver malignancies, primary or metastatic, who underwent MWA therapy at CMC between June 2008 and March 2009.
Patient demographics (age, gender, primary indication for treatment, number of lesions) were recorded in parallel with operative details (tumour diameter MWA system employed, power settings, duration of application, number of applications per lesion). Ablation times and tumour sizes were also analysed to allow a direct comparison of the two systems that would consider the actual total energy applied per burn, the total energy applied per lesion, and the total energy applied per centimetre diameter. Calculations were performed to determine whether any significant difference was present as a result of estimated power loss. To estimate power loss, the power displayed on the screen is converted to actual power at the device tip by multiplying the power displayed on the screen by a factor of 0.76 for the 915-MHz system and 0.78 for the 2.45-GHz system.8,18
All data were collected from the patients' electronic medical records. Data were analysed using GraphPad prism Version 5.0 (GraphPad Software, Inc., San Diego, CA, USA) and stratified by the issue of interest, which was treatment type (915-MHz system or 2.45-GHz system). Significance was indicated by a P-value of < 0.05. Groups were compared using Student's t-tests and analysis of variance (anova) with Tukey's honestly significant differences (HSD) post hoc tests. Outcomes of interest included: energy applied per burn; total energy applied per lesion, and total ablation time per lesion for both absolute power and adjusted for estimated power loss with each system.
Results
Data for 48 patients (28 male, 20 female) were reviewed in this study. The mean ± standard deviation (SD) age of the patients was 58 ± 1.24 years (median: 61 years; range: 35–82 years). Thirty-three patients underwent a laparoscopic procedure and 15 an open procedure. An open approach was chosen when a concomitant liver resection could not be completed laparoscopically, or a separate procedure (e.g. colectomy) was undertaken simultaneously. Thirteen concomitant hepatic resections were performed (three laparoscopic and 10 open). Concomitant liver resection was performed at the discretion of the attending surgeon when the patient had multiple lesions and adequate liver reserve. Final pathology revealed hepatocellular carcinomas (HCCs) in 18 patients, colorectal metastases in 12 patients and other liver lesions in 18 patients.
Using the 915-MHz system, duration of total ablation time per lesion ranged from 2 min to 40 min and utilized one to three antennae per burn for lesions measuring 0.3–5.0 cm in diameter. Using the 2.45-GHz system, total ablation time was 2–24 min for lesions measuring 0.3–6.5 cm in diameter. Up to four burns per lesion were performed with either system based on lesion size and appearance on ultrasound after each burn to ensure complete ablation. In total, 124 tumours were ablated (Table 1). All ablation times were greater for the 915-MHz than the 2.45-GHz system (Table 1).
Table 1.
Tumour and burn characteristics in patients treated with the 915-MHz and 2.45-GHz microwave ablation systems
| Parameter | Microwave ablation system | P-value | |
|---|---|---|---|
| 915-MHz | 2.45-GHz | ||
| Number of tumours ablated | 72 | 52 | N/A |
| Number of lesions per patient, mean | 3 | 2 | 0.25 |
| Lesion size, cm, mean ± SD (median) | 1.66 ± 0.12 (1.5) | 2.46 ± 0.22 (2.0) | <0.01 |
| Power per burn, W | 45 W (n = 69) | 100 W (n = 45) | |
| 40 W (n = 3) | 80 W (n = 7) | ||
| Number of antennae used per burn | 1 antenna: 37% | 1 | |
| 2 antennae: 24% | |||
| 3 antennae: 39% | |||
| Number of burns per lesion, mean ± SD (median) | 2.03 ± 0.11 (1) | 1.65 ± 0.14 (1) | <0.05 |
| Ablation time per antenna burn, min, mean ± SD (median) | 8.19 ± 0.31 (10) | 4.00 ± 0.14 (4) | <0.01 |
| Energy applied per burn, J, mean ± SD (median) | 25 278 ± 1910 (27 000) | 22 920 ± 1018 (24 000) | 0.33 |
| Total energy applied per lesion, J, mean ± SD (median) | 54 719 ± 6080 (40 500) | 38 818 ± 3895 (24 000) | <0.05 |
| Energy applied per cm, J, mean ± SD (median) | 32 111 ± 1975 (27 000) | 17 750 ± 1540 (14 057) | <0.01 |
| Total ablation time per lesion, min, mean ± SD (median) | 9.66 ± 0.71 (10.0) | 6.56 ± 0.64 (4.5) | <0.05 |
| Total energy applied per cm/min/lesion, J, mean ± SD (median) | 2510 ± 227 (1800) | 3645 ± 433 (2864) | <0.05 |
SD, standard deviation; N/A, not applicable.
On in-depth analysis, total ablation time and tumour diameter demonstrated a closer correlation for the 2.45-GHz system (r2 = 0.622) compared with the 915-MHz system (r2 = 0.070) (Fig. 1). It should be noted that five tumours measuring >5 cm in diameter were ablated with the 2.45-GHz system, whereas no tumours of this size were ablated using the 915-MHz system. Mean total energy application per lesion was similarly correlated for the 2.45-GHz system (r2 = 0.625) and the 915-MHz system (r2 = 0.681) (Fig. 2). Finally, the evaluation of energy applied adjusted for estimated power loss revealed a significant difference for energy applied per cm for both systems (P < 0.05 for the 2.45-GHz system; P < 0.01 for the 915-MHz system) (Fig. 3), but no differences for total energy applied or energy applied per cm/min (P > 0.05).
Figure 1.
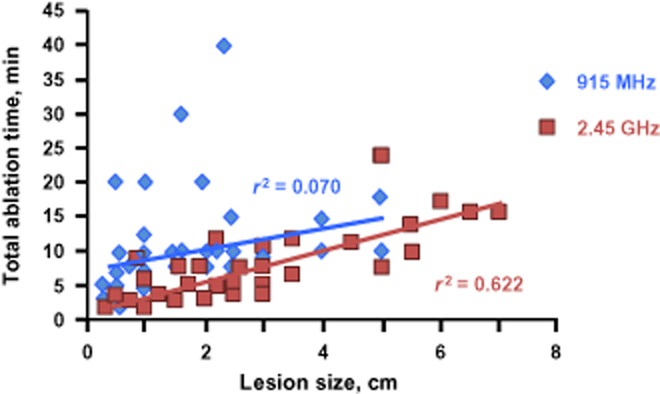
Total ablation time according to lesion size using the 2.45-GHz and 915-MHz microwave ablation systems
Figure 2.
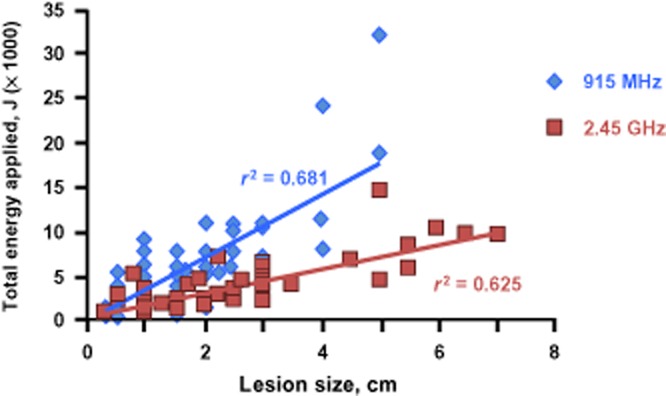
Total energy applied according to lesion size using the 2.45-GHz and 915-MHz microwave ablation systems
Figure 3.
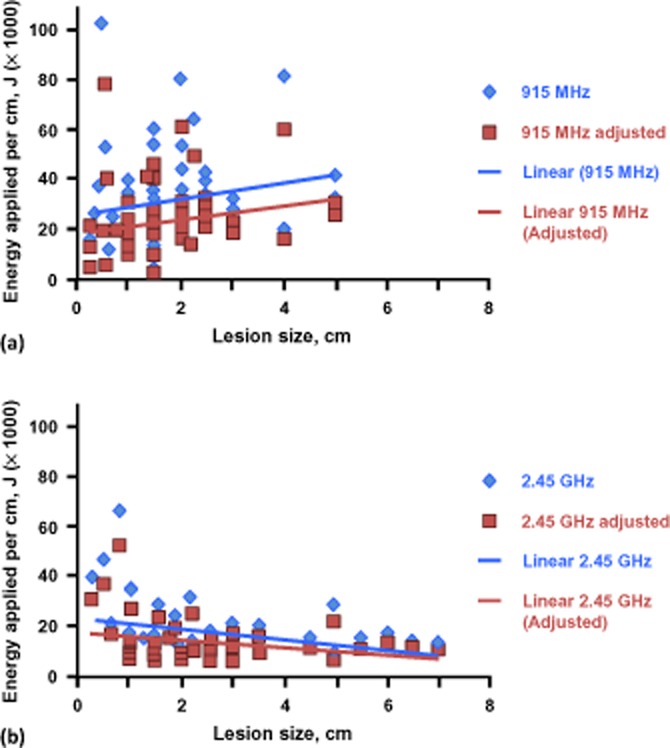
(a) Energy applied per cm of lesion using the 915-MHz system and the 915-MHz system adjusted for estimated power loss. (b) Energy applied per cm of lesion using the 2.45-GHz system and the 2.45-GHz system adjusted for estimated power loss
Discussion
This report summarizes an experience with two different methods of MWA in the treatment of hepatic tumours, using 915-MHz and 2.45-GHz systems, over a 10-month period. The 915-MHz system allows the performance of up to three simultaneous ablations because this system comprises three separate 915-MHz generators. The authors prefer this system for multiple small-volume ablations because, in their experience, this system provides a comparatively smaller ablation zone. Recall that an observed decrease in power-handling ability leads to notably restricted power delivery characteristics as a result of the impedance mismatch between the cable and antenna. This, in turn, results in decreased energy delivery efficiency with the 915-MHz system. Conversely, the 2.45-GHz system has a higher power output as a result of increased efficiency, which results in the ablation of a comparatively larger zone in a shorter time period. However, this system comprises one generator, which necessitates serial ablations. As a result, the authors prefer this system for large-volume and single lesion ablations.
The data revealed that the ablation time per burn required by the 2.45-GHz system was significantly shorter than that required using the 915-MHz system (4.0 ± 0.1 min/burn versus 8.2 ± 0.3 min/burn; P < 0.01), even when total ablation time per lesion is considered (6.6 ± 0.6 min versus 9.7 ± 0.7 min; P < 0.05). Although the energy applied per burn does not differ significantly between the systems (P = 0.33), the energy applied per lesion (P < 0.05) and energy applied per cm (P < 0.01) were greater with the 915-MHz system. Conversely, the total energy applied per cm/min/lesion was greater for the 2.45-GHz (3645 ± 434 J/cm/min) than for the 915-MHz (2510 ± 228 J/cm/min) system (P < 0.05). Thus, the 2.45-GHz system delivered more energy per burn than the 915-MHz system when power loss was taken into account as a result of the higher frequency employed (optimum on the frequency scale), and provided a correspondingly shorter ablation time. However, this effect is not evident after adjusting for total energy applied per lesion as the total energy delivered is greater with the 915-MHz system because this system employs more antennae per burn, and more burns per lesion.
Less time was required for ablation with the 2.45-GHz system given equal power as a result of higher antenna power efficiency. Effective power delivery was also greater for the 2.45-GHz system than for the 915-MHz system in a single-antenna burn. This effect reflects not only differences between generators, but also differences in efficiency of energy transfer from MWA to heat within the ablation zone. Additionally, substantially more ‘radiation’ and heating in the near field than in the surrounding ‘far field’ occurs when using the 2.45-GHz than when using the 915-MHz system.8
Estimations of power loss for both the 915-MHz and 2.45-GHz systems are rudimentary as the reflections (which reduce energy delivery) vary according to impedance mismatches.18 Further, impedance for MWA derives from the interaction of the antenna with the tissue because tissue impedance changes as it undergoes ablation.8,18 It is the impedance of the antenna submerged in tissue and the impedance mismatch between the transmission cable to the antenna and the antenna itself that change.8,18 These effects, in turn, change the efficiency of power delivery.8 Put another way, reflection loss is related to impedance mismatch between a system's impedance (usually 50 Ohm for the cable and generator) and the antenna's impedance.8 This mismatch depends on antenna-characteristic impedance, as well as on the electrical properties of surrounding substances within the near field and their temperature.8 However, these estimates of power loss serve to demonstrate the overriding concept that power loss occurs with both systems, but this loss does not substantially affect the total energy applied to lesions as the systems are currently employed in clinical practice.
The pursuit of the ideal energy source for the thermoablation of hepatic tumours is ongoing. The perfect ablative technology should provide a reliable, predictable, powerful source of energy for tumour destruction, regardless of local tissue conditions and the surrounding environment.10,19 In selecting the most appropriate system for MWA, several factors must be considered, including lesion location, energy deposition and associated application time. It is important to realize the differences in the mechanism of heat generation at different frequencies, which can be translated to provide increased, more focal energy delivery per burn at the centre of the ablation zone, partly as a result of decreased perfusion-related energy dissipation.15 Similarly, at lower frequencies the microwave near field is smaller and surrounded by a far field, which results in a concentration of energy in a smaller region and increased penetration.17
The 915-MHz system has several positive compensatory features, including the fact that multiple antennae can be employed concomitantly to allow the creation of a customizable microwave near-field configuration. This can be especially useful when the ablation must be tailored secondary to surrounding anatomic landmarks, such as when a lesion presents near hepatic veins or close to the confluence of the left and right hepatic ducts. This ability to use multiple antennae also allows for multiple small ablations to be performed simultaneously, such as in multiple small colorectal metastases.
Nevertheless, there are several reasons why the 915-MHz system is less efficient in terms of energy deposition, as a result of which it requires increased application time (although it is still faster than RFA).2 At this lower frequency more energy is dissipated in perfusion (an increased amount is wasted) secondary to the less focal nature of the energy deposition pattern. Moreover, the small coaxial cable employed within the 915-MHz system also results in more power loss as a result of coaxial transmission line losses.8 A ‘comet effect’ is a primarily electromagnetic effect in which the near-field configuration looks like a comet tail, and thus heating happens in this pattern and occurs as a result of hot gases pushing back up the applicator tract with resultant heating of the antenna tip.20 This shaft heating is caused by reflection of the microwave energy secondary to an impedance mismatch, as previously discussed, as well as by a negligible conduction of heat from the ablation zone along the shaft, which must be cooled when using an antenna percutaneously to avoid skin burns.8,21
Likewise, the 2.45-GHz system offers certain clinical advantages, including a shorter ablation time (for the same wattage) and the creation of a reproducible 4-cm MWA with each application. Because of this efficiency, the 2.45-GHz system is better suited than the 915-MHz system to the ablation of single, large lesions (e.g. focal HCC tumours of >5 cm). Additionally, because this system is subject to minimal power loss (secondary to a low degree of shaft heating and good insulation of the shaft by a fluorinated ethylene polymer sheath), there is no risk for skin burns from the antenna shaft.8,18,21
Upon initial evaluation, there appear to be several distinct advantages associated with the 2.45-GHz system. However, on closer inspection, it is apparent that more total energy is applied per lesion with the 915-MHz system secondary to its design. Given the particular strengths and weaknesses of each of these MWA systems, it is recommended that the surgeon should choose the system based on lesion size and location, and with consideration for surrounding structures.
It is important to highlight the potential limitations of this study, such as its retrospective nature and the diversity of the primary and metastatic liver cancers present in the study population. In conducting similar comparative studies in the future, the use of stratified selection criteria for patients and underlying pathologies will be necessary to fully evaluate the relative benefits and limitations of different MWA systems.
Finally, this study did not include a cost-effectiveness analysis because it focused on technical differences between systems. Reported generator costs are essentially similar, at US$35 000 and US$40 000 for the 915-MHz and 2.45-GHz systems, respectively, but three 915-MHz generators are typically required. The cost of using one antenna is US$1815 for the 915-MHz system and US$1950 for the 2.45-GHz system. Thus, the cost of using three 915-MHz antennae is potentially triple that of using a single 2.45-GHz antenna. However, actual costs are determined by a contract between the institution and the individual distributor. An accurate cost comparison would also need to consider the monetary savings to be derived from the decrease in operating room time associated with the use of the 2.45-GHz system.
In conclusion, the use of emerging and effective thermoablative modalities continues to expand the treatment options available to patients presenting with hepatic tumours. The current study confirms that MWA delivered by either of the two systems evaluated in this study is a clinically efficacious therapy. Further, the 2.45-GHz system achieves equivalent, but more predictable and faster ablations, given a single-antenna system.
Conflicts of interest
None declared.
References
- 1.Padma S, Martinie JB, Iannitti DA. Liver tumour ablation: percutaneous and open approaches. J Surg Oncol. 2009;100:619–634. doi: 10.1002/jso.21364. [DOI] [PubMed] [Google Scholar]
- 2.Simo KA, Sereika SE, Newton KN, Gerber DA. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol. 2011;104:822–829. doi: 10.1002/jso.21933. [DOI] [PubMed] [Google Scholar]
- 3.Liang P, Dong BW, Yu X, Yang Y, Yu D, Su L, et al. Prognostic factors for percutaneous microwave coagulation therapy of hepatic metastases. AJR Am J Roentgenol. 2003;181:1319–1325. doi: 10.2214/ajr.181.5.1811319. [DOI] [PubMed] [Google Scholar]
- 4.Liang P, Wang Y, Yu X, Dong B. Malignant liver tumours: treatment with percutaneous microwave ablation – complications among a cohort of 1136 patients. Radiology. 2009;251:933–940. doi: 10.1148/radiol.2513081740. [DOI] [PubMed] [Google Scholar]
- 5.Mack MG, Straub R, Eichler K, Engelmann K, Zangos S, Roggan A, et al. Percutaneous MR imaging-guided laser-induced thermotherapy of hepatic metastases. Abdom Imaging. 2001;26:369–374. doi: 10.1007/s002610000197. [DOI] [PubMed] [Google Scholar]
- 6.Knigge U, Hansen CP, Stadil F. Interventional treatment of neuroendocrine liver metastases. Surgeon. 2008;6:232–239. doi: 10.1016/s1479-666x(08)80033-9. [DOI] [PubMed] [Google Scholar]
- 7.Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466–486. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen A, Rosen HD, Edwards SD. New frontiers in radio frequency (RF)/microwaves in therapeutic medicine. In: Golio M, editor. Microwave and RF Product Applications. Boca Raton, FL: CRC Press; 2003. pp. 16-1–16-23. [Google Scholar]
- 9.Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Arch Surg. 2002;137:422–426. doi: 10.1001/archsurg.137.4.422. discussion 427. [DOI] [PubMed] [Google Scholar]
- 10.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38:135–143. doi: 10.1067/j.cpradiol.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iannitti DA, Heniford T, Hale J, Grundfest-Broniatowski S, Gagner M. Laparoscopic cryoablation of hepatic metastases. Arch Surg. 1998;133:1011–1015. doi: 10.1001/archsurg.133.9.1011. [DOI] [PubMed] [Google Scholar]
- 12.Iannitti DA, Martin RC, Simon CJ, Hope WW, Newcomb WL, McMasters KM, et al. Hepatic tumour ablation with clustered microwave antennae: the US Phase II Trial. HPB. 2007;9:120–124. doi: 10.1080/13651820701222677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hope WW, Schmelzer TM, Newcomb WL, Heath JJ, Lincort AE, Norton HJ, et al. Guidelines for power and time variables for microwave ablation in a porcine liver. J Gastrointest Surg. 2008;12:463–467. doi: 10.1007/s11605-007-0248-2. [DOI] [PubMed] [Google Scholar]
- 14.Sindram D, Lau KN, Martinie JB, Iannitti DA. Hepatic tumour ablation. Surg Clin North Am. 2010;90:863–876. doi: 10.1016/j.suc.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng. 2010;38:65–78. doi: 10.1615/critrevbiomedeng.v38.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumours: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 17.Schmink JR, Leadbeater NE. Microwave heating as a tool for sustainable chemistry: an introduction. In: Leadbeater NE, editor. Microwave Heating as a Tool for Sustainable Chemistry. Boca Raton, FL: CRC Press; 2010. pp. 1–21. [Google Scholar]
- 18.Mehdizadeh M, editor. Microwave/RF Applicators and Probes for Material Heating, Sensing and Plasma Generation. A Design Guide. Oxford: Elsevier; 2010. Fundamentals of field applicators and probes at RF and microwave frequencies; pp. 35–66. [Google Scholar]
- 19.Brace CL. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol. 2009;38:61–67. doi: 10.1067/j.cpradiol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanus G, Boetto R, Gringeri E, Vitale A, D'Amico F, Carraro A, et al. Microwave thermal ablation for hepatocarcinoma: six liver transplantation cases. Transplant Proc. 2011;43:1091–1094. doi: 10.1016/j.transproceed.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 21.Sindram D, Haley K, McKillop IH, Martinie JB, Iannitti DA. Determination of angle, depth, and distance of antennae as skin burn risks in microwave ablation in a porcine model. J Interv Oncol. 2010;3:46–52. [Google Scholar]


