Abstract
Introduction
Few tools predict survival from pancreatic cancer (PAC). The McGill Brisbane Symptom Score (MBSS) based on symptoms at presentation (weight loss, pain, jaundice and smoking) was recently validated. The present study compares the ability of four strategies to predict 9-month survival: MBSS, carbohydrate antigen 19-9 (CA 19-9) alone, CA19-9-to-bilirubin ratio and a combination of MBSS and the CA19-9-to-bilirubin ratio.
Methodology
A retrospective review of 133 patients diagnosed with PAC between 2005 and 2011 was performed. Survival was determined from the Quebec civil registry. Blood CA 19-9 and bilirubin values were collected (n = 52) at the time of diagnosis. Receiver-operating characteristic (ROC) curves were used to determine a cutoff for optimal test characteristics of CA 19-9 and CA19-9-to-total bilirubin ratio in predicting survival at 9 months. Predictive characteristics were then calculated for the four strategies.
Results
Of the four strategies, the one with the greatest negative predictive value was the MBSS: negative predictive value (NPV) was 90.2% (76.9–97.3%) and the positive likelihood ratio (LR) was the greatest. The ability of CA 19-9 levels alone, at baseline, to predict survival was low. For the CA19-9-to-bilirubin ratio, the test characteristics improved but remained non-significant. The best performing strategy according to likelihood ratios was the combined MBSS and CA19-9 to the bilirubin ratio.
Conclusion
CA19-9 levels and the CA19-9-to-bilirubin ratio are poor predictors of survival for PAC, whereas the MBSS is a far better predictor, confirming its clinical value. By adding the CA19-9-to-bilirubin ratio to the MBSS the predictive characteristics improved.
Introduction
Pancreatic adenocarcinoma (PAC) is the 13th most common cancer worldwide and the 8th commonest cause of cancer mortality.1 Upon presentation, 80% of patients have locally advanced or metastatic disease and are not immediate surgical candidates.2 Predicting survival in patients who have resectable and unresectable PAC is a crucial step in optimizing personalized patient management.3,4
Few clinical tools predict PAC survival and most of these are designed for resectable disease. The Karnofsky performance scale, while easy to use, is not specific to PAC.5 The Memorial Sloan-Kettering nomogram is specific to PAC but remains fastidious, only applies to resected patients and relies on histopathological findings.6–8 The Glasgow prognostic score, which assesses the systemic inflammatory response (C-reactive protein and albumin), was shown to predict survival in unresectable9,10 and resectable11,12 PAC. However, any concomitant infectious process might yield falsely elevated scores. The McGill surgical department has published and validated a new symptom-based score that accurately identifies PAC patients at baseline who will have increased survival, in both resected and unresected groups.4,13 The McGill Brisbane Scoring System (MBSS) is assessed at the time of first encounter and is based on smoking status, pain, jaundice and a reported weight loss of more than 10% (Table 1). Although this score was strongly associated with survival in multiple cohorts, being even more accurate than radiology in non-resected patients, its predictive characteristics have not been formally compared with those of other strategies.
Table 1.
McGill-Brisbane Symptom Score (adapted from Jamal et al.4)
Pain: any pain, whether abdominal or back pain.
Jaundice: subjectively observed by the patient or healthcare worker.
Smoking: over the past 5 years.
The carbohydrate antigen 19-9 (CA 19-9) was first discovered by Koporowski et al.14 in a colorectal cancer cell line in 1979. Since then, serum CA 19-9 elevations were linked to 27 different disease processes, the most common being gastrointestinal malignancies, hepato-biliary and pancreatic cancers.15 CA 19-9 is expressed on the surface of cancer cells as a glycolipid and as a glycoprotein. Carcinogenesis leads to an abnormal synthesis and accumulation of this byproduct.15 CA 19-9 when expressed on the tumour cell surface, may play a role in the invasion and metastasis process as it is involved in cell adhesion pathways.16
CA 19-9 has become a well-established diagnostic tumour marker for PAC.17 Increasing evidence points to its potential as a peri-operative prognostic marker for survival from PAC.18 However, adenocarcinoma of the pancreatic head often presents with biliary obstruction. In this context, a CA 19-9 elevation can in fact be as a result of biliary obstruction, the cancer itself or a combination of both.19 Hence a standardized method correcting for obstruction-related CA 19-9 elevations is necessary to decrease confounding. One method described in the literature is the CA 19-9-to-bilirubin ratio;20 however, this method remains to be validated or compared with alternate strategies.
The aim of this study was to assess and compare the test characteristics of four strategies to predict survival in patients with head PAC upon presentation: the MBSS, the CA 19-9, the CA 19-9/bilirubin ratio and the combined score of MBSS and CA 19-9/bilirubin ratio.
Methods
A retrospective review of the McGill University Health Center (MUHC) tumour registry was performed. Between 2005 and 2010, data on 133 patients diagnosed with pancreatic adenocarcinoma (PAC) of the head, as confirmed by histopathology, were available. These patients underwent either resectional or palliative treatment. The diagnosis of PAC in patients with unresectable disease was confirmed through biopsies taken at the time of the exploratory laparotomy or during endoscopic ultra-sound. Patient demographics and clinical symptoms and characteristics at presentation were recorded from charts and routine nutritional assessments. The serum CA 19-9 and bilirubin levels prior to any intervention were collected. At the MUHC, CA 19-9 is measured using the Uni-Cell®DXI-800 Beckman Couter immunoassay (Beckman Coulter, Inc., Brea, CA, USA). Note that there was very poor access to CA 19-9 prior to 2007 in the province of Quebec, thus simultaneous measurements were available in only 52 patients (40%) that were included in the final analysis. The Quebec Civil Registry was used at the time of censoring to determine the date of death. If patients were not reported as deceased, they were censored on 14 February 2011, allowing a minimum of 14-month follow-up for the last patients included in this study. The primary outcome of this study was mortality at 9 months, a clinically significant endpoint based on previous studies.4 Overall survival was also assessed.
Statistical analysis
Receiver-operating characteristic (ROC) curves were used to determine an optimal cutoff for CA 19-9 and the CA 19-9-to-bilirubin ratio for mortality at 9 months. The distribution of baseline characteristics in each of the four strategies was compared. These include demographics, resectable disease, resection margins, whether patients had metastatic or locally advanced disease and whether chemotherapy was administrated. The predictive characteristics for each of the four strategies were also determined: sensitivity (Sen), specificity (Spec), positive predictive value (PPV), negative predictive value (NPV), positive and negative likelihood ratios (LR + and LR–, respectively) and ROC. Particular attention was paid to the LR +, the LR– and the NPV as these characteristics have more of a direct clinical impact.21
LR enlighten the clinician as to the accuracy with which a test improves our diagnostic impression.21 The positive LR (LR+) is calculated using the formula sensitivity/(1-specificity) whereas the negative LR (LR-) is calculated using the formula (1-sensitivity)/specificity.21 The initial impression is represented by the pre-test probability and the usefulness of the test leads to a post-test probability. For a diagnostic test, a LR of 1 means that the post-test probability is identical to the pretest probability, i.e. the test does not improve our original diagnostic acumen. The usefulness of a test is thus corroborated by how the LR affects the post-test probability: as LRs increase above 1, the post-test probability progressively increases in relation to the pretest probability; as LRs decrease below 1, the post-test probability decreases. In the case of MBSS, the LR + informs the clinician on how the probability of mortality at 9 months shifts when the MBSS is high, whereas the LR– informs the clinician on how the probability of mortality at 9 months changes when the MBSS is low. An ideal test will have a very high LR + and very low LR–. An LR is considered significant when the 95% confidence interval (CI) does not cross 1. Moreover, a high NPV indicates that a high proportion of patients with a low MBSS survive beyond 9 months. For the NPV to be significant, the test must perform better than chance, thus the 95% CI of the NPV must not cross 50%.
Kaplan–Meier survival curves were used to visually evaluate the predictive ability of each strategy. Wilcoxon's test was used to detect statistical significant (P < 0.050) differences between high and low tests results for each strategy and a univariate COX analysis was used to assess their association with survival.
In order to assess for selection bias, baseline characteristics of the patients included in the study were compared with those of the excluded patients, through chi-square, Fisher's exact test and anova when appropriate. Statistical analysis was performed using STATA 13® (Statacorp, College Station, TX, USA).
Results
Patient characteristics are presented in Table 2. The 24 patients (48%) who did not undergo a resection underwent palliative intervention (endoscopic, surgical or both).
Table 2.
Patient characteristics
| Total (n = 52) | Number of patients (%) |
|---|---|
| Median age (years) | 67.5 (60.5–76.0) |
| Male | 28 (53%) |
| Underwent resection | 28 (53%) |
| R0 | 21 (40.4%) |
| R1 | 7 (13.5%) |
| Metastatic disease | 13 (25%) |
| Locally advanced | 9 (17.3%) |
| Chemotherapy | 38 (73.1%) |
| Size of the tumour (cm) | 3.35 (2.6–4.0) |
| High MBSS | 32 (62%) |
| MBSS symptoms | |
| Weight loss >10% | 32 (62%) |
| Pain | 31 (60%) |
| Jaundice | 41 (79%) |
| Smoking | 11 (21%) |
| Bilirubin (μmol/l) | 45.7 (15.0–181.8) |
| CA 19-9 (U/ml) | 365.9 (107–1111.5) |
| CA 19-9/bilirubin ratio | 4.67 (1.63–39.8) |
| Median overall survival (months) | 11.0 (5.5–22.0) |
| Resected patients | 16.5 (11.0–31.5) |
| Unresectable patients | 11.0 (5.5–22) |
MBSS, McGill Brisbane Scoring System; CA 19-9, carbohydrate antigen 19-9.
For CA 19-9, the optimal cutoff value was 100 U/ml with a sensitivity of 80.0% and a specificity of 34.8% for mortality at 9 months (ROC area 0.584). For the CA 19-9/bilirubin ratio, the optimal cutoff value was a ratio of 4.36 exhibiting a sensitivity and specificity of 60.0% and 60.9%, respectively (ROC area 0.548). The combined MBSS and CA 19-9/bilirubin ratio was defined as ‘high’ when the MBSS was high and the CA 19-9/bilirubin ratio was greater than the cut offs. The distribution of baseline characteristics was comparable among the groups for each of the four strategies used (Table 3).
Table 3.
Distribution of patient characteristics by strategy use
| Median age (years) | Male | Underwent resection | R1 | Metastatic PAC | Locally advanced PAC | Chemotherapy | Median overall survival (months) | |
|---|---|---|---|---|---|---|---|---|
| High MBSS (n = 32) | 69.5 (60.5–76.0) | 18 (56.2%) | 16 (50.0%) | 6 (18.8%) | 10 (31.3%) | 4 (12.5%) | 21 (65.6%) | 8.0 (3.5–12.5) |
| Low MBSS (n = 20) | 66.0 (60.5–75.0) | 10 (50.0%) | 12 (60.0%) | 1 (5.0%) | 3 (15.0%) | 5 (25.0%) | 17 (85.0%) | 17.5 (11.5–35.0) |
| P-value | 0.314 | 0.660 | 0.358 | 0.184 | 0.324 | 0.280 | 0.200 | 0.002* |
| High CA 19-9 (n = 40) | 69.0 (60.5–77.0) | 21 (52.5%) | 19 (47.5%) | 6 (15.0%) | 11 (27.5%) | 8 (20.0%) | 28 (70.0%) | 10.5 (4.5–18.5) |
| Low CA 19-9 (n = 12) | 65.5 (60.5–69.5) | 7 (58.3%) | 9 (75.0%) | 1 (8.3%) | 2 (16.7%) | 1 (8.3%) | 10 (83.3%) | 17.0 (8.5–33.5) |
| P-value | 0.126 | 0.722 | 0.113 | 0.371 | 0.706 | 0.666 | 0.475 | 0.117 |
| High CA 19-9/bilirubin ratio (n = 29) | 68.0 (60.0–77.0) | 15 (51.7%) | 12 (41.4%) | 4 (13.0%) | 10 (34.5%) | 5 (17.2%) | 19 (65.5%) | 8.0 (3.0–12.0) |
| Low CA 19-9/bilirubin ratio (n = 23) | 67.0 (61.0–74.0) | 13 (56.5%) | 16 (69.5%) | 3 (13.7%) | 3 (13.0%) | 4 (17.4%) | 19 (82.6%) | 17.0 (9.0–32.0) |
| P-value | 0.462 | 0.730 | 0.0643* | 0.980 | 0.111 | 1.000 | 0.217 | 0.026* |
| High combined MBSS and CA19-9/Bilirubin Ratio (n = 23) | 66.0 (57.0–70.0) | 13 (56.5%) | 12 (41.4%) | 4 (17.3%) | 8 (34.7%) | 2 (8.7%) | 14 (60.9%) | 8.0 (2.0–11.0) |
| Low combined MBSS and CA19-9/Bilirubin Ratio (n = 29) | 68.0 (62.0–75.0) | 15 (51.7%) | 12 (52.2%) | 3 (10.3%) | 5 (17.2%) | 7 (24.1%) | 24 (82.8%) | 17.0 (11.0–32.0) |
| P-value | 0.961 | 0.730 | 0.483 | 0.381 | 0.147 | 0.268 | 0.116 | 0.001* |
The predictive characteristics for all four strategies are presented in Table 4. The MBSS alone had the best NPV at 90.2%, making it the most successful predictor of survival at 9 months. The combined MBSS and CA 19-9/bilirubin ratio also had a significant NPV of 76.7%. This combined score also showed the highest PPV at 58.3%; however, it remained not significant.
Table 4.
Predictive characteristics of the four strategies
| Value (95% CI) | MBSS | CA 19-9 | CA 19-9/Bilirubin Ratio | MBSS and Ca19-9/Bilirubin Ratio |
|---|---|---|---|---|
| PPV | 45.6% (32.4–59.3%) | 40.5% (25.6–56.7%) | 50.0% (31.3–68.7%) | 58.3% (36.6–77.9%) |
| NPV | 90.2% (76.9–97.3%)* | 69.2% (38.6–90.9%) | 75.0% (53.3–90.2%)* | 76.7% (57.7–90.1%)* |
| LR + | 1.90 (1.42-2.55)* | 1.10 (0.82-1.47) | 1.57 (0.99-2.49) | 2.20 (1.21-4.01)* |
| LR - | 0.25 (0.10-0.63)* | 0.72 (0.25-2.05) | 0.52 (0.25-1.10) | 0.48 (0.25-0.91)* |
| Sensitivity | 86.7% (69.3–96.2%)* | 81% (58.1–94.6%)* | 71.4% (47.8–88.7%) | 66.7% (43.0–85.4%) |
| Specificity | 54.4% (41.9–64.4%) | 54.4% (41.9–64.4%) | 54.5% (36.4–71.9%) | 69.7% (51.3–84.4%)* |
| ROC area | 0.71 (0.62-0.79)* | 0.54 (0.42-0.65) | 0.62 (0.49-0.75) | 0.68 (0.55-0.81)* |
statistically significant. MBSS, McGill Brisbane Scoring System; CA 19-9, carbohydrate antigen 19-9.
The combined score (MBSS with CA 19-9/bilirubin ratio) emerged as the most informative predictive strategy with the highest LR + (2.20, 95% CI 1.21–4.01), followed by MBSS alone. The MBSS alone also had the best LR– (0.25, 95% CI 0.10–0.63), followed by the combined score (MBSS with CA 19-9/bilirubin ratio). CA 19-9 alone had the worst predictive characteristics. When correcting for the CA 19-9 for bilirubin at baseline, both LR + and LR– improved, but remained non-significant.
The Kaplan–Meier survival analyses illustrate the predictive ability for each strategy (Figs 1–4). CA 19-9 alone was not able to discriminate between survivors and non-survivors (P = 0.423; Fig. 2). Correcting for bilirubin improved the predictive ability of CA 19-9 and patients with a high CA 19-9/bilirubin ratio exhibited a greater survival (Fig. 3). But again the combined MBSS and CA 19-9/bilirubin ratio was the most accurate strategy in predicting overall survival (P = 0.000; Fig. 4). The results of the univariate survival analysis (Table 5) also found that only CA 19-9 and combined MBSS and the CA 19-9/bilirubin ratio were significant independent predictors of survival, whereas CA 19-9 alone was not.
Figure 1.
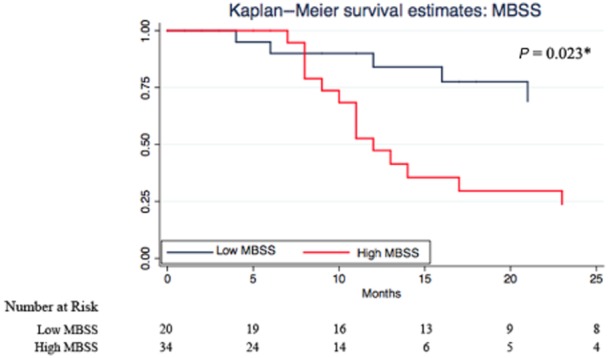
Kaplan–Meier survival estimates for high and low McGill Brisbane Scoring System (MBSS)
Figure 4.
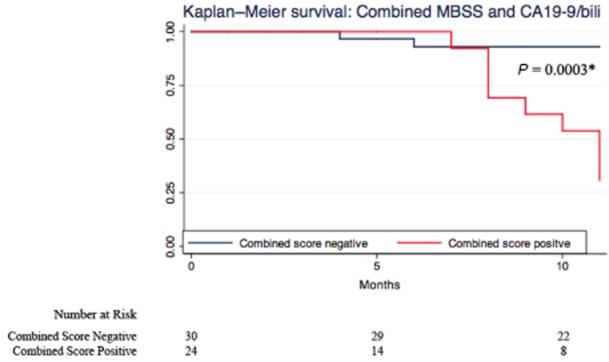
Kaplan–Meier survival estimates for a high and low combined score
Figure 2.
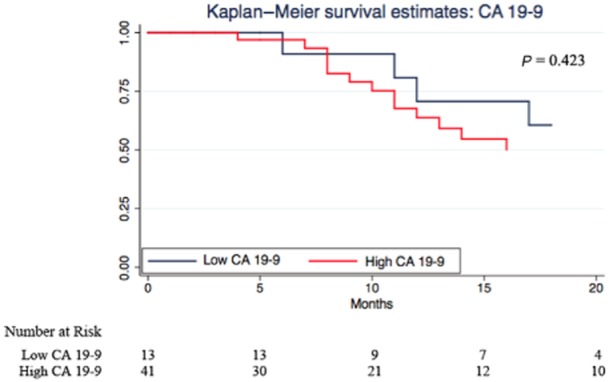
Kaplan–Meier estimates for high and low carbohydrate antigen 19-9 (CA 19-9)
Figure 3.
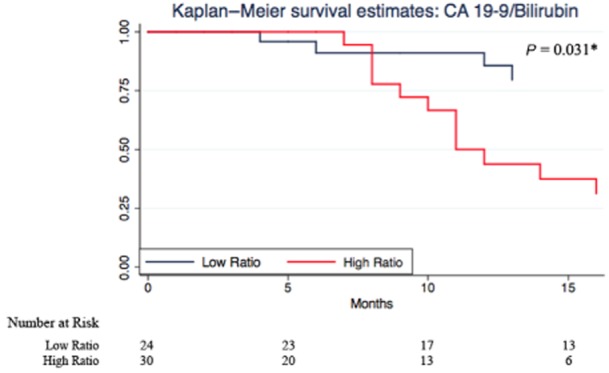
Kaplan–Meier survival estimates for a high and low carbohydrate antigen 19-9 (CA 19-9)/bilirubin ratio
Table 5.
Univariate Cox analysis (results presented in hazard ratios with 95% confidence intervals)
| MBSS | CA 19-9 | CA 19-9/bilirubin ratio | MBSS and Ca19-9/bilirubin ratio | |
|---|---|---|---|---|
| HR | 2.96 (1.19–7.32) | 1.52 (0.55–4.14) | 2.34 (0.99–5.55) | 4.55 (1.91–10.82) |
| P-value | 0.019* | 0.409 | 0.052 | 0.001* |
MBSS, McGill Brisbane Scoring System; CA 19-9, carbohydrate antigen 19-9; HR, hazard ratio.
The bias analysis comparing baseline characteristics in the selected patients and those excluded for the lack of CA 19-9 found no significant differences (Table 6).
Table 6.
Bias analysis: comparison of baseline characteristics of the included and excluded patients
| Included (n = 52) | Excluded (n = 78) | P-value | |
|---|---|---|---|
| Median age (years) | 67.5 (60.5–76.0) | 66.0 (68.0–74.0) | 0.582 |
| Male | 28 (53.0%) | 43 (55.1%) | 0.886 |
| Underwent resection | 28 (53.0%) | 48 (53.9%) | 1.00 |
| R0 | 21 (40.4%) | 29 (37.2%) | 0.689 |
| R1 | 7 (13.5%) | 13 (17.7%) | 0.589 |
| Metastatic disease | 13 (25%) | 18 (23.1%) | 0.836 |
| Locally advanced | 9 (17.3%) | 17 (21.8%) | 0.531 |
| High MBSS | 32 (62.0%) | 41 (52.6%) | 0.312 |
| MBSS symptoms | |||
| Weight loss >10% | 32 (62.0%) | 38 (48.7%) | 0.151 |
| Pain | 31 (60.0%) | 45 (57.7%) | 0.827 |
| Jaundice | 41 (79.0%) | 59 (75.6%) | 0.671 |
| Smoking | 11 (21.0%) | 23 (29.5%) | 0.280 |
| Chemotherapy | 38 (73.1%) | 59 (75.6%) | 0.926 |
| Size of the tumour (cm) | 3.3 (2.6–4.0) | 3. (2.6–4.0) | 0.707 |
| Median overall survival (months) | 11.0 (5.5–22.0) | 13.0 (7.0–27.0) | 0.144 |
MBSS, McGill Brisbane Scoring System.
Discussion
The present study shows that the combination of MBSS and the CA 19-9/bilirubin ratio has the greatest ability of any strategy to predict mortality at 9 months and overall survival from head PAC. Although CA 19-9 has been increasingly used in the diagnosis and prognosis of pancreatic cancer, it has many inherent limitations. CA 19-9 has a limited use as a universally applicable biomarker as it is related to the sialylated Lewis blood group antigens, absent in 5–10% of the population making it inapplicable in these patients whose status is not routinely known upon initial clinical presentation.15,17,22 Moreover, a particular mechanism of CA 19-9 elevation is reduced excretion secondary to biliary obstruction which is nearly ubiquitous in patients with head PAC;19,23,24 this process can lead to confounding in the interpretation of the elevated value. In spite of these significant and often overlooked limitations, there is an increasing interest in the use of CA 19-9 to predict survival in both resectable15,18,25–28 and unresectable patients with PAC.5,22,29,30 In the present cohort, CA 19-9 alone did not perform as well in predicting survival, and exhibited the least useful test characteristics, which highlights the need for a more accurate predictive strategy. This phenomenon can be explained by the fact that 76% of patients presented with jaundice, a significant confounder of high CA 19-9 values. Several methods have been used in an attempt to adjust for hyperbilirubinaemia. A study by Mann et al. analysed the change in CA 19-9 levels after biliary decompression.19 Although conceptually appealing, the use of such a drop in bilirubin (slope) might be clinically limited. The most prevalent method used to adjust CA 19-9 involves dividing the pre-operative levels by the total bilirubin serum level when the latter is elevated.20,31 Unfortunately, none of these studies have validated or compared their results with CA 19-9 alone or to other strategies. The current study is the first to validate the use of CA 19-9 to the bilirubin ratio by demonstrating that its predictive abilities are superior to that of CA 19-9 alone in adenocarcinomas of the head of the pancreas.
This study also confirms the superior usefulness of the MBSS, in both resectable and non-resectable patients.4 The MBSS is a bedside, user-friendly score that predicts survival better than pre-operative radiological findings or resection margin status.13 These results demonstrate a significant clinical advantage in using the combined MBSS and the CA 19-9/bilirubin ratio. Indeed the LR + is higher for the combined score when compared with MBSS alone or the CA 19-9/bilirubin ratio alone enabling the clinician to predict who is at greater risk of dying. This could improve the stratification of patients into therapeutic trials by removing the heterogeneity of survival associated with the diagnosis of head PAC. The combined MBSS and CA 19-9/bilirubin ratio thus opens the door to a more personalized treatment approach, perhaps akin to ‘clinical’ microarray.
The limitations of this study are those inherent to retrospective chart reviews. There is a potential for misclassification bias with respect to some of the MBSS criteria. Using a prospectively collected database may have reduced some of these biases. Another potential bias is the exclusion of patients who did not have simultaneous CA 19-9 and bilirubin levels available at presentation. However, when comparing the baseline characteristics for the included compared with all patients there were no statistically significant differences suggesting that no significant selection bias was introduced through this exclusion criteria. One potential confounder could be the simultaneous study of both palliative and resected patients. Both resectable and unresectable patients were considered together in this analysis as previous work demonstrated that the hazard ratio for the MBSS was strikingly similar in both groups. This suggests that the MBSS performs just as well among resectable and unresectable patients. In order to determine the NPV, the prevalence of ‘death at 9 months’ was thus chosen as a convenient measure, and has also been previously reported on.4 A thorough analysis of the distribution of the MBSS, the CA 19-9 and the CA 19-9-bilirubin ratio according to resection status failed to reveal major differences.
The combined score is thus useful in predicting outcomes, irrespective of resectability status. In fact, the advantage of using a mixed cohort of resectable and unresectable PAC patients underscores its applicability to actual clinical practice.2
Conclusion
The study presented here study shows that the combination of MBSS and the CA 19-9/bilirubin ratio has very strong predictive characteristics for mortality in head PAC patients and is the most clinically meaningful predictor of survival. These results emphasize the importance of clinical symptoms in determining patient prognosis, and support the evaluation of PAC patients by measuring the CA 19-9 and bilirubin levels.
Conflicts of interest
None declared.
References
- 1.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Benson AB., 3rd Adjuvant therapy for pancreatic cancer: one small step forward. JAMA. 2007;297:311–313. doi: 10.1001/jama.297.3.311. [DOI] [PubMed] [Google Scholar]
- 3.Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994;344:1655–1660. doi: 10.1016/s0140-6736(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 4.Jamal MH, Doi SA, Simoneau E, Abou Khalil J, Hassanain M, Chaudhury P, et al. Unresectable pancreatic adenocarcinoma: do we know who survives? HPB. 2010;12:561–566. doi: 10.1111/j.1477-2574.2010.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan S, Rana V, Janjan NA, Abbruzzese JL, Gould MS, Das P, et al. Prognostic factors in patients with unresectable locally advanced pancreatic adenocarcinoma treated with chemoradiation. Cancer. 2006;107:2589–2596. doi: 10.1002/cncr.22328. [DOI] [PubMed] [Google Scholar]
- 6.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Castro SM, Biere SS, Lagarde SM, Busch OR, van Gulik TM, Gouma DJ. Validation of a nomogram for predicting survival after resection for adenocarcinoma of the pancreas. Br J Surg. 2009;96:417–423. doi: 10.1002/bjs.6548. [DOI] [PubMed] [Google Scholar]
- 8.Ferrone CR, Kattan MW, Tomlinson JS, Thayer SP, Brennan MF, Warshaw AL. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol. 2005;23:7529–7535. doi: 10.1200/JCO.2005.01.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glen P, Jamieson NB, McMillan DC, Carter R, Imrie CW, McKay CJ. Evaluation of an inflammation-based prognostic score in patients with inoperable pancreatic cancer. Pancreatology. 2006;6:450–453. doi: 10.1159/000094562. [DOI] [PubMed] [Google Scholar]
- 10.Shimoda M, Katoh M, Kita J, Sawada T, Kubota K. The Glasgow Prognostic Score is a good predictor of treatment outcome in patients with unresectable pancreatic cancer. Chemotherapy. 2010;56:501–506. doi: 10.1159/000321014. [DOI] [PubMed] [Google Scholar]
- 11.Garcea G, Cairns V, Berry DP, Neal CP, Metcalfe MS, Dennison AR. Improving the diagnostic yield from staging laparoscopy for periampullary malignancies: the value of preoperative inflammatory markers and radiological tumor size. Pancreas. 2012;41:233–237. doi: 10.1097/MPA.0b013e31822432ee. [DOI] [PubMed] [Google Scholar]
- 12.La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G. The glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2917–2923. doi: 10.1245/s10434-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 13.Jamal M, Jad A, Dumitra S, Simoneau E, Doi S, Barkun J. The McGill-Brisbane score is an excellent predictor of survival in patients undergoing a pancreatico-duodenectomy for pancreatic adenocarcinoma. J Am Coll Surg. 2011;213:S15–16. [Google Scholar]
- 14.Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 15.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012;3:105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kannagi R. Carbohydrate antigen sialyl Lewis a – its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J. 2007;30:189–209. [PubMed] [Google Scholar]
- 17.Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R, et al. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21:441–447. doi: 10.1093/annonc/mdp332. [DOI] [PubMed] [Google Scholar]
- 18.Hallemeier CL, Botros M, Corsini MM, Haddock MG, Gunderson LL, Miller RC. Preoperative CA 19-9 level is an important prognostic factor in patients with pancreatic adenocarcinoma treated with surgical resection and adjuvant concurrent chemoradiotherapy. Am J Clin Oncol. 2011;34:567–572. doi: 10.1097/COC.0b013e3181f946fc. [DOI] [PubMed] [Google Scholar]
- 19.Mann DV, Edwards R, Ho S, Lau WY, Glazer G. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol. 2000;26:474–479. doi: 10.1053/ejso.1999.0925. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz-Gonzalez J, Alvarez-Aguila NP, Medina-Castro JM. Adjusted carbohydrate antigen 19-9. Correlation with histological grade in pancreatic adenocarcinoma. Anticancer Res. 2005;25:3625–3627. [PubMed] [Google Scholar]
- 21.McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17:646–649. doi: 10.1046/j.1525-1497.2002.10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasan HS, Springett GM, Chodkiewicz C, Wong R, Maurel J, Barone C, et al. CA 19-9 as a biomarker in advanced pancreatic cancer patients randomised to gemcitabine plus axitinib or gemcitabine alone. Br J Cancer. 2009;101:1162–1167. doi: 10.1038/sj.bjc.6605243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris-Stiff G, Teli M, Jardine N, Puntis MC. CA19-9 antigen levels can distinguish between benign and malignant pancreaticobiliary disease. Hepatobiliary Pancreat Dis Int. 2009;8:620–626. [PubMed] [Google Scholar]
- 24.Marrelli D, Caruso S, Pedrazzani C, Neri A, Fernandes E, Marini M, et al. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009;198:333–339. doi: 10.1016/j.amjsurg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 25.Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology. 2006;70:255–264. doi: 10.1159/000094888. [DOI] [PubMed] [Google Scholar]
- 26.Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897–2902. doi: 10.1200/JCO.2005.05.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hata S, Sakamoto Y, Yamamoto Y, Nara S, Esaki M, Shimada K, et al. Prognostic impact of postoperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2012;19:636–641. doi: 10.1245/s10434-011-2020-9. [DOI] [PubMed] [Google Scholar]
- 28.Kondo N, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Hashimoto Y, et al. Prognostic impact of perioperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol. 2010;17:2321–2329. doi: 10.1245/s10434-010-1033-0. [DOI] [PubMed] [Google Scholar]
- 29.Maisey NR, Norman AR, Hill A, Massey A, Oates J, Cunningham D. CA19-9 as a prognostic factor in inoperable pancreatic cancer: the implication for clinical trials. Br J Cancer. 2005;93:740–743. doi: 10.1038/sj.bjc.6602760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saad ED, Machado MC, Wajsbrot D, Abramoff R, Hoff PM, Tabacof J, et al. Pretreatment CA 19-9 level as a prognostic factor in patients with advanced pancreatic cancer treated with gemcitabine. Int J Gastrointest Cancer. 2002;32:35–41. doi: 10.1385/IJGC:32:1:35. [DOI] [PubMed] [Google Scholar]
- 31.Kang CM, Kim JY, Choi GH, Kim KS, Choi JS, Lee WJ, et al. The use of adjusted preoperative CA 19-9 to predict the recurrence of resectable pancreatic cancer. J Surg Res. 2007;140:31–35. doi: 10.1016/j.jss.2006.10.007. [DOI] [PubMed] [Google Scholar]


