Abstract
Macrophage inhibitory cytokine (MIC-1), a divergent member of the transforming growth factor-β (TGF-β) superfamily and activation associated cytokine, is secreted as a 28 kDa dimer. To understand its secretion, we examined its processing in MIC-1-transfected Chinese hamster ovary cells. Mature MIC-1 dimer arises post-endoplasmic reticulum (ER) by proteolytic cleavage of dimeric pro-MIC-1 precursor at a furin-like site. Unlike previously characterized TGF-β superfamily members, MIC-1 dimers are also secreted in constructs lacking the propeptide. A clue to the function of the propeptide came from the observation that a range of proteasome inhibitors, including lactacystin and MG132, cause major increases in levels of undimerized pro-MIC-1 precursor. There was no effect of proteasome inhibitors on cells expressing mature MIC-1 without the propeptide, suggesting that the propeptide can signal misfolding of MIC-1, leading to proteasomal degradation. Deletion mutagenesis showed the N-terminal 28 amino acids of the propeptide are necessary for proteasomal degradation. This is the first demonstration, to our knowledge, of a quality control function in a propeptide domain of a secretory protein and represents an additional mechanism to ensure correct folding of proteins leaving the ER.
Keywords: cytokine/misfolding/propeptide/proteasome/secretion
Introduction
Macrophage inhibitory cytokine 1 (MIC-1) was first isolated from a U937 subtraction cDNA library enriched for genes associated with macrophage activation (Bootcov et al., 1997). It is a divergent member of the transforming growth factor-β (TGF-β) superfamily that is highly expressed in placenta and can also be detected at low levels in several other tissues including kidney, pancreas, prostate and colon (Lawton et al., 1997; Yokoyama-Koyabashi et al., 1997; Fairlie et al., 1998). The induction of MIC-1 mRNA in monocytoid cells by pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6, and lack of induction by interferon-γ ± lipopolysaccharide (LPS) suggests that MIC-1 may represent an autocrine regulatory molecule, whose expression may serve to limit later phases of macrophage activation by responding largely to secreted pro-inflammatory monokines (Bootcov et al., 1997).
MIC-1, like other TGF-β superfamily proteins, is synthesized as an inactive protein precursor that undergoes disulfide-linked dimerization in the endoplasmic reticulum (ER) before transport to the Golgi. The dimeric precursor is then cleaved proteolytically by a furin-like convertase at an RXXR site that separates the propeptide from the mature domain. The mature peptide of MIC-1 arises by cleavage of the dimeric precursor at a conserved RXXR site that can be found at either amino acid 193 or 196 (Bootcov et al., 1997). Proteolytic cleavage by a furin-like pro-protein convertase separates the propeptide from the C-terminal mature domain, and MIC-1 is secreted as a disulfide-linked dimer of 24.5 kDa.
MIC-1, like all TGF-β superfamily proteins, contains a highly conserved seven-cysteine domain spanning ∼80 amino acids. This encompasses most of the mature protein and forms the cysteine knot, a structural hallmark of this superfamily. In most superfamily members, the propeptide is glycosylated and secreted (reviewed by Kingsley, 1994). For all characterized members, the propeptide is required for the secretion of the mature peptide dimer (Gray and Mason, 1990; Israel et al., 1992). It is thought that the propeptide facilitates the correct folding of the mature peptide as in-frame deletion of the propeptide causes an intracellular accumulation of disulfide-linked aggregates (Gray and Mason, 1990). Quality control mechanisms in the ER ensure that nascent proteins that fail to fold or oligomerize correctly are targeted from the ER to the proteasome for degradation.
The proteasome is a multisubunit protease complex that specifically degrades targeted proteins. Among these are cell cycle and growth regulators, components of signal transduction pathways, enzymes of housekeeping and cell-specific metabolic pathways, and mutated or post-translationally damaged proteins. These proteins are tagged with ubiquitin on lysine residues, which targets them to the proteasome for degradation (reviewed by Kopito, 1997; Ciechanover, 1998). Proteasomes have been localized to the nucleus and cytoplasm and also stud the cytosolic face of the ER (Peters et al., 1994; Palmer et al., 1996; Reits et al., 1997). It is currently thought that export of misfolded or mutant secreted proteins from the ER to the cytosol occurs through the translocation channel formed by the Sec61 protein complex (Wiertz et al., 1996; Plemper et al., 1997). Several wild-type secreted proteins are also regulated post-translationally by targeting to the proteasome from the ER. Apolipoprotein B100 is targeted to the proteasome when lipid substrates are limiting for the formation of low-density lipoprotein (LDL; Fisher et al., 1997), and precursors for both parathyroid hormone-related protein (Meerovitch et al., 1998) and clotting factor VIII (Pipe et al., 1998) are targeted to the proteasome, which is thought to be a mechanism for regulating their concentration.
This study examines the processing of MIC-1 and demonstrates that unlike other characterized members of the TGF-β superfamily, the propeptide is not required for secretion of disulfide-linked mature MIC-1 dimers. The MIC-1 propeptide is glycosylated and, after processing, can be detected in the extracellular medium. Examination of the effect of the proteasome inhibitors lactacystin and MG132 on MIC-1-transfected cells reveals that the propeptide acts as a quality control mechanism, through epitopes close to the N-terminus, resulting in proteasomal degradation of monomeric pro-MIC-1.
Results
The MIC-1 propeptide is glycosylated
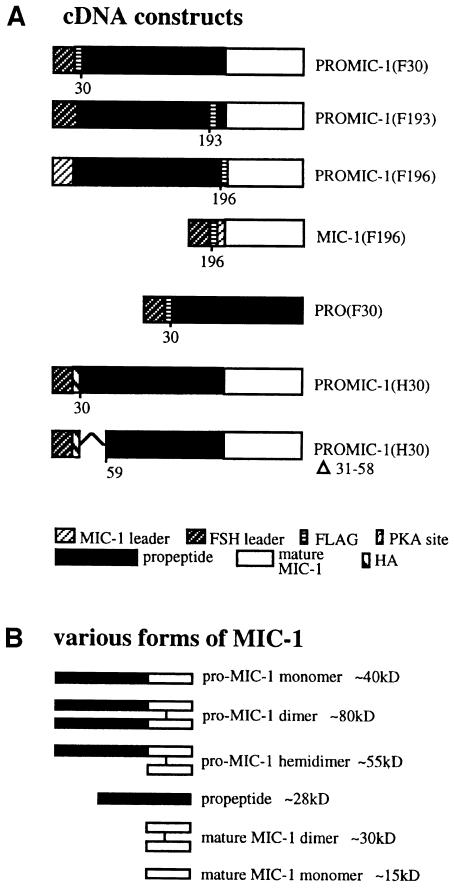
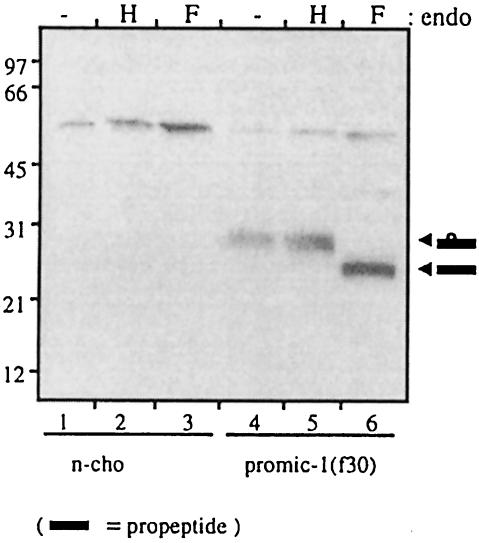
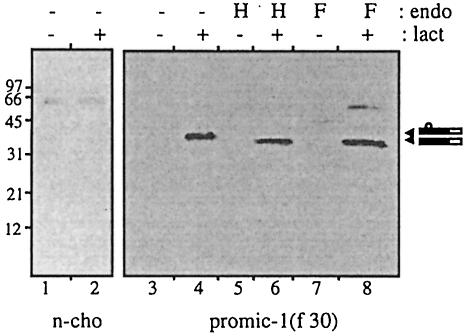
To allow identification of the propeptide, the MIC-1 cDNA (coding region minus the predicted leader sequence) was modified by the addition of nucleotides encoding an eight amino acid FLAG epitope (DYKDDDDK). The follicle-stimulating hormone (FSH) leader peptide was added to ensure signal peptide cleavage at the N-terminal side of the FLAG epitope [PROMIC-1(F30), see Figure 1]. This construct was cloned into the eukaryotic expression vector pIRES-neo and stably transfected into Chinese hamster ovary (CHO) cells. Serum-free conditioned medium was collected over 2 days from both transfected and untransfected CHO cells. The conditioned medium was characterized by immunoprecipitation with the M2 anti-FLAG monoclonal antibody linked to agarose gel (anti-FLAG M2 agarose gel), then SDS–PAGE followed by immunoblotting with anti-FLAG (Figure 2). A large number of transfected cells (confluent 150 cm2 flask) was needed to enable visualization of the propeptide, which is secreted as a 28 kDa monomer (Figure 2, lane 4). The size of the propeptide is larger than that predicted by the amino acid sequence, suggesting that it may be glycosylated at a potential N-linked glycosylation site at amino acid 70. Additionally, the FLAG epitope can retard migration on SDS–PAGE by ∼2–3 kDa (Knappick and Pluckthun, 1994). To determine whether the propeptide is glycosylated, propeptide eluted from M2 affinity gel was treated with endoglycosidase H (endo H) and N-glycosidase F (N-glyc F). N-glyc F treatment, which cleaves both high mannose and complex N-linked oligosaccharides, resulted in the propeptide migrating more quickly at ∼23–25 kDa (Figure 2, lane 6). This is close to the predicted size of the FLAG-propeptide sequence. Treatment of the propeptide with endo H, which removes only high-mannose N-linked oligosaccharides, has no effect (Figure 2, lane 5), indicating that the oligosaccharide moiety is only of the complex type, consistent with its passage through the Golgi.
Fig. 1. (A) Schematic representation of MIC-1 constructs used in this study. Numbers represent residue positions. (B) Schematic representation of the various forms of recombinant MIC-1 produced in CHO cells (both reduced and non-reduced).
Fig. 2. The MIC-1 propeptide is glycosylated. Conditioned medium from untransfected control (n-cho, lanes 1–3) and PROMIC-1(F30)-transfected CHO cells (lanes 4–6) was collected following a 2 day serum starvation period. This was immunoprecipitated with M2 anti-FLAG agarose gel and the precipitated proteins were eluted and incubated with endo H (H), N-glycosidase F (F) or without treatment (–). Samples were analysed by SDS–PAGE (10% gel) and immunoblotting with anti-FLAG (M2) antibody. Arrowheads indicate glycosylated and non-glycosylated propeptide. The band at ∼50 kDa most probably represents IgG heavy chain from the immunoprecipitation.
Proteolytic processing of pro-MIC-1 occurs post-ER at amino acid 196
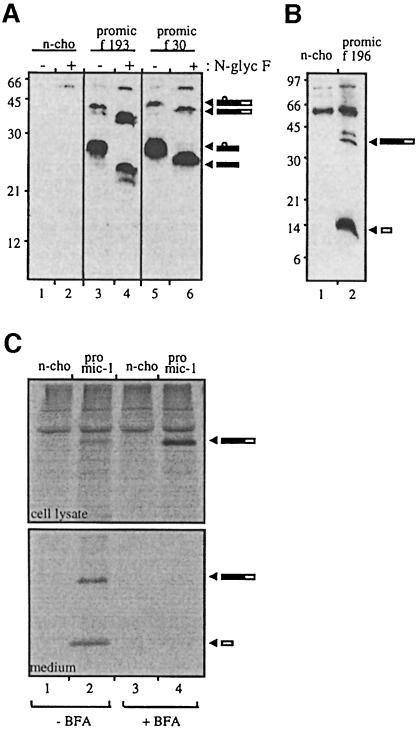
To investigate where in the precursor the proteolytic processing of pro-MIC-1 occurs, the FLAG epitope was inserted downstream of the two predicted proteolytic cleavage sites at amino acids 193 and 196 [PROMIC-1 (F193), PROMIC-1(F196), see Figure 1]. These are the most C-terminal potential pro-protein convertase cleavage sites upstream of the seven-cysteine domain. The FLAG epitope is not likely to interfere with enzyme cleavage, as it has been shown that this epitope, when inserted on the C-terminal side of the autoproteolytic maturation site of furin, does not interfere with its cleavage (Molloy et al., 1994). Immunoblot analysis of conditioned medium from CHO cells transfected with PROMIC-1(F193) demonstrates that the FLAG label remains in the 28 kDa propeptide (Figure 3A, lane 3), and treatment with N-glyc F reduces the molecular weight to 25 kDa (Figure 3A, lane 4), correlating with the predicted size of the FLAG-propeptide. These bands essentially co-migrate with propeptide in PROMIC-1(F30)-transfected cells (Figure 3A, lanes 5 and 6). The minor difference in migration is most probably due to the FLAG epitope, which can variably retard the mobility of fusion proteins (Knappick and Pluckthun, 1994; Bootcov et al., 1997). Also visible in the conditioned medium is unprocessed pro-MIC-1 precursor (∼40 kDa under reducing conditions; Figure 3A, lanes 3 and 5), which also migrates more quickly after N-glyc F digestion (∼37 kDa; Figure 3A, lanes 4 and 6). In contrast, the immunoblot of conditioned medium from CHO cells transfected with PROMIC-1(F196) (Figure 3B) demonstrates that the FLAG label is cleaved from the propeptide and migrates at the molecular weight of mature MIC-1 (∼15 kDa under reducing conditions). Some unprocessed pro-MIC-1 precursor is also labelled by the FLAG epitope (Figure 3B, lane 2). This shows that proteolytic processing occurs at amino acid 196 and not at amino acid 193, resulting in the production of a mature MIC-1 dimer with a predicted molecular weight of 25 kDa. Subsequent N-terminal sequencing of purified mature MIC-1 (without the FLAG tag) confirmed that proteolytic cleavage occurs after amino acid 196 (data not shown).
Fig. 3. Proteolytic processing of pro-MIC-1 occurs at amino acid 196. (A) Insertion of the FLAG epitope at residue 193 tags the propeptide. Conditioned medium from untransfected control (n-cho, lanes 1 and 2) and from PROMIC-1(F193)- (lanes 3 and 4) and PROMIC-1(F30)-transfected CHO cells (lanes 5 and 6) was collected following a 2 day serum starvation period. This was immunoprecipitated with M2 anti-FLAG agarose gel, the precipitated proteins eluted and incubated with (+) and without (–) N-glycosidase F and then analysed by reducing SDS–PAGE (10% gel) and immunoblotting with M2 anti-FLAG antibody. Arrowheads indicate glycosylated and unglycosylated forms of both unprocessed pro-MIC-1 precursor and propeptide. (B) Insertion of the FLAG epitope at residue 196 tags the mature MIC-1. Conditioned medium from untransfected control (n-cho, lane 1) and PROMIC-1(F196)-transfected CHO cells (lane 2) was immunoprecipitated with M2 anti-FLAG agarose gel and analysed by reducing SDS–PAGE (15% gel) and immunoblotting with M2 anti-FLAG antibody. Arrowheads indicate mature MIC-1 and incompletely processed pro-MIC-1 precursor. (C) Proteolytic processing of pro-MIC-1 precursor occurs post-ER. Untransfected (n-cho, lanes 1 and 3) and PROMIC-1(F30)-transfected CHO cells (proMIC-1, lanes 2 and 4) were pulse-labelled for 15 min with [35S]methionine and [35S]cysteine, then chased for 2 h with excess cold methionine and cysteine in the presence (+) or absence (–) of BFA. Cell lysate and conditioned medium were immunoprecipitated with sheep polyclonal anti-MIC-1 serum and analysed by SDS–PAGE under reducing conditions followed by autoradiography.
To investigate whether proteolytic processing occurs post-ER, we inhibited exit from the ER with brefeldin A (BFA). This drug also induces a disassembly of the proximal part of the Golgi, resulting in a redistribution of cis-, medial- and trans-Golgi resident enzymes back into the ER, and blocks forward transport to the trans-Golgi network (TGN; Klausener et al., 1992). CHO cells transfected with PROMIC-1(F30) were pulse labelled for 15 min with [35S]methionine and [35S]cysteine and then chased with excess cold methionine/cysteine in the presence or absence of BFA for 2 h (Figure 3C). In the presence of BFA, there is an accumulation of the pro-MIC-1 precursor in cell lysate (Figure 3C, upper panel, compare lane 4 with lane 2) but no mature MIC-1 is visible (upper panel, lane 4). In the absence of BFA, there is a minor amount of pro-MIC-1 precursor in the cell lysate (Figure 3C, upper panel, lane 2) with both pro-MIC-1 precursor and processed mature MIC-1 appearing in the chase medium (lower panel, lane 2). This shows that processing in the cell occurs post-ER.
The propeptide is not required for the secretion of disulfide-linked MIC-1 dimers
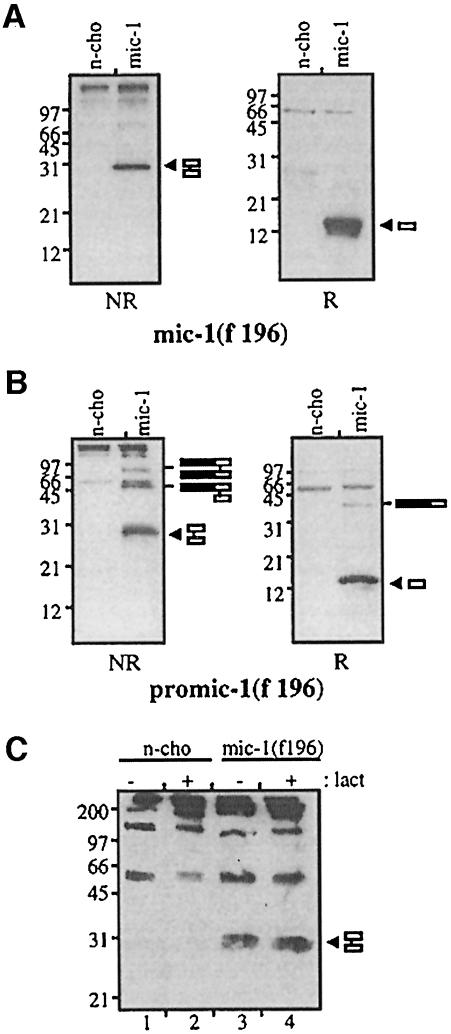
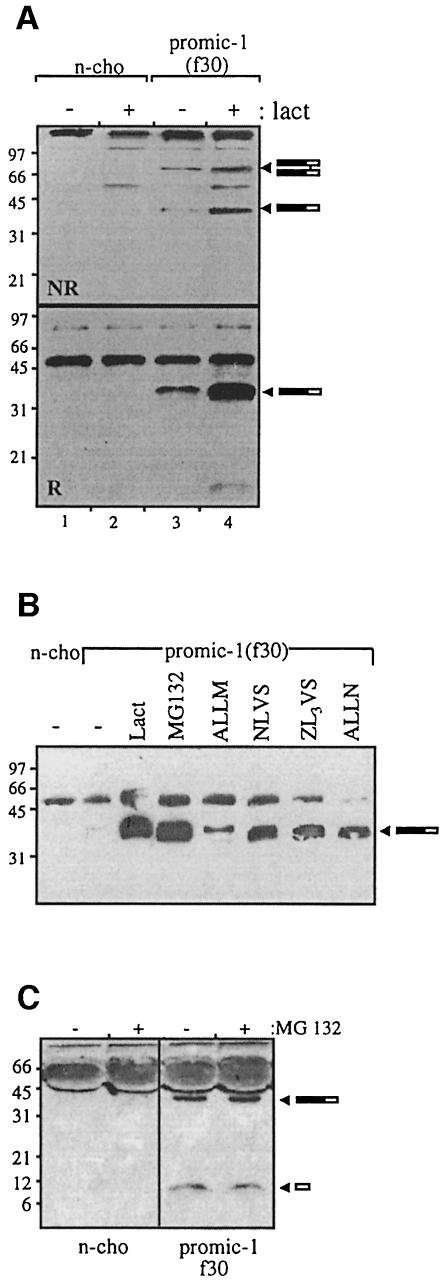
For all previously characterized members of the TGF-β superfamily, the propeptide is required for the secretion of mature disulfide-linked dimers (Wise et al., 1988; Gray and Mason, 1990; Israel et al., 1992). To determine whether the propeptide is required for the secretion of MIC-1, the propeptide region was deleted and the mature region of MIC-1 was linked directly to an FSH leader sequence. Both a protein kinase A (PKA) phosphorylation site (RRASVA) and a FLAG epitope tag were attached to the N-terminus of the mature peptide [MIC-1(F196), see Figure 1]. This construct was stably transfected into CHO cells, and conditioned medium (Figure 4A) was compared with that obtained from cells transfected with PROMIC-1(F196) that contains the propeptide (Figure 4B). The serum-free conditioned medium collected over 2 days was analysed by immunoprecipitation with M2 agarose gel, SDS–PAGE and immunoblot analysis with anti-FLAG antibody. MIC-1 is secreted as a mature disulfide-linked dimer into the medium from both transfectants (∼30 kDa), which, under reducing conditions, migrates at ∼15 kDa. In the presence of lactacystin, a specific proteasome inhibitor (Dick et al., 1997; Fenteany and Schreiber, 1998), there is essentially no accumulation of cell-associated MIC-1 (a 1.12-fold increase) in MIC-1(F196)-transfected cells (Figure 4C, compare lanes 3 and 4). As incorrectly folded proteins in the ER are targeted to the proteasome for degradation (Hiller et al., 1996; reviewed by Kopito, 1997), this provides further confirmation that the MIC-1 dimer produced in the absence of propeptide is folded correctly.
Fig. 4. The propeptide is not required for secretion of disulfide-linked MIC-1 dimer. Conditioned medium from untransfected (n-cho) and (A) MIC-1(F196)- and (B) PROMIC-1(F196)-transfected CHO cells was collected following a 2 day serum starvation period. This was immunoprecipitated with M2 anti-FLAG agarose gel and analysed by SDS–PAGE (15% gel) under both non-reducing (NR) and reducing (R) conditions followed by immunoblotting with M2 anti-FLAG antibody. Arrowheads indicate reduced and non-reduced mature MIC-1. Bars represent incompletely processed pro-MIC-1 precursor. (C) Mature MIC-1 expressed without propeptide does not accumulate in the presence of lactacystin. Untransfected control (n-cho, lanes 1 and 2) and MIC-1(F196)-transfected CHO cells (lanes 3 and 4) were treated with (+) and without (–) 10 µM lactacystin for 20 h. Cell lysate was immunoprecipitated with M2 affinity gel, analysed by SDS–PAGE under non-reducing conditions (15% gel) and immunoblotted with M2 anti-FLAG antibody. The arrowhead indicates mature MIC-1.
Lactacystin causes an accumulation of monomeric pro-MIC-1 precursor in the cell
To investigate whether the proteasome plays a role in the quality control of MIC-1 cytokine secretion, we treated PROMIC-1(F30)-transfected CHO cells with the proteasome inhibitor lactacystin. Lactacystin covalently modifies the active site threonine of the 20S catalytic core and inhibits the chymotrypsin- and trypsin-like activities of the proteasome. Treatment was for 20 h, and the amount of pro-MIC-1 precursor in these cells was compared with that in untreated cells. Following lactacystin treatment, there is a 10-fold increase in the amount of undimerized pro-MIC-1 monomer in the cell lysate (Figure 5A, NR, compare lanes 3 and 4), which can also be observed under reducing conditions (Figure 5A, R, lanes 3 and 4). This indicates that monomeric pro-MIC-1 behaves like other incorrectly folded proteins in being targeted to the proteasome for degradation. In contrast, only a minor increase (1.6-fold) in dimeric pro-MIC-1 precursor can also be observed following lactacystin treatment (Figure 5A, NR, lanes 3 and 4), which is most likely to be due to dimerization of the accumulating monomeric pro-MIC-1 in the ER.
Fig. 5. (A) Lactacystin treatment enhances intracellular levels of non-dimerized pro-MIC-1 precursor. Untransfected control (n-cho, lanes 1 and 2) and PROMIC-1(F30)-transfected CHO cells (lanes 3 and 4) were treated with (+) and without (–)10 µM lactacystin for 20 h. Cell lysate was immunoprecipitated with M2 anti-FLAG agarose gel and analysed by SDS–PAGE under both non-reducing (NR) (10% gel) and reducing (R) (15% gel) conditions, followed by immunoblotting with M2 anti-FLAG antibody. (B) A wide range of proteasome inhibitors cause an intracellular accumulation of pro-MIC-1 precursor. PROMIC-1(F30)-transfected cells were treated with 10 µM lactacystin, 50 µM MG132, 100 µM ALLM, 30 µM NLVS, 30 µM ZL3VS or 100 µM ALLN and without treatment (–). n-cho = untransfected CHO cell control. Densitometry analysis shows that lactacystin and MG132 cause a 100-fold increase in the levels of MIC-1 precursor; ALLM, a 4-fold increase; and NLVS, ZL3VS and ALLN, a 20-fold increase. (C) MG132 does not affect the levels of secreted MIC-1 forms. Untransfected CHO cells and PROMIC-1(F30)-transfected CHO cells were incubated with (+) and without (–) 50 µM MG132 for 20 h. n-cho = untransfected CHO cell control. Medium from these cells was analysed by reducing SDS–PAGE (12.5% gel) and immunoblotting with polyclonal anti-MIC-1 serum. Arrowheads indicate pro-MIC-1 precursor and mature MIC-1. Densitometry analysis shows that MG132 causes a 1.21-fold increase in pro-MIC-1 precursor and no effect on mature MIC-1.
A range of proteasome inhibitors increase the levels of pro-MIC-1 precursor in the cell
To confirm the role of the proteasome in the degradation of the pro-MIC-1 precursor, several other proteasome inhibitors were used to inhibit degradation (Figure 5B). The most effective inhibitor of degradation was MG132, with similar inhibition to that obtained with lactacystin, while other proteasome inhibitors ZL3VS, NLVS (Bogyo et al., 1997) and ALLN inhibited degradation to a lesser extent. Although peptidyl aldehydes ALLN and MG132 competitively inhibit the chymotrypsin-like and peptidyl glutamyl sites on the 20S proteasome, they are also inhibitors of proteases such as cathepsins and calpains (Traenckner et al., 1994). The calpain II inhibitor (ALLM) is a potent inhibitor of the cathepsins and calpains but has little effect on the proteasome (Vinitsky et al., 1992). It is used frequently as a negative control for MG132 and ALLN, and it had little effect on pro-MIC-1 degradation. While proteasome inhibitors prevent the degradation of monomeric pro-MIC-1 precursor, there is little effect of proteasome inhibitor on the levels of secreted MIC-1 forms (Figure 5C).
The pro-MIC-1 precursor is targeted to the proteasome from a pre-Golgi compartment
To determine the origin of enhanced pro-MIC-1 precursor in lactacystin-treated cells, pro-MIC-1-transfected cells were lysed and immunoprecipitated with anti-FLAG, then eluted and subjected to endoglycosidase analysis. The accumulated pro-MIC-1 monomer is endo H sensitive (Figure 6), demonstrating that it is present in a pre-medial Golgi compartment. Thus, pro-MIC-1 monomer, similarly to other incorrectly folded proteins, is likely to be removed from the ER by retrograde translocation into the cytosol for degradation by proteasomes either in the cytosol (Peters et al., 1994; Reits et al., 1997) or on the cytosolic face of the ER (Palmer et al., 1996).
Fig. 6. Intracellular undimerized pro-MIC-1 precursor is targeted to the proteasome from a pre-medial Golgi compartment. Untransfected (n-cho, lanes 1 and 2) and PROMIC-1(F30)-transfected CHO cells (lanes 3–8) were incubated for 20 h with (+) and without (–) 10 µM lactacystin. Cell lysate was immunoprecipitated with M2 anti-FLAG agarose gel, eluted and incubated with endo H (H) and N-glycosidase F (F) or without treatment (–). Samples were analysed by reducing SDS–PAGE (12.5% gel) and immunoblotting with anti-M2 antibodies. Arrowheads indicate glycosylated and unglycosylated pro-MIC-1 monomer.
The sequences involved in misfolding and proteasomal degradation lie in the N-terminus of the propeptide
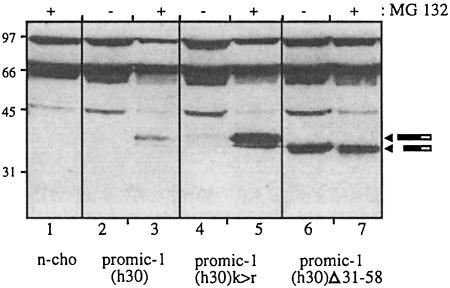
Lactacystin treatment of CHO cells stably transfected with the truncated MIC-1 construct lacking the propeptide do not accumulate MIC-1 in the cell lysate (Figure 4C), suggesting that sequences signalling misfolding and proteasomal degradation lie in the propeptide. To determine whether ubiquitylation of the propeptide is required for proteasomal degradation, the only lysine residue (K59) in the propeptide was mutated to an arginine residue. Additional lysines in the FLAG epitope of the PROMIC-1(F30) construct were removed by replacing the FLAG sequence with the haemagglutinin (HA) epitope [PROMIC-1(H30), see Figure 1]. CHO cells expressing the lysine mutant PROMIC-1(H30)K>R did not accumulate pro-MIC-1 precursor in the cell in the absence of proteasome inhibitor (Figure 7, lane 4), while treatment with MG132 caused an accumulation of pro-MIC-1 precursor similar to that obtained with cells expressing wild-type PROMIC-1(H30) [Figure 7, compare lanes 4 and 5 with 2 and 3; the differences in the levels of accumulated pro-MIC-1 precursor are due to the different levels of expression of the transfected cells as assessed by enhanced green fluorescent protein (EGFP) fluorescence]. The failure to protect against degradation in the mutant containing no lysine residues showed that direct ubiquitylation of the propeptide was not required for proteasomal degradation.
Fig. 7. Sequences in the propeptide determine proteasomal targeting and degradation of pro-MIC-1 precursor. PROMIC-1(H30)- (lanes 2 and 3), PROMIC-1(H30)K>R- (lanes 4 and 5) and PROMIC-1(H30)Δ31–58-transfected CHO cells (lanes 6 and7) were incubated with (+) and without (–) 50 µM MG132 for 20 h. n-cho = untransfected CHO cell control. Cell lysates were analysed by reducing SDS–PAGE (12.5% gel) and immunoblotting with anti-HA antibodies. Arrowheads indicate pro-MIC-1 monomer.
The sequences in the propeptide involved in misfolding and proteasomal degradation were further analysed by deletion mutagenesis. Deletions of increasing size were introduced into the N-terminal end of the PROMIC-1(H30) construct, while retaining the FSH leader peptide, signal peptidase cleavage site and HA tag. These constructs were stably transfected into CHO cells, the cells treated with the proteasome inhibitor MG132, and cell lysates compared with untreated cells (Figure 7). Immunoblot analysis with anti-HA antibody shows that while MG132 caused an accumulation of wild-type pro-MIC-1 precursor (Figure 7, compare lanes 2 and 3), there was no effect on pro-MIC-1 precursor with an N-terminal deletion in the propeptide (amino acids 31–58) (compare lanes 6 and 7). Larger deletions of the propeptide (containing the N-terminal 31–58 amino acid deletion) also prevent targeting to the proteasome (data not shown). Similar results were obtained when immunoblot analysis was performed with polyclonal anti-MIC-1 antibody (data not shown). These data demonstrate a role for the N-terminal region of the propeptide in signalling misfolding and proteasomal degradation of the pro-MIC-1 precursor.
The presence of the mature peptide is required for proteasomal degradation
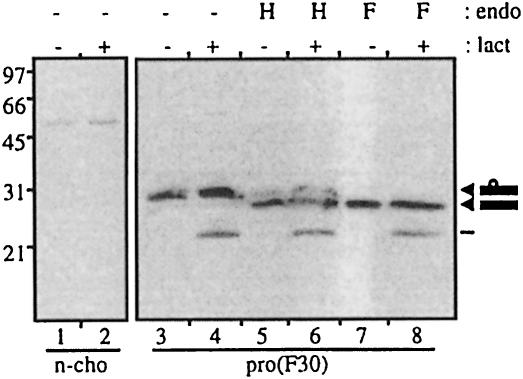
To investigate whether the propeptide alone has sequences determining misfolding and proteasomal degradation, the propeptide was expressed independently of the mature MIC-1 peptide. A FLAG-tagged propeptide domain was attached to the FSH leader [PRO(F30), see Figure 1] and stably transfected into CHO cells. Analysis of the propeptide expressed in these cells shows that the propeptide is largely retained inside the cell (Figure 8, lane 3), with only very low levels being secreted (data not shown). Endoglycosidase analysis of intracellular propeptide demonstrates that most of the intracellular propeptide is endo H sensitive (Figure 8, lane 5). This shows that most of the intracellular propeptide has high-mannose N-linked glycosylation, characteristic of retention in a pre-medial Golgi compartment. Confocal immunofluoresence microscopy of PRO(F30)-transfected cells using fluorescein isothiocyanate (FITC)-labelled anti-FLAG antibody shows a reticulated staining pattern characteristic of the ER (data not shown), suggesting retention of the propeptide in the ER.
Fig. 8. Independently expressed propeptide is insensitive to lactacystin treatment. Untransfected control (n-cho, lanes 1 and 2) and PRO(F30)-transfected CHO cells (lanes 3–8) were treated with (+) and without (–) lactacystin for 20 h. Cell lysate was immunoprecipitated with M2 anti-FLAG agarose gel, eluted and incubated with endo H (H) or N-glycosidase F (F) or without (–) treatment for 2 h. Samples were analysed by reducing SDS–PAGE (10% gel) and immunoblot analysis with M2 antibody. Arrowheads indicate glycosylated and unglycosylated propeptide.
Lactacystin treatment of these cells has no effect on intracellular propeptide levels, although there is some accumulation of a truncated propeptide species (Figure 8, lanes 4, 6 and 8). This suggests that although the propeptide contains sequences involved in proteasomal degradation, the presence of the mature peptide is required for this sequence to be unmasked. It is therefore likely that the N-terminal region of the propeptide interacts with the mature peptide and thereby exposes a misfolding signal and/or proteasomal targeting region on the propeptide.
Discussion
MIC-1, like all TGF-β superfamily proteins, is synthesized as an inactive precursor (Bootcov et al., 1997). During secretion, mature MIC-1 arises by proteolytic processing of a dimeric precursor following the RRAR sequence at amino acid 196, a typical pro-protein convertase site. Following cleavage from the propeptide, mature MIC-1 is secreted as a 25 kDa disulfide-linked dimer. Like other members of the TGF-β superfamily, the secreted propeptide of MIC-1 is glycosylated. Glycosylation is on the only potential N-linked site within the propeptide sequence at amino acid 70 and is insensitive to digestion with endo H, showing that the carbohydrate is of the complex type, consistent with passage through the Golgi.
Our studies have revealed a major difference in the assembly and secretion of MIC-1 compared with other TGF-β superfamily proteins. In the case of activin, TGF-β and BMP-2, deletion of the propeptide inhibits secretion of the mature peptide dimer (Gray and Mason, 1990; Israel et al., 1992). In the absence of propeptide, activin forms large disulfide-linked aggregates, either with itself or with other proteins in the ER (Gray and Mason, 1990). For both TGF-β and activin, co-transfection of constructs lacking the propeptide with constructs independently expressing their propeptide regions is able partly to rescue dimer secretion (Gray and Mason, 1990). In contrast, a similar deletion of the propeptide in MIC-1 has no effect on the amount of MIC-1 secreted or on the formation of misfolded proteins in the cell. At first glance, this might be thought to be due to the absence of cysteine residues in the MIC-1 propeptide. In the case of another secreted protein, von Willebrand factor (vWF), the propeptide, which forms transient disulfide bonds with the mature peptide, is required for appropriate disulfide bonding and assembly of the mature vWF subunits into multimers (Wise et al., 1988). However, similarly to MIC-1, the BMP-2 propeptide, which is required for secretion of mature BMP dimer (Israel et al., 1992), also has no cysteines, indicating that disulfide bond formation is not the only reason for requiring a propeptide for secretion. The requirement for a propeptide may also be linked to the biological properties of the secreted peptide, for example, mature TGF-β is secreted as a latent complex in which the propeptide associates non-covalently with the mature peptide, inhibiting its bioactivity. Activation of TGF-β requires dissociation of the propeptide either by heat, acid treatment or protease digestion (reviewed by Kingsley, 1994). It is also likely that the propeptide of TGF-β, activin and BMP-2 is required for the correct folding of the mature peptide, acting similarly to a chaperone. Since MIC-1 propeptide is not required for secretion or stabilization of mature MIC-1 dimer, we investigated in more detail its role in the assembly and exit of pro-MIC-1 from the ER.
As the site of entry of proteins into the secretory pathway, the ER is responsible for the correct folding of these proteins and the proofreading of mutated, unnecessary or incompletely assembled proteins that have to be eliminated (Sommer and Wolf, 1997). The ubiquitin–proteasome system in the cytoplasm is currently recognized as the major site for this process. Our data demonstrate that the pro-MIC-1 precursor must dimerize in order to exit the ER, and only after exit from the ER does it undergo proteolytic processing. Thus, cleavage by a furin-like protease most probably occurs in a late Golgi fraction, similar to TGF-β (Dubois et al., 1995).
Cells expressing MIC-1 without the propeptide do not accumulate intracellular MIC-1 in the presence of lactacystin, indicating that the sequences signalling misfolding and proteasomal degradation must lie in the propeptide. Deletion mutagenesis demonstrated that the N-terminal 28 amino acids of the propeptide are necessary for proteasomal degradation. However, expression of just the propeptide (without mature peptide) did not result in accumulation in the presence of lactacystin. One interpretation of these data is that the N-terminal region of the propeptide interacts with the mature peptide in the pro-MIC-1 monomer thereby exposing a misfolding signal, which leads to proteasomal degradation. It is also possible that a direct proteasomal targeting signal is exposed. In either case, this signal is masked in the dimeric precursor, leading to a specific degradation of undimerized pro-MIC-1 monomer. The minor accumulation of dimeric precursor in the presence of lactacystin is most likely to be due to dimerization of some of the monomeric precursor accumulating in the ER. It could, however, be due to the misfolding/proteasomal targeting signal not being entirely masked.
Mutation of the only lysine residue in the propeptide did not cause an accumulation of the pro-MIC-1 precursor in the cell, demonstrating that direct ubiquitylation of the pro-MIC-1 precursor is not required for proteasomal degradation. Pro-MIC-1, similarly to HMG-CoA reductase (McGee et al., 1996), may not require ubiquitylation for proteasomal degradation. It is also possible that the misfolding/proteasomal targeting signal interacts with other protein(s) that are ubiquitylated and then targeted for degradation. One such protein, shown to facilitate proteasomal degradation of mutant α-1 antitrypsin, is the transmembrane chaperone calnexin (Qu et al., 1996). However, as we have shown that mutation of the single N-linked glycosylation site in the MIC-1 propeptide domain does not prevent targeting to the proteasome for degradation (data not shown), interaction with calnexin is very unlikely. Other potential binding proteins that may interact with the incorrectly folded pro-MIC-1 precursor to facilitate proteasomal degradation are the molecular chaperone BiP and the Sec61p complex, which functions in the retrograde transport of proteins from the ER lumen to the cytosol for degradation by the proteasome (Plemper et al., 1997; Chen et al., 1998; Meerovitch et al., 1998).
The difference between the secretion of MIC-1 and that of other TGF-β superfamily cytokines has made it possible to define a novel role for the propeptide. Unlike other characterized members of the superfamily, the MIC-1 propeptide is not required for folding and secretion of mature MIC-1 dimers. Rather, its function may be to ensure that only dimeric precursor exits the ER. It does this by targeting monomeric precursor in the ER for degradation by the proteasome. This is the first time, to our knowledge, that a sequence signalling misfolding and proteasomal degradation has been identified within a propeptide, suggesting a quality control function rather than a chaperone-like function.
Materials and methods
Cell culture
CHO cells were maintained as recommended by the American Type Culture Collection in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco-BRL) supplemented with 10% fetal bovine serum (FBS) (Gibco-BRL). For collection of conditioned medium from transfected CHO cells, 80–90% confluent cells were cultured in the absence of FBS for 48 h. For treatment with proteasome inhibitors, 90% confluent cells were incubated with either 10 µM lactacystin, 100 µM ALLN, 50 µM MG132, 100 µM ALLM (all from Calbiochem), 30 µM ZL3VS, 30 µM NLVS (a kind gift from Hidde L.Ploegh) or equivalent dimethylsulfoxide vehicle as control. Incubations were in medium supplemented with 5% FBS for 20 h, after which the cells were washed with cold phosphate-buffered saline (PBS) then lysed as described below. For proteasome inhibitor experiments, cells were plated in 6-well plates. For all other experiments, cells were plated in either 75 or 150 cm2 flasks.
Immune reagents
Anti-FLAG M2 monoclonal antibodies used for immunoblotting were obtained from Sigma Chemicals, and anti-FLAG M2 antibody affinity gel used in immunoprecipitation was obtained from Eastman Kodak Company. The affinity gel comprises the anti-FLAG M2 monoclonal antibody coupled to agarose gel. Anti-HA monoclonal antibodies (clone 12CA5) were obtained from Boehringer Mannheim. Sheep polyclonal antibodies to the mature domain of MIC-1 were obtained by immunizing with purified recombinant MIC-1.
Preparation of MIC-1 cDNA constructs (see Figure 1)
All constructs were prepared using a two-step PCR protocol to generate the FLAG epitope. For the PROMIC-1(F193) and PROMIC-1(F196) constructs, a FLAG-tagged mature MIC-1 domain was linked to the MIC-1 prepro region. To generate a FLAG-tagged mature domain, the common 3′ PCR primer used was TCCACGCAGACTCGAGTCA TATGCAGTGGCAGTCTTTGGC, while the 5′ PCR primer used to generate the FLAG epitope for PROMIC-1(F196) was ACAAGGACG ACGATGACAAGGCGCGCAACGGGGACCACTG and for PROMIC-1(F193) was ACAAGGACGACGATGACAAGAGAGCGCGTGCGC GCAACGG.
A second 5′ PCR primer CTCGCCAGCCGAATTCGACTACAA GGACGACGATGACAAG was used to complete the FLAG epitope and generate a 5′ EcoRI restriction site to use for ligation with the prepro region of MIC-1. The EcoRI restriction site was added to the 3′ end of the MIC-1 prepro region using a 3′ PCR primer ATATTTGAATTC ACGCGCTCTGCGGCGCCC for PROMIC-1(F196) and ATATTT GAATTCGCGGCGCCCCCTGGC for PROMIC-1(F193). The common 5′ PCR primer used was AGATCTCTCGAGAAGCTTATGCCC GGGCAAGAACTCAG. For the PROMIC-1(F30) construct, an FSH leader with an additional five amino acids was linked to the predicted pro-MIC-1 region to ensure cleavage of the FSH leader. A FLAG sequence was attached to the 3′ end of the FSH leader sequence to allow FLAG tagging of the pro domain, following signal peptide cleavage. To generate this, the common 5′ PCR primer was AGATCTCTCGAG GCTAGCGCCATGGATTACTACAGAAAA (for the pCEP4 expression vector) and CGCTCGAGGATATCGCCATGGATTACTACAGAAAA (for pIRES-neo). The first 3′ PCR primer used to generate the FLAG sequence was GTCGTCCTTGTAGTCGAATTCATCAGGAGCGGA ATGGAG. A second 3′ PCR primer ATATTCGGCCAGAGACAG CTTGTCATCGTCGTCCTTGT was used to complete the FLAG epitope and generate a 3′ BsmAI restriction site to use for ligation with the pro-MIC-1 region. A BsmAI restriction site is present at the 5′ end of the pro-MIC-1 region. For the MIC-1(F196) construct, using the PROMIC-1(F30) as a template, the PKA site was added to the 3′ end of the FLAG sequence using a two-step PCR protocol. The first 3′ PCR primer used was CACGGAGGCGCGGCGAAGCTTGTCGTCGTC GTCCTTGTAGTC and the second was ATTATTATGCGCGCCACG GAGGCGCGGCGCAAGAA to both complete the PKA site and add a BssHII site to the 3′ end of the fragment. This was then ligated to the BssHII site occurring at the 5′ end of the mature MIC-1 domain. The resulting cDNA constructs were cloned into the HindIII–XhoI unique restriction sites of the pCEP4 vector (modified to remove the EBNA gene, InVitrogen) for the PROMIC-1(F193) construct and the EcoRV–BstXI unique sites of the pIRES1-Neo bicistronic vector (Clontech) for the MIC-1(F196), PROMIC-1(F196) and PROMIC-1(F30) constructs.
The constructs containing the HA epitope were all made using a whole plasmid PCR technique similar to that described by Fisher and Pei (1997) but excluding the the DpnI digestion step, which was found to be unnecessary. Essentially, forward and reverse primers were phosphorylated using T4 polynucleotide kinase (Boehringer Mannheim) before PCR using Pfu DNA polymerase (Promega). The PCR products were isolated and re-ligated using T4 DNA ligase (Boehringer Mannheim). To replace the FLAG tag in the PROMIC-1(F30) construct with the HA epitope, PROMIC-1(F30) was cloned into the XhoI and BglII sites of the pOCUS-2 vector (Novagen) and used as the template. The primers used were 5′TGCCCGACTACGCCCTCTCTCTGGCCGAGGCG3′ (forward) and 5′CGTCGTAGGGGTAGAATTCATCAGGAGCGG3′ (reverse). This PROMIC-1(H30) construct was used as template for the K>R mutant and the propeptide deletion mutants. The primers used to mutate lysine (59) to arginine for the PROMIC-1(H30)K>R construct were 5′GACGCTACGAGGACCTGCTAACCAGGC3′ (forward) and 5′TCC GCAACTCTCGGAATCTGGAGTCTTCG3′ (reverse). For the propeptide deletion mutants, the common reverse primer 5′GGCGTAGTC GGGCACGTCGTAGG3′ was used with forward primers 5′AAACGC TACGAGGACCTGCTAACC3′ (Δ31–58) or 5′CAGCTCAGCCTT GCAAGACCCCAGG3′ (Δ31–144). The XhoI–BssHII inserts containing the mutated/deleted propeptide domains were subcloned into the XhoI–BssHII sites of PROMIC-1(F30) in the pIRES2-EGFP vector.
All constructs were sequenced bidirectionally by the dideoxy chain termination method using the dye terminator cycle sequencing kit (Perkin Elmer) and the ABI377 automated sequencer, according to the manufacturer’s instructions.
Transfection
CHO cells were stably transfected with the various constructs using lipofectamine (Gibco-BRL) as described previously (Bootcov et al., 1997). CHO transfectants were selected with either 1000 µg/ml geneticin (G418) (Gibco-BRL) for the pIRES1-Neo and pIRES2-EGFP vectors or 400 µg/ml hygromycin B (Boehringer Mannheim) for the pCEP4 vector.
Endoglycosidase analysis
Immunoprecipitated protein was eluted from the anti-FLAG M2 affinity gel (Kodak Eastman Company) as previously described (Bauskin et al., 1991) and then digested with N-glyc F or endo H (Boehringer Mannheim) essentially according to the manufacturer’s instructions.
N-terminal sequencing
Purified mature MIC-1 was absorbed onto PVDF membrane using a ProSorb sample preparation cartridge (Perkin Elmer Applied Biosystems) then sequenced by Edman degradation on a Procise 494 Protein Sequencer (Applied Biosystems) using standard PVDF blot cycles.
Immunoprecipitation, gel electrophoresis, immunoblot analysis and metabolic labelling
Conditioned medium was collected and cells were washed with ice-cold PBS, then lysed as previously described (Bauskin et al., 1991). Immunoprecipitation with M2 anti-FLAG agarose and elution of precipitated proteins with SDS–PAGE sample buffer was performed essentially as previously described (Bootcov et al., 1997). Immunoblot analysis with anti-FLAG M2 antibody was as previously described (Bootcov et al., 1997). Samples were analysed by reducing or non-reducing SDS–PAGE (Bauskin et al., 1991). Immunoblot analysis with anti-HA was essentially as described for anti-FLAG, except that anti-HA was used at a concentration of 200 ng/ml. For metabolic labelling, confluent monolayers of transfected CHO cells were washed twice with PBS, then incubated in methionine/cysteine-free DMEM (Gibco-BRL) for 30 min. The medium was then replaced with this medium containing [35S]methionine/cysteine (200 µCi/ml) for 15 min. The labelling medium was removed and the cells were chased for different lengths of time with medium containing excess cold methionine/cysteine. For incubation with BFA (Sigma), 10 µg/ml BFA was added to the chase directly after pulsing. Conditioned medium was collected and cells were washed with ice-cold PBS, then lysed as previously described (Bauskin et al., 1991). For immunoprecipitation with sheep polyclonal anti-MIC-1, the antibodies were used at a dilution of 1:1000 on ice overnight, then immunoprecipitations incubated with protein G–Sepharose (Pharmacia) at 4°C for 2 h. Immunoprecipitated proteins were washed five times in ice-cold PBS/1% Triton X-100, then eluted at 90°C in SDS–PAGE sample buffer.
Acknowledgments
Acknowledgements
We thank Dr Rohit Cariappa for advice on pulse chase and Dr John Zaunders for providing brefeldin A. This work has been funded in part by grants from St Vincent’s Hospital and by Meriton Apartments Pty Ltd through an R&D syndicate arranged by Macquarie Bank Ltd. In addition, this project was partially funded by a New South Wales Health Research and Development infrastructure grant.
References
- Bauskin A.R., Alkalay,I. and Ben Neriah,Y. (1991) Redox regulation of a tyrosine kinase in the ER. Cell, 66, 685–696. [DOI] [PubMed] [Google Scholar]
- Bogyo M., McMaster,J.S., Gaczynska,M., Tortorella,D., Goldberg,A.L and Ploegh,H. (1997) Covalent modification of the active site threonine of proteasomal β-subunits and the E.coli homolog HslV by a new class of inhibitors. Proc. Natl Acad. Sci. USA, 94, 6629–6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootcov M.R. et al. (1997) MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-β superfamily. Proc. Natl Acad. Sci. USA, 94, 11514–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Le Caherec,F. and Chuck,S.L. (1998) Calnexin and other factors that alter translocation affect the rapid binding of ubiquitin to apoB in the Sec61 complex. J. Biol. Chem., 273, 11887–11894. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. (1998) The ubiquitin–proteasome pathway: on protein death and cell life. EMBO J., 17, 7151–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick L.R. et al. (1997) Mechanistic studies on the inactivation of the proteasome by lactacystin in cultured cells. J. Biol. Chem., 272, 182–188. [DOI] [PubMed] [Google Scholar]
- Dubois C.M., Laprise,M.-H., Blanchette,F., Gentry,L.E. and Leduc,R. (1995) Processing of transforming growth factor β1 precursor by human furin convertase. J. Biol. Chem., 270, 10618–10624. [DOI] [PubMed] [Google Scholar]
- Fairlie W.D., Moore,A.G., Bauskin,A.R., Russell,P.K., Zhang,H.-P. and Breit,S.N. (1998) MIC-1 is a novel TGF-β superfamily cytokine associated with macrophage activation. J. Leukoc. Biol., 65, 2–5. [DOI] [PubMed] [Google Scholar]
- Fenteany G. and Schreiber,S.L. (1998) Lactacystin, proteasome function and cell fate. J. Biol. Chem., 273, 8545–8548. [DOI] [PubMed] [Google Scholar]
- Fisher C.L. and Pei,G.K. (1997) Modification of a PCR-based site directed mutagenesis method. Biotechniques, 23, 570–574. [DOI] [PubMed] [Google Scholar]
- Fisher E.A., Zhou,M., Mitchell,D.M., Wu,X., Omura,S., Wang,H., Goldberg,A.L. and Ginsberg,H.N. (1997) The degradation of apolipoprotein B100 is mediated by the ubiquitin–proteasome pathway and involves heat shock protein 70. J. Biol. Chem., 272, 20427–20434. [DOI] [PubMed] [Google Scholar]
- Gray A.M. and Mason,A.J. (1990) Requirement for activin A and transforming growth factor-β1 pro-regions in homodimer assembly. Science, 247, 1328–1330. [DOI] [PubMed] [Google Scholar]
- Hiller M.M., Finger,A., Schweiger,M. and Wolf,D.H. (1996) ER degradation of a misfolded luminal protein by the cytosolic ubiquitin–proteasome pathway. Science, 273, 1725–1728. [DOI] [PubMed] [Google Scholar]
- Israel D.I., Nove,J., Kerns,K.M., Moutsatsos,I.K. and Kaufman,R.J. (1992) Expression and characterization of bone morphogenetic protein-2 in Chinese hamster ovary cells. Growth Factors, 7, 139–150. [DOI] [PubMed] [Google Scholar]
- Kingsley D.M. (1994) The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev., 8, 133–146. [DOI] [PubMed] [Google Scholar]
- Klausener R.D., Donaldson,J.G. and Lippencott-Schwartz,J. (1992) Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol., 116, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappick A. and Pluckthun,A. (1994) An improved affinity tag based on the FLAG peptide for the detection and purification of recombinant antibody fragments. Biotechniques, 17, 754–761. [PubMed] [Google Scholar]
- Kopito R.R. (1997) ER quality control: the cytoplasmic connection. Cell, 88, 427–430. [DOI] [PubMed] [Google Scholar]
- Lawton L.N. et al. (1997) Identification of the novel TGF-β superfamily highly expressed in human placenta. Gene, 203, 17–26. [DOI] [PubMed] [Google Scholar]
- McGee T.P., Cheng,H.H., Kumagi,H., Omura,S. and Simoni,R.D. (1996) Degradation of 3-hydroxyl-3-methylglutaryl-CoA reductase in endoplasmic reticulum membranes is accelerated as a result of increased susceptibility to proteolysis. J. Biol. Chem., 271, 25630–25638. [DOI] [PubMed] [Google Scholar]
- Meerovitch K., Wing,S. and Goltzman,D. (1998) Proparathyroid hormone-related protein is associated with the chaperone protein BiP and undergoes proteasome mediated degradation. J. Biol. Chem., 273, 21025–21030. [DOI] [PubMed] [Google Scholar]
- Molloy S.S., Thomas,L., VanSlyke,J.K., Stenberg,P.E. and Thomas,G. (1994) Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J., 13, 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A., Rivett,A.J., Thomson,S., Hendkil,K.B., Butchers,G.W., Fuertes,G. and Knecht,E. (1996) Subpopulations of proteasomes in rat liver nuclei, microsomes and cytosol. Biochem J., 316, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J.M., Franke,W.W. and Kleinschmidt,J.A. (1994) Distinct 19S and 20S subcomplexes of the 26S proteasome and their distribution in the nucleus and the cytoplasm. J. Biol. Chem., 269, 7709–7718. [PubMed] [Google Scholar]
- Pipe S.W., Morris,J.A., Shah,J. and Kaufman,R.J. (1998) Differential interaction of coagulation factor VIII and factor V with protein chaperones calnexin and calreticulin. J. Biol. Chem., 273, 8537–8544. [DOI] [PubMed] [Google Scholar]
- Plemper R.K., Bohmler,S., Bordallo,J., Sommer,T. and Wolf,D.H. (1997) Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature, 388, 891–895. [DOI] [PubMed] [Google Scholar]
- Qu D., Teckman,J.H., Omura,S. and Perlmutter,D.H. (1996) Degradation of a mutant secretory protein, α1-antitrypsin Z, in the endoplasmic reticulum requires proteasome activity. J. Biol. Chem., 271, 25630–25638. [DOI] [PubMed] [Google Scholar]
- Reits E.A.J., Benham,A.M., Plougastel,B., Neefjies,J. and Trowsdale,J. (1997) Dynamics of proteasome distribution in living cells. EMBO J., 16, 6087–6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T. and Wolf,D.H. (1997) Endoplasmic reticulum degradation: reverse protein flow of no return. FASEB J., 11, 1227–1233. [DOI] [PubMed] [Google Scholar]
- Traenckner E.B., Wilk,S. and Baeuerle,P.A. (1994) A proteasome inhibitor prevents activation of NF-κB and stabilises a newly phosphorylated form of IκB-α that is still bound to NF-κB. EMBO J., 13, 5433–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinitsky A., Michaud,C., Powers,J.C. and Orlowski,M. (1992) Inhibition of the chymotrypsin-like activity of the pituitary multicatalytic proteinase complex. Biochemistry, 31, 9421–9428. [DOI] [PubMed] [Google Scholar]
- Wiertz E.J., Tortorella,D., Bogyo,M., Yu,J., Mothes,W., Jones,T.R., Rapoport,T.A. and Ploegh,H.L. (1996) Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature, 384, 432–438. [DOI] [PubMed] [Google Scholar]
- Wise R.J., Pittman,D.P., Handin,R.I., Kaufman,R.J. and Orkin,S.H. (1988) The propeptide of von Willebrand factor independently mediates the assembly of von Willebrand multimers. Cell, 52, 229–236. [DOI] [PubMed] [Google Scholar]
- Yokoyama-Kobayashi M., Saeki,M., Sekine,S. and Kato,S. (1997) Human cDNA encoding a novel TGF-β superfamily protein highly expressed in placenta. J. Biochem., 122, 622–626. [DOI] [PubMed] [Google Scholar]