Abstract
Background
Tumour permittivity feedback control is a novel method for microwave ablation (MWA) that theoretically allows for larger, more predictable ablations. This prospective case series evaluates the feasibility and efficacy of MWA of liver malignancies using a device with tumour permittivity feedback control.
Methods
Ten consecutive patients initially determined to be candidates for surgical resection of a liver malignancy underwent intra-operative MWA with tumour permittivity feedback control followed by a surgical resection. A 14-gauge Medwaves microwave antenna was used to deliver a single treatment according to the manufacturer's recommendations. Tumours were assessed grossly as well as by haematoxylin and eosin (H&E) and tetrazolium chloride staining. The primary end point was per cent tumour necrosis.
Results
The median maximum ablation diameter measured was 4.1 cm (range 3.0–6.8). The median ablation volume was 8.7 cm3 (range 4.84–17.55). Six of the 10 tumours demonstrated a pathological complete response (CR). Six of seven tumours ≤3 cm demonstrated a pathological CR. Zero of the three tumours ≥3 cm had a pathological CR, but all had ≥50% tumour necrosis. All patients survived and there were no ablation-related morbidities.
Discussion
MWA of liver tumours with tumour permittivity feedback control is feasible and appears effective for the treatment of small (<3 cm) liver tumours.
Introduction
Thermal ablation by either microwave (MW) or radiofrequency (RF) has proven efficacy in the treatment of primary and metastatic liver cancer and has become a standard of care treatment for selected, small liver tumours not amenable to surgical resection.1 Microwave ablation (MWA) offers several advantages over radiofrequency ablation (RFA), including faster heating over a larger volume, simultaneous multiple applicator use and no requirement for ground pads.2
Recent advances in engineering have allowed for the development of an advanced MW device that may allow more uniform ablations to be created in a shorter period of time compared with currently available RF and MW systems. The newly designed Medwaves (San Diego, CA, USA) MWA system has tumour permittivity feedback control which allows real-time monitoring of ablation conditions and modulation of the power and frequency of delivered MW energy.3 As biologically active tissues are heated, properties of permittivity change. Thus, energy deposition can be maximized and reverse power, a measure of reflectivity, is minimized. By maximizing the amount of delivered forward power, active heating increases and may induce cellular death more uniformly in the target area.3
Because it uses heat, this new device should have complication rates similar to currently available RF and MW energy devices, and lower than cryoablation.1 In a recent study in pulmonary tumours, Wolf et al. 3 demonstrated that MWA with tumour permittivity feedback control results in cytotoxicity and extension of the ablation zone into aerated peri-tumoral pulmonary parenchyma. The purpose of this ablate and resect study is to determine the feasibility and efficacy of MWA with tumour permittivity feedback for the treatment of liver tumours.
Materials and methods
Patient population
This ablate and resect prospective study was Institutional Review Board approved and HIPAA compliant. Between June 2010 and October 2011, a tumour permittivity feedback control MWA system was used on 10 patients (5 men, 5 female) that were scheduled to undergo curative resection of a liver malignancy by a single surgeon with 10 years experience in liver tumour ablation. Informed consent was obtained.
Microwave ablation system
A Medwaves tumour permittivity feedback control MWA system was used in the ‘temperature control’ mode. This generator adapts to the changing conditions within the ablation zone by continuously and automatically adjusting the power (10–32 Watts) and frequency (902–928 MHz) of delivered MW energy to maintain a temperature of 110–120 °C at the microwave antenna active-tip. A single 14-guage, 2-cm active tip, 15-cm straight antenna was used in each session to deliver a single 10-min application at the target temperature. Multiple, overlapping ablations were not permitted in this study to allow adequate characterization of the ablation created by a single antennae with a single treatment.
Microwave ablation protocol: ablation and resection
All operations were performed at a single institution by a single surgeon, and patients were routinely placed under general anaesthesia. Using ultrasound guidance, a single, straight microwave antenna was placed into the tumour, and the generator was powered in the temperature control mode in accordance with the manufacturer's recommendations for 10 min. Upon completion, the antenna was removed. The tumour including the zone of ablation and the tract of the antenna was then resected as initially planned to achieve an R0 resection.
Pathological correlation
The resected liver containing the ablated tumour was transported en-bloc to the surgical pathology department for analysis. Specimens were grossly inspected by the pathologist. Specimens were sectioned at 5-mm intervals allowing the ablation zone to be measured in three dimensions. Scaled digital photos were also taken. Triphenyl tetrazolium chloride (TTC) staining was performed of representative sections to determine the extent of cellular death. With binary staining characteristics, TTC staining assays were used to assess viability in regions deemed equivocal on standard haematoxylin and eosin (H&E) examination. Positive TTC staining was indicative of tissue viability. A lack of staining was consistent with cellular death.4 Resection margins were also analysed in accordance with standard pathological protocol. Complete necrosis was defined as the absence of neoplastic cells along with the presence of amorphous material while the diagnosis of active neoplasm was based on the demonstration of neoplastic cells. Similar to Pompilli et al.,5 the amount of necrosis within still-viable tumours was estimated on a percentage basis by a pathologist during microscopic assessment; the nodules were divided into 4 groups based on the demonstration of complete necrosis, partial necrosis greater than 50%, partial necrosis less than 50% and absent necrosis.
Volumetric calculations and data analysis
All tumours were measured in two-dimensions on intra-operative ultrasound enabling calculation of the median maximum tumor diameter. Gross measurements in three-dimensions of the ablation zone were made. Approximate ablation zone diameters and volumes were calculated based upon gross post-resection measurements and the assumption of an ellipsoid geometric shape: V = 1/6•piy•z where V is volume and x, y, and z represent the diameter along three orthogonal axes.
Results
Between June 2010 and October 2011, a tumour permittivity feedback control MWA system was used on 10 patients (5 men and 5 female) that were scheduled to undergo curative resection of a liver malignancy. Their median age was 55 years (range 42–83).
Three of the tumors were primary hepatocellular carcinomas (HCCs), two associated with cirrhosis. Seven were metastatic lesions from colorectal primaries. The median maximal tumour diameter was 1.9 cm (range 0.7–5.0). On gross inspection, a core of ablated tan tissue with a surrounding narrow hyperemic rim of coagulation necrosis was observed (Fig. 1A). The ablated zone was encompassed by normal parenchyma. With TTC staining, the non-viable tissue is highlighted (Fig. 1B). The zone of cell death measured by TTC staining was always greater than the macroscopic appearing area by approximately 25%. H&E staining demonstrates findings of coagulation necrosis and ablation-related architecture alteration (Fig. 2). No large vessels were identified in or immediately adjacent to any of the ablation zones thereby prohibiting any comment regarding a heat sink effect in this series.
Figure 1.
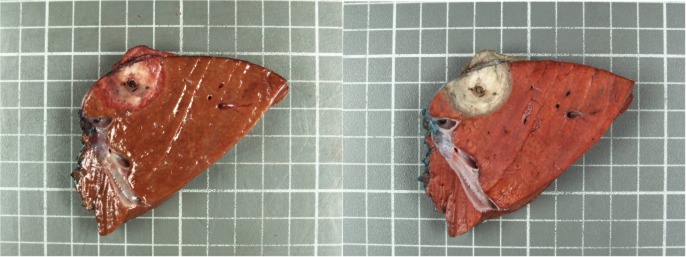
(A,B) Grossly seen is a post-microwave ablation (MWA) and resection hepatic tumour within the lobectomy specimen. (A) Fresh, without staining and (B) with triphenyl tetrazolium chloride (TTC) staining highlighting the non-viable area
Figure 2.
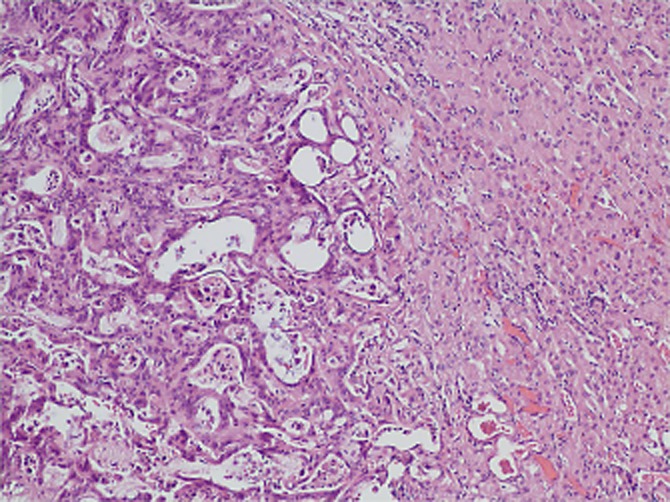
Haematoxylin and eosin (H&E) (100×) staining of metastatic hepatic nodule post-microwave ablation (MWA) and resection. Ablated metastatic adenocarcinoma (left) with normal liver tissue (right)
Ablation zones were measured with a median ablation long-axis diameter of 4.1 cm (range 3.0–6.8) and median estimated volume of 8.7 cm3 (range 4.8–17.6). Six tumours had complete necrosis and four tumours had partial ablation >50% after MWA (Table 1). Six of the seven tumours <3 cm had a complete necrosis response, and all three of the tumours >3 cm had a partial ablation response >50% (Table 2).
Table 1.
Tumour and ablation characteristics in 10 patients treated with microwave ablation using tumour permittivity feedback control then subjected to surgical resection
| Patient number | Tumour size (cm) | Ablation size (cm) 3 dimensions | Tumour ablation volume (cm3) | Complete ablation | Partial ablation >50% | Partial ablation <50% |
|---|---|---|---|---|---|---|
| 1 | 0.9 × 0.9 | 3.6 × 3 × 1.6 | 9.05 | X | ||
| 2 | 0.6 × 0.7 | 5 × 2.3 × 1.4 | 8.43 | X | ||
| 3 | 4.6 × 3.8 | 5.7 × 2.8 × 2.1 | 17.55 | X | ||
| 4 | 0.7 × 0.7 | 6.8 × 2 × 1.8 | 12.82 | X | ||
| 5 | 3.2 × 3.1 | 3.3 × 2.5 × 2.1 | 9.07 | X | ||
| 6 | 1.1 × 1.5 | 5.1 × 1.7 × 1.6 | 7.26 | X | ||
| 7 | 5 × 3 | 4.1 × 3.5 × 2 | 15.03 | X | ||
| 8 | 2.8 × 1.9 | 4 × 2.5 × 1.6 | 8.38 | X | ||
| 9 | 1.2 × 1 | 4 × 2.1 × 1.1 | 4.84 | X | ||
| 10 | 2.3 × 1.6 | 3 × 2.5 × 1.5 | 5.89 | X | ||
Table 2.
Results of pathological examination of 10 microwave ablation-treated nodules
| Complete necrosis | Partial necrosis >50% | Partial necrosis <50% | Total | |
|---|---|---|---|---|
| <3 cm | 6 | 1 | 0 | 7 (7/10) |
| >3 cm | 0 | 3 | 0 | 3 (3/10) |
| Total | 6 (6/10) | 4 (4/10) | 0 | |
Discussion
RFA, the most commonly used thermal ablative modality, has an established role in the treatment of a variety of unresectable malignancies owing to its proven efficacy and safety.6,7 RFA as a complementary or alternative therapy provides a minimally invasive treatment option for selected patients. It is an effective treatment for small (<3 cm) HCCs in cirrhotics awaiting orthotopic liver transplantation.5,8 RFA provides complete necrosis in approximately 65% of treated, small hepatomas (<3 cm) in patients awaiting liver transplant.5,8 Complete response rates fall to 11–25% when tumour size exceeds 3 cm.5,8
Early data suggest equivalency between RFA and MWA for ablation of small HCC in terms of both efficacy and morbidity.9–12 MWA has the potential advantage over RFA of being less dependent on tissue properties, as well as, allowing for the generation of consistently higher intra-tumoural temperatures resulting in faster ablations, requiring fewer applications per treatment session.13 MW energy also minimizes the increase in impedance caused by RFA. Radiofrequency induced tissue charring results in increased impedance limiting the spread of energy and therefore, decreasing ablation size.14
RF electrodes currently available commercially have ablation algorithms based on temperature or tissue impedance.15 A criticism of the early MWA systems is the lack of feedback from the tissue level to the antennae as the treatment is being delivered. MW energy delivered at a frequency of 915 MHz results in thermal cytotoxic changes at the cellular level that alter the dielectric properties of the tissue. The amount of energy deposited depends on tissue temperature as well as permittivity. As tissue temperatures approach 60–70 °C, electrical conductivity increases by a factor of 1.5–2.0 while permittivity increases only by 5–10%.3
MWA with tumour permittivity feedback attempts to overcome this limitation by allowing the generator to adapt to the changing conditions within the ablation zone by continuously and automatically adjusting the power (10–32 Watts) and frequency (902–928 MHz) of delivered MW energy to maintain a temperature of 110–120 °C at the microwave antenna active-tip and maximize tissue permittivity. Tumour permittivity feedback offers the theoretical advantage of faster, more uniform ablations with less charring and therefore, less impedance compared with conventional MWA systems that do not provide feedback from the target area tissue during the ablation. A recent study evaluating MWA with tumor permittivity feedback in lung tissue, showed ablation around vessels <4 mm without evidence of heat sink or collateral damage/thrombosis suggesting the possibility of improved outcomes compared with conventional forms of thermal ablation.3
The current data shows excellent results with MWA using tumour permittivity feedback for the treatment of small liver tumours (<3 cm) using a single antennae, single treatment approach with complete tumour necrosis in 6/7 treated tumours. Imaging studies overestimate complete ablation rates.5 Pathological evaluation is a more accurate way to determine the effectiveness of ablation and has been reported after RFA as bridging therapy in patients undergoing liver transplantation.5,8 Ablate and resect studies offer another way to more accurately define completeness of ablation through pathological evaluation. This is the first ablate and resect study evaluating a MWA system with tumour permittivity feedback control for the treatment of liver tumours.
The one tumour <3 cm in the current series that did not have a complete ablation was the result of a technical failure. After further evaluation, this may be the result of a satellite tumour adjacent to the target tumour, although simple misalignment of the antennae cannot be entirely excluded as a cause.
None of the tumours >3 cm had a complete pathological response in the current series. This is consistent with previous reports using other thermal ablation modalities. With a larger sample size and allowing multiple overlapping ablations these results would be expected to reach the 10–25% complete response rate for liver tumours >3 cm previously reported using other methods of thermal liver tumour ablation.5,8
Furthermore, the current data shows that MWA using a device with tumour permittivity feedback control is feasible for the treatment of selected liver tumours. In the current series, no immediate ablation related morbidity was noted. The study design of resection after ablation has several limitations. The small sample size and study design does not allow comment on local recurrence rates, delayed complications and disease-free survival for the treatment of liver tumours after MWA with tumour permittivity feedback control.
In conclusion, the current data supports the feasibility of using MWA with tumour permittivity feedback in the treatment of selected liver tumours. A larger, prospective, multicentre study should be performed to better investigate the safety and efficacy in treating patients who are not candidates for hepatic resection and to define delayed complications and oncological outcomes after MWA with tumour permittivity feedback in the treatment of liver tumours.
Acknowledgments
All of the authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
The research was supported by a grant from Medwaves, Inc., San Diego, CA.
References
- 1.Poon R, Fan S, Tsang F, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466–486. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brace CL. Microwave ablation technology: what every user should know (Review) Curr Probl Diagn Radiol. 2009;38:61–67. doi: 10.1067/j.cpradiol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf FJ, Aswad B, Ng T, Dupuy DE. Intraoperative microwave ablation of pulmonary malignancies with tumor permittivity feedback control: ablation and resection study in 10 consecutive patients. Radiology. 2011;262:353–360. doi: 10.1148/radiol.11110015. [DOI] [PubMed] [Google Scholar]
- 4.Neumann RA, Knobler RM, Pieczkowski F, Gebhart W. Enzyme histochemical analysis of cell viability after argon laser-induced coagulation necrosis of the skin. J Am Acad Dermatol. 1991;25:991–998. doi: 10.1016/0190-9622(91)70296-e. [DOI] [PubMed] [Google Scholar]
- 5.Pompili M, Mirante VG, Rondinara G, Fassati LR, Piscaglia F, Agnes S, et al. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117–1126. doi: 10.1002/lt.20469. [DOI] [PubMed] [Google Scholar]
- 6.Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, et al. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2008;207:20–29. doi: 10.1016/j.jamcollsurg.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Kessler A, Blank A, Merhav H, Orron D, Konikoff F, Oren R, et al. Minimally invasive techniques in the treatment of liver tumors. Isr Med Assoc J. 2002;4:1106–1110. [PubMed] [Google Scholar]
- 8.Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohmoto K, Miyake I, Tsuduki M, Shibata N, Takesue M, Kunieda T, et al. Percutaneous microwave coagulation therapy for unresectable hepatocellular carcinoma. Hepatogastroenterology. 1999;46:2894–2900. [PubMed] [Google Scholar]
- 10.Matsukawa T, Yamashita Y, Arakawa A, Nishiharu T, Urata J, Murakami R, et al. Percutaneous microwave coagulation therapy in liver tumors. A 3-year experience. Acta Radiol. 1997;38:410–415. doi: 10.1080/02841859709172092. [DOI] [PubMed] [Google Scholar]
- 11.Seki S, Sakaguchi H, Kadoya H, Morikawa H, Habu D, Nishiguchi S, et al. Laparoscopic microwave coagulation therapy for hepatocellular carcinoma. Endoscopy. 2000;32:591–597. doi: 10.1055/s-2000-9014. [DOI] [PubMed] [Google Scholar]
- 12.Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- 13.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25(Suppl 1):S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 14.Skinner MG, Iizuka MN, Kolios MC, Sherar MD. A theoretical comparison of energy sources – microwave, ultrasound and laser – for interstitial thermal therapy. Phys Med Biol. 1998;43:3535–3547. doi: 10.1088/0031-9155/43/12/011. [DOI] [PubMed] [Google Scholar]
- 15.Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: a literature review. Int J Hepatol. 2011;2011:104685. doi: 10.4061/2011/104685. [DOI] [PMC free article] [PubMed] [Google Scholar]


