Abstract
Dictyostelium development starts with the chemotactic aggregation of up to 106 amoebae in response to propagating cAMP waves. cAMP is produced by the aggregation stage adenylyl cyclase (ACA) and cells lacking ACA (aca null) cannot aggregate. Temperature-sensitive mutants of ACA were selected from a population of aca null cells transformed with a library of ACA genes, a major segment of which had been amplified by error-prone PCR. One mutant (tsaca2) that can complement the aggregation null phenotype of aca null cells at 22°C but not at 28°C was characterized in detail. The basal catalytic activity of the enzyme in this mutant was rapidly and reversibly inactivated at 28°C. Using this mutant strain we show that cell movement in aggregates and mounds is organized by propagating waves of cAMP. Synergy experiments between wild-type and tsaca2 cells, shifted to the restrictive temperature at various stages of development, showed that ACA plays an important role in the control of cell sorting and tip formation.
Keywords: adenylyl cyclase/cAMP relay chemotaxis/cell movement/cell sorting/differentiation
Introduction
During the initial stages of aggregation of the social amoeba Dictyostelium discoideum the cells move chemotactically in response to waves of cAMP, which are initiated periodically by aggregation centres and relayed outward by surrounding cells. The cells detect this extracellular cAMP signal via highly specific serpentine seven-transmembrane cAMP receptors. Upon binding cAMP these receptors activate a signal transduction cascade that results in the activation of the aggregation stage adenylyl cyclase (ACA), an enzyme that spans the plasma membrane 12 times and contains two large cytoplasmic domains (designated C1 and C2) (Devreotes, 1989; Pitt et al., 1992; Parent and Devreotes, 1996b). While some of the cAMP produced is thought to function inside the cell by activating cAMP-dependent protein kinase (PKA), most is secreted and degraded by a cAMP phosphodiesterase. The secreted cAMP can bind to the cell-surface cAMP receptors, resulting in amplification of the cAMP signal (Parent and Devreotes, 1996b; Aubry and Firtel, 1999). Activation of the cAMP receptors by binding of cAMP also induces an adaptation process. Together, excitation and adaptation result in cAMP oscillations.
The cells not only amplify the cAMP signal, but also move in response to cAMP gradients as long as the amplitude of the gradient that they detect over their length increases with time. Since the waves propagate from the aggregation centre outward, the cells move inward. cAMP wave propagation thus leads to the periodic inward movement of the cells and their accumulation in a multicellular aggregate. Waves of moving cells can be observed under dark field optics, because when the cells are moving they change shape, resulting in alteration in their light-scattering properties (Alcantara and Monk, 1974; Gross et al., 1976). The waves are organized in intricate spiral and target patterns. By the use of isotope dilution fluorography it has been shown that the optical density (OD) waves are a faithful representation of the underlying cAMP waves (Tomchik and Devreotes, 1981). The bright bands correspond to cells moving in response to the rising phase of the cAMP wave, and the darker bands to cells recovering during the descending phase of the wave.
We have previously been able to observe propagating OD waves in aggregation streams and mounds, and in the slugs of some strains (Siegert and Weijer, 1989, 1995; Dormann et al., 1997). However, it has not yet been possible to show beyond doubt that these waves are also caused by propagating waves of cAMP. This problem could in principle be overcome by using conditional mutants, such as temperature-sensitive ACA mutants. This would allow one to switch off ACA activity at any stage of development and investigate the immediate consequences for cell movement. It has been shown that development of aca null cells can be rescued by expression of the ACA gene under a strong actin promoter. Using this system it proved possible to select mutants with altered activation and catalytic properties from libraries made by random mutagenesis of a region of the ACA gene (Parent and Devreotes, 1995, 1996a). We have used one such library to screen for mutants that show temperature-sensitive development.
Results
Isolation of temperature-sensitive ACA mutants
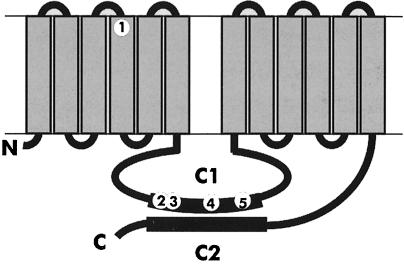
A randomly mutagenized library of the ACA gene in an autonomously replicating plasmid (see Materials and methods) was transformed into aca null cells. Transformants harbouring a plasmid with a temperature-sensitive form of ACA were selected by plating ∼104 independent transformants clonally on bacterial lawns. Clones that developed at 22°C but not at 27°C were selected. Closer examination of four such strains showed that tsaca2 and tsaca3 had very similar phenotypes. Both developed normally and with normal timing at 22°C, while their development was completely blocked at 27°C. The other two strains, tsaca1 and tsaca4, did not show such clean temperature sensitivity and we decided to concentrate on the tsaca2 mutant. The plasmid from this strain was recovered and sequenced. Five missense mutations were identified, four of which are located within the C1 loop, which forms half of the catalytic core (Figure 1).
Fig. 1. Schematic diagram of adenylyl cyclase and the mutations conferring temperature sensitivity. The cyclase is a 12 transmembrane catalytic domain. The mutations in tsaca2 are F306L, S498G, K504E, E593V and I649T. The F306L mutation is in the fourth transmembrane domain and the others are in the C1 domain.
Development of tsaca2 can be reversibly blocked at the aggregation and mound stages
At 22°C, development of tsaca2 was indistinguishable from wild-type cells (Figure 2A and B). To investigate at what stages of development a shift to the non-permissive temperature (28°C) would block development, we plated tsaca2 at a density of 106/cm2 on KK2-buffered agar plates and shifted them to the restrictive temperature at various times of development. Development could be blocked by a shift to 28°C at the pre-aggregation stage, as expected, since this was the basis for the selection. If cells were shifted at the aggregation or loose mound stage they tended to disperse. When the cells had reached the tight mound stage before transfer, development was again arrested at 28°C (Figure 2C). Arrest of development was largely reversible since if the mounds were shifted down to 22°C after incubation for 12 h at 28°C most of them went on to develop into fruiting bodies (Figure 2D). Microscopic examination showed that the fruiting bodies consisted of normal looking stalk cells and spores (not shown). As control strains, we used the original parent of the aca null cell line, DH1, and a strain, acan, expressing ACA under the control of the actin 15 promoter. The acan strain was isolated in the original screen as a colony whose development was not temperature sensitive. At room temperature, development of acan is relatively normal, although it forms somewhat smaller aggregation territories and shows less prominent aggregation streams, as is characteristic of strains that overexpress ACA. When these cell lines were shifted to 28°C at the beginning of development or at the mound stage, their subsequent development was normal (Figure 2E and F).
Fig. 2. Temperature sensitivity of development in strains tsaca2 and acan and their parent DH1. (A and B) A tsaca2 aggregate after 8 and 24 h of development at 22°C. (C) tsaca2 mounds were shifted to 28°C after 8 h of development at 22°C and photographed at 24 h. Development is arrested at the mound stage. (D) After 8 h of development at 22°C, tsaca2 cells were shifted to 28°C for 12 h and then returned to 22°C and left to develop for another 16 h. (E and F) Fruiting bodies formed by acan and DH1 cells, respectively, after development for 8 h at 22°C followed by 16 h at 28°C.
We are currently examining in detail the behaviour of tsaca2 cells after transfer to restrictive temperature at the late mound and slug stage, and the results will be the subject of a later report.
The cAMP relay response and the activation of ACA basal activity are temperature sensitive in tsaca2
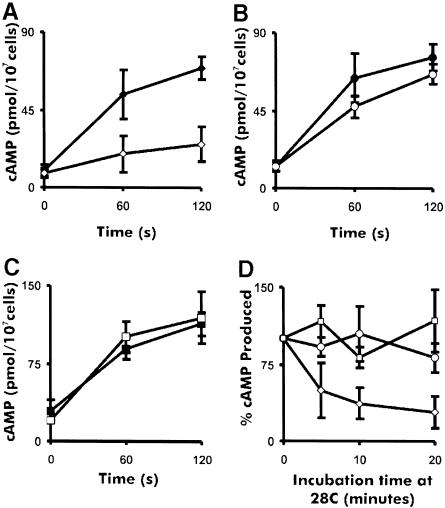
To test the effect of temperature on the activity of the mutant cyclase, we performed cAMP relay assays (Figure 3). tsaca2 cells (Figure 3A) gave a comparable response to that of DH1 cells (Figure 3B) at the permissive temperature, but showed severe inhibition of the relay response after pre-incubation at 28°C for 20 min. DH1 and acan cells gave comparable relay responses at 22 and 28°C (Figure 3B and C) with the acan cells consistently producing more cAMP than DH1 cells. The residual cAMP produced by tsaca2 cells at 28°C may result from incomplete inactivation of the cAMP relay response or from activity of one or other of the adenylyl cyclases (ACB or ACG) known to be expressed at various stages of development and spore germination (Pitt et al., 1992; Kim et al., 1998; Meima and Schaap, 1999). These experiments show that substantial inactivation of tsaca2 occurs within 20 min of exposure to the restrictive temperature. To examine the time course of inactivation, we measured the relay response after pre-incubation of the cells for 5, 10 and 20 min at 28°C. Inactivation of the relay response is very rapid, with a half-time of <5 min (Figure 3D).
Fig. 3. cAMP relay by tsaca2 and control strains at permissive and restrictive temperature. Shown are the cAMP relay responses in cells starved for 4.5 h as a function of temperature in tsaca2, DH1 and acan (A, B and C). Closed symbols correspond to cAMP relay at 22°C and open symbols to relay measurements after pre-incubation for 20 min at 28°C. (D) cAMP relay response after various times at 28°C for tsaca2 (diamonds), DH1 (circles) and acan (squares). The data were collected from at least four different experiments in each case. The error bars indicate the SEM.
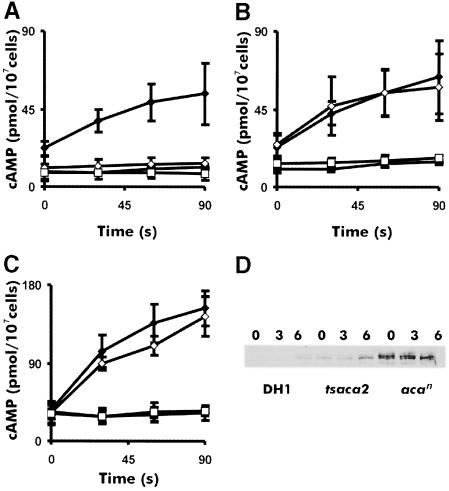
The assay of cAMP receptor-induced activation of ACA used above is rather complex since it involves many different factors. It was therefore of interest to determine whether it is the catalytic activity of ACA itself that is temperature sensitive or rather its activation. To investigate this, we measured the effect of temperature on the basal and unregulated cyclase activity in cell lysates of aggregation-competent cells. The cells were shaken in buffer for 4.5 h and then incubated for 20 min at either 22 or 28°C followed by lysis and immediate measurement of adenylyl cyclase activity. We measured activity in the presence of Mg2+ ions to determine the basal activity and in the presence of Mn2+ ions to determine the unregulated activity. The experiments showed that the unregulated cyclase activity in tsaca2 was comparable to that of its parent strain DH1 at 22°C, but severely reduced at 28°C, where it was hardly measurable (Figure 4A and B). On the other hand the control strain acan showed 2- to 3-fold higher cyclase activity than tsaca2 and DH1 (Figure 4C). These results show that the catalytic activity of ACA is temperature sensitive rather than its activation.
Fig. 4. Temperature sensitivity of basal ACA activity in DH1, acan and tsaca2. ACA activity was measured in cells starved for 4.5 h in the presence of Mg2+ or Mn2+ ions, at 22 and 28°C, for strains tsaca2 (A), DH1 (B) and acan (C). The diamonds are activities measured in the presence of 2.5 mM Mn2+, the squares in the presence of 2 mM Mg2+. The solid symbols are activities measured at 22°C and open symbols are activities measured at 28°C. ACA activity was on average three times higher in acan compared with tsaca2 and DH1. (D) ACA expression in DH1, tsaca2 and acan at 0, 3 and 6 h of development, as determined by Western blot analysis. The results are representative of four experiments. Quantitation of the light emission showed that the level of ACA protein expression in acan was on average seven times higher than in tsaca2, while the levels of ACA in cells of tsaca2 and DH1 starved for 6 h were similar.
Somewhat surprisingly, the basal level of ACA activity in tsaca2 was much lower than in acan, and similar to that in DH1, despite the fact that tsaca2 is expressed under a strong actin 15 promoter. This suggests that the tsaca2 enzyme is partially defective at 22°C or that it is unstable. To check ACA protein expression levels we performed Western blots on DH1, tsaca2 and acan during the early stages of development. These blots showed that ACA expression was detectable in vegetative stage cells of both tsaca2 and acan, in accord with the fact that the actin 15 promoter is active in vegetative cells. Expression in DH1, which depends on the endogenous ACA promoter, was first detectable in cells starved for 3 h. The expression of tsaca2 was much lower than that of acan and similar to that of DH1 cells starved for 6 h. It is, therefore, likely that a considerable fraction of the tsaca2-encoded enzyme is degraded at 22°C (see Discussion).
ACA activity is required for OD wave propagation at the aggregation and the mound stage
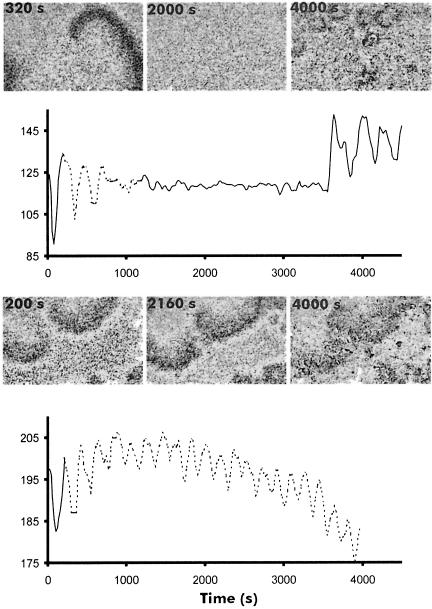
As mentioned, it has been shown previously that the OD waves observed during early aggregation directly reflect the underlying cAMP waves (Tomchik and Devreotes, 1981; Devreotes et al., 1983). At that stage of development the cells are still isolated and have to communicate by an extracellular signal that diffuses through the medium. Analysis of OD waves is a convenient way to analyse cell–cell signalling in vivo. We have observed OD waves not only during early aggregation, but also in aggregation streams when the cells make characteristic end-to-end contacts, at the mound stage and even in slugs of some species (Siegert and Weijer, 1995; Rietdorf et al., 1996; Dormann et al., 1998). Since we showed that the activity of adenylyl cyclase in tsaca2 is temperature sensitive at the early aggregation stage we anticipated that the appearance and persistence of the dark field waves would also be temperature sensitive. Figure 5 shows that, as expected, once dark field waves have appeared they can be reversibly inhibited by increasing the temperature of the plate of aggregating cells to the restrictive temperature, while in DH1 the waves are virtually unaffected. It is noticeable that the effect is rapid: it takes <20 min for the waves to disappear, in agreement with the short half-time for inactivation of the mutant enzyme (Figure 3D). In fact, it seems likely that the main factor involved in the disappearance of the waves is the time taken for the agar plate and heat stage to reach 28°C. The effect is almost completely reversible: if the heat stage is switched off, the waves reappear after 20 min. It is noticeable that when the waves return they do so in the form of many competing centres (Figure 5). However, the dynamics of the waves are only slightly changed, indicating almost full recovery of ACA activity (Figures 5 and 6A).
Fig. 5. Temperature sensitivity of OD waves at the aggregation stage. Upper panel: OD waves in tsaca2 before shifting (320 s), during incubation at 28°C (2000 s) and 20 min after lowering the temperature (4000 s). The solid line represents time spent at 22°C and the stippled line time at 28°C. Lower panel: aggregation stage DH1 before (200 s) and after transfer (2160 s, 4000 s) to 28°C. The lower graph shows OD oscillations during the course of the experiment, as measured in the centre of the image field.
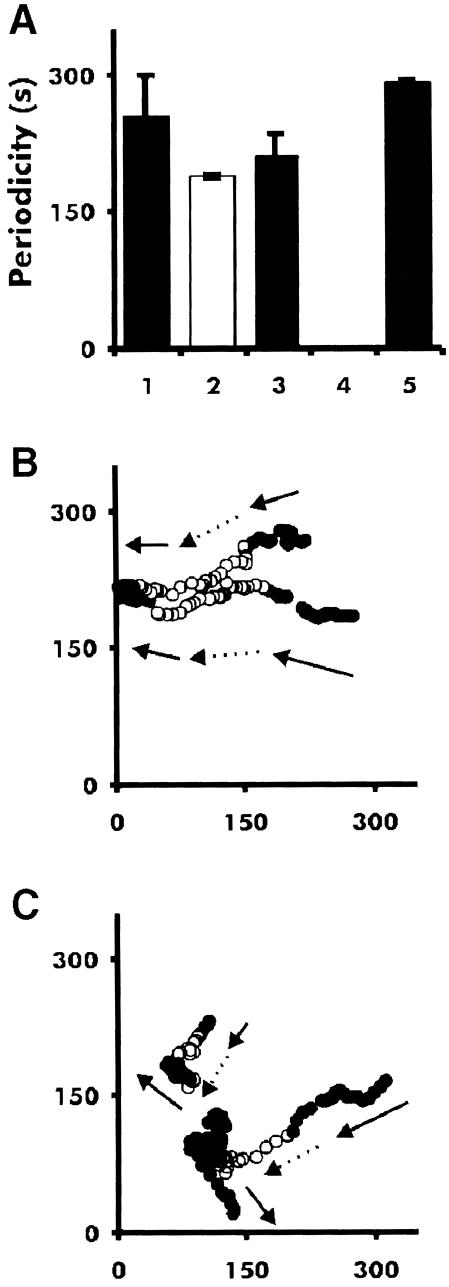
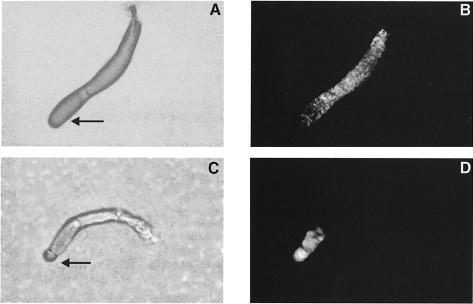
Fig. 6. Period of dark field waves and cell movement in tsaca2 and DH1 at 22 and 28°C. Periodicity was measured during aggregation (A). Bar 1 is the period of DH1 at 22°C, bar 2 is the period at 28°C, bar 3 is tsaca2 at 22°C, bar 4 is tsaca2 at 28°C (where no waves were measured) and bar 5 is the period of tsaca2 after return to 22°C. The data are the mean of at least three experiments. (B) Tracks of two cells in an aggregation field of DH1 cells during a temperature shift experiment. Solid symbols: movement at 22°C; open symbols: movement at 28°C. (C) Tracks of two cells in a field of aggregating tsaca2 cells. The arrows mark the direction of movement of the cells. At the elevated temperature the cells lose their sense of direction. Images were taken every 20 s and, for clarity, the location of the centre of mass of each cell is show in every fourth image (80 s).
Measurement of the movement of individual fluorescent cells shows that the tsaca2 cells lose their sense of direction once the waves disappear, providing further evidence that cell movement is guided by propagating cAMP signals (Figure 6C). During early aggregation (∼4–5 h after induction of starvation) at permissive temperature, DH1 and tsaca2 cells both move in a directed and periodic fashion towards aggregation centres. Increasing the temperature to 28°C has no effect on DH1 cells, whereas the aggregation centres of tsaca2 cells disperse and the movement of individual cells becomes random (Figure 6C). When the cells are returned to permissive temperature, periodic and directed cell movement towards newly formed aggregation centres is re-established. In many cases, periodic movement can be seen at the permissive temperature and correlates well with the period of visible OD waves. These results confirm that the OD waves formed at the aggregation stage depend on cAMP produced by ACA.
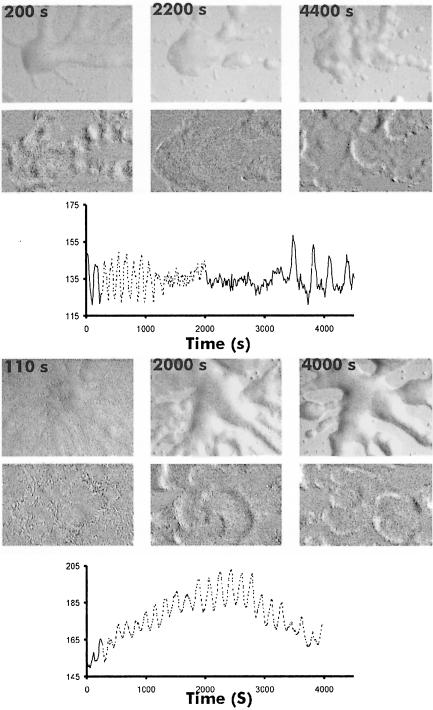
Next we investigated whether the OD waves, which we have previously observed in mounds, also reflect propagating cAMP waves. Figures 7 and 8A show that these waves also disappear and reappear when tsaca2 cells are shifted from 22 to 28°C and back to 22°C. As soon as the waves disappear the mound starts to flatten and finally disperses completely; the cells then move randomly since there is no signalling centre to move to, and, as a result, the cells disperse. After return to the permissive temperature several competing oscillating centres appear and set up new centres. OD waves in the parent strain DH1, on the other hand, are hardly affected by the increase in temperature (Figures 7 and 8A). Mounds of this strain also do not undergo the flattening characteristic of tsaca2. Comparison of cell movement in the two strains shows that cell movement in tsaca2 mounds becomes disoriented upon shift to 28°C, whereas cells in mounds of DH1, while slowing down, keep moving in their original direction (Figure 8B and C). Quantitative analysis of the movement of a number of individual cells in mounds of tsaca2 and DH1 shows that cell movement slows down in both strains during the period of increased temperature, and that it takes >30 min to recover after shift down (not shown).
Fig. 7. Temperature sensitivity of the OD waves at the mound stage. OD waves present in tsaca2 mounds before shifting (200 s) and during incubation (2200 s) at restrictive temperature, together with their reappearance as many small centres 20 min after lowering the temperature (4400 s). The period of the OD waves is shown in the graph; the solid line represents time at 22°C and the stippled line time at 28°C. The lower panel shows images of a DH1 mound before (110 s) and during (2000 s, 4000 s) incubation at 28°C.
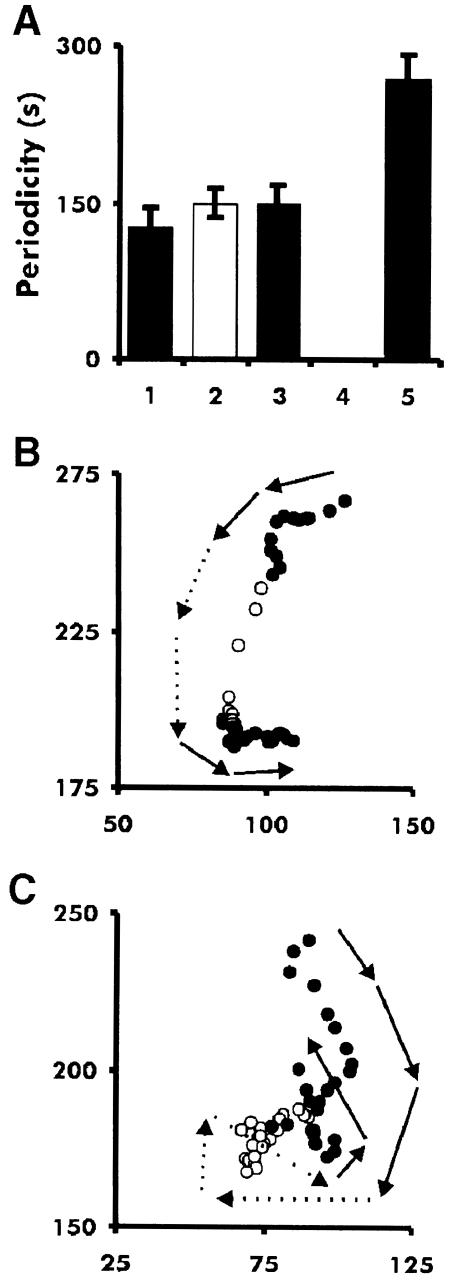
Fig. 8. Period of dark field waves and persistence of cell movement in tsaca2 and DH1 at 22 and 28°C. (A) Periods of OD waves in mounds of DH1 and tsaca2 at 22 and 28°C. bar 1 is the period of DH1 at 22°C, bar 2 is the period at 28°C, bar 3 is tsaca2 at 22°C, bar 4 is tsaca2 at 28°C (where no waves were measured) and bar 5 is the period after return to 22°C. Data are means of at least three experiments. (B) Tracks of a fluorescently labelled DH1 cell at 22°C (solid symbols) and 28°C (open symbols) and the same for tsaca2 (C). The arrows show the direction of cell movement. The images were taken every 20 s; for clarity, the location of the centre of mass of each cell is shown every fourth image (80 s).
Cell sorting at the restrictive and permissive temperatures
After the tight mound is formed, a tip appears. The tip is a distinct morphological structure, consisting of prestalk cells that control the movement of the slug as well as culmination. It thus acts as an organizer for later development (Rubin and Robertson, 1975). Cells start to differentiate into prespore and prestalk cells during aggregation and enter the mound in a random fashion. After a period of relatively disorganized movement, prestalk cells sort out from prespore cells to form the tip. There is circumstantial evidence that cell sorting involves differential chemotaxis towards a cAMP source. Thus, prespore and prestalk cells are chemotactically responsive to cAMP (Mee et al., 1986) and isolated prestalk cells move more rapidly in response to an external cAMP gradient than prespore cells (Early et al., 1995). Moreover, in slug tissue prestalk cells sort preferentially towards a micropipette emitting cAMP pulses (Matsukuma and Durston, 1979; Traynor et al., 1992; Rietdorf et al., 1998). Since the tip controls the movement of all other cells, it is likely that the tip is itself a source of cAMP signals during later development.
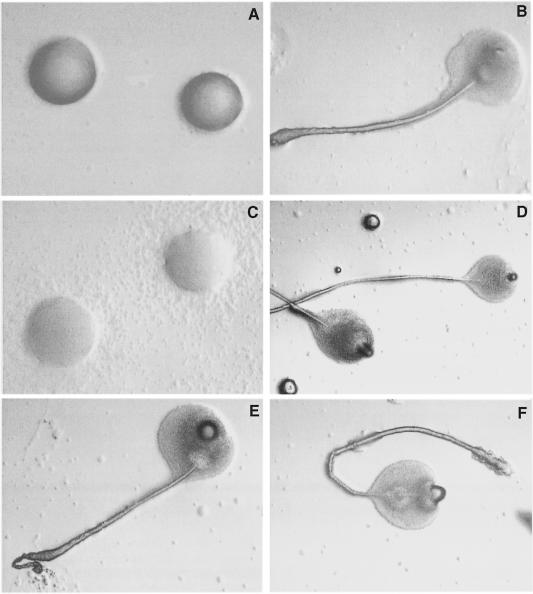
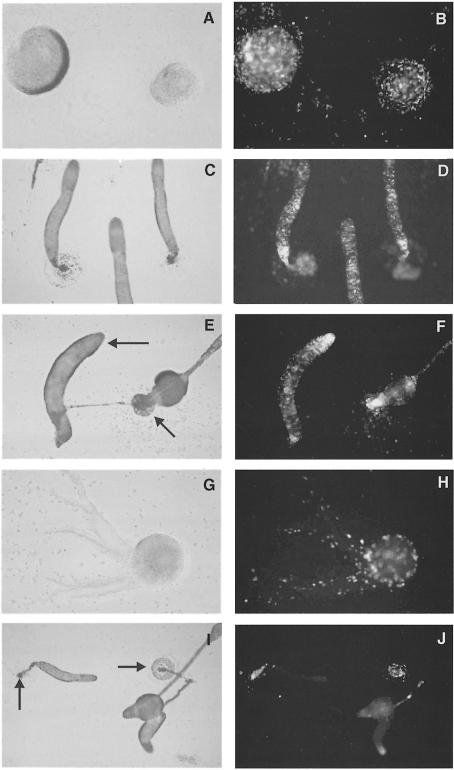
We decided to investigate whether the cAMP produced by ACA plays an important role in the process of cell sorting and tip formation. To this end we performed a series of synergy experiments between the tsaca2 strain and DH1, in which one or the other was marked by expressing the green fluorescent protein (GFP) under the control of the actin 15 promoter. In synergy experiments between a minority of labelled DH1 cells and unlabelled tsaca2 cells at 22°C we observed no appreciable sorting of the DH1 cells from the tsaca2 cells in the slug (Figure 9A and B). However, at the restrictive temperature, the DH1 cells accumulated preferentially in the prestalk zones of slugs (Figure 9C and D). We noted that even in mixtures where 25% of the cells were wild type only a few slugs were formed, and they were generally small. We conclude that 25% wild-type cells was not sufficient for efficient rescue of aggregation.
Fig. 9. Effect of temperature on synergy and cell sorting. Twenty-five per cent DH1 cells expressing red-shifted GFP under the control of the actin 15 promoter were mixed with 75% tsaca2 cells and allowed to develop to the slug stage at either 22°C (A and B) or 28°C (C and D). (A) and (C) are bright field images; (B) and (D) are fluorescence images of the structures shown in (A) and (C) to show the localization of the labelled DH1 cells. The black arrows in (A) and (C) point towards the tips of the slugs.
We decided to perform a further series of experiments in which we let the cells aggregate and develop to the early mound stage at permissive temperature and then shifted them to restrictive temperature (Figure 10A and B). Under these conditions slugs formed efficiently in mixtures containing only 10% DH1 cells. If the cells were left to develop at 22°C there was no appreciable sorting of the DH1 from the tsaca2 cells (Figure 10C and D). If the cells were shifted to the restrictive temperature, the DH1 cells took up positions in the prestalk zone of the slugs (Figure 10E and F). In the reverse experiment, in which 10% labelled tsaca2 cells were mixed with unlabelled DH1, the tsaca2 cells happily co-aggregated with DH1 cells to form mounds (Figure 10G and H), but if shifted at that stage of development they were subsequently excluded from the tip and the major part of the prespore zone. Instead, they accumulated at the back of the slug and ended up in the basal disc of the fruiting bodies (Figure 10I and J). If we waited for slugs to form from these mixtures at the permissive temperature and then shifted them to the restrictive temperature, the slugs went on to culminate. Most of the tsaca2 cells in the prestalk zone were lost from the tip and finally from the slug, some of the tsaca2 cells in the very tip moved into the stalk tube mouth and differentiated into stalk cells. The tsaca2 cells in the prespore zone were mostly lost from the slug; they did not differentiate into mature spores (not shown). These experiments indicate that in such mixtures cells require ACA activity in order to participate in tip formation, and furthermore that ACA activity may play a role in differentiation.
Fig. 10. Effect of temperature on sorting from the mound stage onwards. (A and B) Ten per cent DH1 cells expressing the red-shifted GFP were mixed with 90% tsaca2 cells and allowed to develop to the mound stage at 22°C. They were then either kept at 22°C until they reached the slug stage (C and D) or shifted to 28°C until they reached the slug stage (E and F). In the reverse experiments, 10% tsaca2 cells expressing the red-shifted GFP were mixed with 90% DH1 cells and allowed to develop to the mound stage (G and H) and then shifted to 28°C (I and J). The black arrows in (E) point towards the tip of a slug and fruit. The black arrows in (I) point towards the back of a slug and the basal disk of a fruit.
Discussion
Tsaca2 shows temperature-sensitive catalytic activity
We have described a temperature-sensitive adenylyl cyclase mutant, tsaca2, whose catalytic activity is rapidly reduced after a shift to 28°C. This property permits a more thorough investigation of the role of ACA in cell movement and differentiation. We have shown that at the aggregation stage the cAMP relay response of tsaca2 cells is substantially reduced at 28°C, and furthermore that the mutational defect affects the Mn2+-dependent unregulated cyclase activity of tsACA2 (Figures 3 and 4).
Rescue and sequencing of the mutated gene showed that tsaca2 carries five mutations (Figure 1). The first mutation, F306L, lies in the fourth transmembrane domain, while the other four mutations lie in the C1 catalytic half-site, which starts at position 396 and ends at position 693. The Dictyostelium C1 domain contains an 85 residue asparagine-rich insertion (Parent and Devreotes, 1995). The mutations S498G, K504E and E593V lie in the poorly conserved parts of the catalytic C1 domain, at the boundary of this insertion, whereas the mutation I649T is located in the highly conserved QY/FDIWG motif, which has been shown to make up half of the forskolin binding site in mammalian adenylyl cyclases (Tesmer et al., 1997; Zhang et al., 1997). Binding of forskolin is thought to promote the dimerization of the catalytic C1 and C2 half-sites and thereby form the catalytically active heterodimer. Although Dictyostelium ACA is not stimulated by forskolin, the I649T mutation may well be responsible for the temperature-sensitive behaviour of tsaca2. It is possible that this highly conserved residue plays an important role in stabilization of the folded state of the cyclase (Pakula and Sauer, 1989). In addition, formation of the C1–C2 dimer may be temperature sensitive in the mutant, and this could explain why the inactivation of the cyclase at the elevated temperature is so fast (t1/2 <5 min) and why it seems relatively easily reversible.
Whatever the precise molecular basis of the lesion in tsaca2, it is clear that the level of the protein is low (Figure 4F), suggesting that it is degraded more rapidly than the wild-type protein. This instability turns out to be an advantage for our biological experiments: the tsaca2 cells produce adequate levels of cAMP during relay and also show a normal developmental phenotype at 22°C. We find (unpublished experiments) that they do not have the sporogenous phenotype characteristic of mutants with elevated cAMP levels or elevated cAMP-dependent protein kinase activity (Abe and Yanagisawa, 1983; Simon et al., 1992; Hopper et al., 1995; Mann et al., 1997).
The role of ACA in the control of cell movement
Previously, high-frequency dark field waves were shown to exist in a variety of axenic and non-axenic strains at the mound stage of development (Siegert and Weijer, 1995; Rietdorf et al., 1996). It also proved possible to initiate waves in mounds by periodic microinjection of cAMP from a micropipette (Rietdorf et al., 1998). On the basis of these findings it was argued that the waves elicited by cAMP were propagated by the same propagator as the endogenous waves, and that this was cAMP. The experiments presented here now show conclusively that the waves are due to cAMP propagation, and that the cAMP is generated by ACA, since we can make them disappear and reappear by shifting tsaca2 mounds from the permissive to the restrictive temperature (Figures 5 and 7). Furthermore, we could show that disappearance–reappearance of the waves correlates with a change from directed to non-directed cell movement and back (Figures 6 and 8). One interesting observation is that as soon as the cells in the mound lose their directionality of movement at the restrictive temperature the mound starts to disperse. At this stage of development the slime sheath is not yet formed and there is no physical envelope that keeps the cells together, as is the case later at the slug stage of development. This observation shows that the inward-directed chemotactic movement of the cells is a main determinant of mound integrity: the cells do not simply stick together by cell–cell contacts, but have to try actively to move to the centre.
Upon lowering of the temperature, many new oscillating centres arise in a tsaca2 mound originally dominated by a spiral wave. This is reminiscent of experiments where aggregating cells distributed over an agar plate are collected and replated at the same density. In that case many more aggregation centres arise than were originally present, since during development many more cells had become capable of setting up centres (Gingle and Robertson, 1976). These cells would normally be prevented from becoming centres by the signals originating in already established centres. The same seems to be true in the mound: after lowering of the temperature, a mound originally organized by one centre becomes organized by many centres, each sending out target waves (Figure 7).
The role of ACA in cell sorting
The present data support the notion that the cAMP produced by ACA is, under normal physiological conditions, responsible for the control of cell movement during aggregation and the mound stage of development. The sorting experiments showed that a lack of ACA function had a significant influence on the cell sorting behaviour of cells in mounds and slugs. In chimeras of 25% labelled DH1 cells with tsaca2 cells that are kept under restrictive conditions from the beginning of development, the wild-type cells populate the prestalk compartment of the slug (Figure 10). This is true even if the cells are allowed to develop to the mound stage at the permissive temperature and then shifted to the restrictive temperature (Figure 10A–F). Evidently, lack of ACA puts cells at a disadvantage in either establishing a signalling centre or responding to its signals. It is possible that ACA activity is not only required for extracellular signalling, but also plays an important role in cell type differentiation by producing cAMP required to activate PKA.
In conclusion, we have isolated a temperature-sensitive adenylyl cyclase mutant whose activity is rapidly and reversibly affected by increasing the temperature from 22°C to 27 or 28°C. Using this mutant we have shown definitively that the OD waves observed in aggregation streams and mounds reflect the propagation of waves of cAMP generated by ACA. Lastly, we have shown that cell sorting and tip formation are affected by ACA activity.
Materials and methods
Cells and developmental conditions
Cells of the strains DH1, tsaca2 and acan were grown in HL5 medium according to standard conditions (Sussman, 1987). tsaca2 and acan were grown with 10 µg/ml G418 selection at a density between 2 and 5.5 × 106 cells/ml. To initiate development, cells were harvested from shaking culture by centrifugation at 14 000 g for 5 s and washed once in KK2 buffer (20 mM KH2PO4, 20 mM K2HPO4 pH 6.8) before plating on 1% KK2 agar plates at a density of 5 × 105 cells/cm2. Their development was examined at 22 and 28°C. Alternatively, cells were starved by shaking in suspension as described below.
Generation and selection of the mutants
The mutagenized ACA cDNA library (Parent and Devreotes, 1995) was made by amplifying the ACA gene between positions 799 and 2462 under error-prone conditions (Cadwell and Joyce, 1992). PCR products made under conditions for strong mutagenesis were subcloned into the ACA gene using XL-1 Blue electrocompetent cells (Stratagene). From there the library was transferred to pCP33, an extrachromosomal Dictyostelium expression vector. This library was then electroporated into aca– cells (Pitt et al., 1992) using a Bio-Rad gene pulsar as described (Howard et al., 1988). Transformants selected on 20 µg/ml G418 were plated clonally on SM agar plates with Aerobacter aerogenes and incubated at 22°C. Clones that showed normal development were picked and gridded onto two equivalent SM agar plates with A.aerogenes. One plate was incubated at 22°C, the other at 27°C. Clones that aggregated and fruited at 22°C, but failed to aggregate at 27°C, were further purified. The four temperature-sensitive strains described were found among several hundred clones that developed at 22°C. The restrictive temperature used for the experiments in this paper was 28°C.
Vectors and constructs
The plasmid pH15rsGFP was transformed into tsaca2 cells [which were under G418 (10 µg/ml) selection] by electroporation (Howard et al., 1988). The vector contains the red-shifted variant of the GFP [rsGFP(S65T)] under the control of the actin 15 promoter, together with a hygromycin resistance cassette. pB15rsGFP plasmid was transformed into DH1 cells via electroporation. The mutants were selected at 10 µg/ml hygromycin. pB15rsGFP contains rsGFP under the control of the actin 15 promoter and a G418 resistance cassette. Transformants were selected at 10 µg/ml G418.
Assay of the cAMP relay response
Cells were grown in HL5 medium. To initiate development they were harvested from shaking culture at a density of 2–5 × 106 cells/ml, washed once in KK2 buffer (20 mM KH2PO4, 20 mM K2HPO4 pH 6.8) and resuspended in DB buffer (5 mM KH2PO4, 5 mM Na2HPO4, 2 mM MgSO4, 0.2 mM CaCl2 pH 6.2) to 2 × 107 cells/ml. The cells were shaken at 22°C for 4.5 h at 150 r.p.m. To obtain cells in which the adenylyl cyclase is in a basal activation state, the cells were shaken for a further 20 min in the presence of 5 mM caffeine at either 22 or 28°C. To start the experiment the caffeine was removed by two washes in DB minus Ca2+ buffer, followed by resuspension of the cells in the same buffer at a density of 2 × 107 cells/ml. After resuspension, the cells were immediately stimulated with 10 µM 2′deoxy-cAMP in the presence of a 5 mM concentration of the cAMP phosphodiesterase inhibitor dithiothreitol (DTT). One hundred microlitres of the resuspended cells were taken every 30 s and lysed in 50 µl of 3.5% HClO4. The samples were neutralized using 25 µl of half-saturated KHCO3 (Van Haastert, 1984). cAMP was measured in the neutralized samples by a radioactive displacement assay using standard methods.
Adenylyl cyclase assay
For the adenylyl cyclase assays the cells were starved and pre-incubated for 20 min in caffeine as described above. After washing out the caffeine they were resuspended in DB minus Ca2+ buffer to a density of 4 × 107 cells/ml. An equal volume of lysis buffer (10 mM Tris, 2 mM MgCl2⋅6H2O) was then added to achieve a final cell density of 2 × 107 cells/ml. The cells were immediately lysed through two 5 µm Isopore (Sartorius) membrane filters. The reaction was started by the addition of a reaction mixture that resulted in a final concentration of 5 mM DTT and 0.5 mM ATP. In some assays MnCl2 was added to a final concentration of 2.5 mM. Every 30 s, 100 µl aliquots were taken and stopped immediately by addition of 50 µl of 3.5% HClO4. The samples were neutralized and cAMP was determined as described above.
Immunoblotting
Cells were allowed to develop at 107/ml in DB buffer for various times. Samples were collected and solubilized in Laemmli sample buffer, and 106 cell equivalents were subjected to 8% SDS–PAGE. Immunoblotting was performed on nitrocellulose membranes using a peptide antibody directed against the last 15 amino acids of ACA (Parent and Devreotes, 1995). The antiserum was diluted 1:3000 in TBST and detection was performed by chemiluminescence using sheep anti-rabbit horseradish peroxidase-coupled antibody (Promega) in 1:10 000 dilution. Chemiluminescence was recorded and quantitated using a Fujifilm LAS 1000 image reader.
Measurement of OD waves and image processing
OD waves were observed under oblique illumination using a Zeiss Axiovert 100 or 135 inverted microscope equipped with 2.5×, 10× and 20× objectives. The illumination was adjusted by shifting the phase ring partly into the light path in such a way that the faint OD signals related to cell shape changes were maximally enhanced. These OD waves became visible only in time-lapse recordings. The waves were recorded using the Hamamatsu C4792-95-cooled CCD camera, adjusted to medium high sensitivity in order to reduce the light intensity. Data collection and storage were controlled by an Openlab system (Improvision). Time-Space Plots were generated and analysed as described (Siegert and Weijer, 1989, 1995) using specially developed macro routines based on the Optimas 6.0 image-processing package.
Cell movement analysis of GFP-labelled cells
To determine the movement of individual cells throughout development, we mixed a low percentage (0.1–0.5%) of cells expressing rsGFP with unlabelled cells of the same strain. Fluorescence was observed using a Zeiss inverted microscope (Axiovert 100) equipped with a Polychrome II (TILL) computer-controlled monochromator. To record the waves, images were collected with a Hamamatsu C4792-95 cooled CCD camera alternated with bright field images. Data collection was co-ordinated by the Openlab program. Images were taken every 10 or 20 s and the cells were tracked manually or automatically as described (Siegert and Weijer, 1991; Rietdorf et al., 1996), using specially developed macro routines also based on the Optimas 6.0 image-processing package. For synergy experiments we mixed tsaca2 cells with wild-type cells in various proportions and let them develop on KK2 or water agar plates at either 22 or 28°C, from various stages of development onwards.
Acknowledgments
Acknowledgements
This work was supported by an MRC postgraduate studentship to H.P., a Wellcome Trust Program Grant to C.J.W. and BBSRC Grant 43/ICR07521 to J.G.
References
- Abe K. and Yanagisawa,K. (1983) A new class of rapid developing mutants in Dictyostelium discoideum: Implications for cyclic AMP metabolism and cell differentiation. Dev. Biol., 95, 200–210. [DOI] [PubMed] [Google Scholar]
- Alcantara F. and Monk,M. (1974) Signal propagation during aggregation in the slime mould Dictyostelium discoideum. J. Gen. Microbiol., 85, 321–334. [DOI] [PubMed] [Google Scholar]
- Aubry L. and Firtel,R. (1999) Integration of signalling networks that regulate Dictyostelium differentiation. Annu. Rev. Cell. Dev. Biol., 15, 469–517. [DOI] [PubMed] [Google Scholar]
- Cadwell R.C. and Joyce,G.F. (1992) Randomization of genes by PCR mutagenesis. PCR Methods Appl., 2, 28–33. [DOI] [PubMed] [Google Scholar]
- Devreotes P. (1989) Dictyostelium discoideum: A model system for cell–cell interactions in development. Science, 245, 1054–1058. [DOI] [PubMed] [Google Scholar]
- Devreotes P.N., Potel,M.J. and MacKay,S.A. (1983) Quantitative analysis of cyclic AMP waves mediating aggregation in Dictyostelium discoideum. Dev. Biol., 96, 405–415. [DOI] [PubMed] [Google Scholar]
- Dormann D., Weijer,C. and Siegert,F. (1997) Twisted scroll waves organize Dictyostelium mucoroides slugs. J. Cell Sci., 110, 1831–1837. [DOI] [PubMed] [Google Scholar]
- Dormann D., Vasiev,B. and Weijer,C.J. (1998) Propagating waves control Dictyostelium discoideum morphogenesis. Biophys. Chem., 72, 21–35. [DOI] [PubMed] [Google Scholar]
- Early A., Abe,T. and Williams,J. (1995) Evidence for positional differentiation of prestalk cells and for a morphogenetic gradient in Dictyostelium. Cell, 83, 91–99. [DOI] [PubMed] [Google Scholar]
- Gingle A.R. and Robertson,A. (1976) The development of the relaying competence in Dictyostelium discoideum. J. Cell Sci., 20, 21–27. [DOI] [PubMed] [Google Scholar]
- Gross J.D., Peacey,M.J. and Trevan,D.J. (1976) Signal emission and signal propagation during early aggregation in Dictyostelium discoideum. J. Cell Sci., 22, 645–656. [DOI] [PubMed] [Google Scholar]
- Hopper N.A., Sanders,G.M., Fosnaugh,K.L., Williams,J.G. and Loomis,W.F. (1995) Protein kinase A is a positive regulator of spore coat gene transcription in Dictyostelium. Differentiation, 58, 183–188. [DOI] [PubMed] [Google Scholar]
- Howard P.K., Ahern,K.G. and Firtel,R.A. (1988) Establishment of a transient expression system for Dictyostelium discoideum. Nucleic Acids Res., 16, 2613–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Chang,W.T., Meima,M., Gross,J.D. and Schaap,P. (1998) A novel adenylyl cyclase detected in rapidly developing mutants of Dictyostelium. J. Biol. Chem., 273, 30859–30862. [DOI] [PubMed] [Google Scholar]
- Mann S.K.O., Brown,J.M., Briscoe,C., Parent,C., Pitt,G., Devreotes,P.N. and Firtel,R.A. (1997) Role of cAMP-dependent protein kinase in controlling aggregation and postaggregative development in Dictyostelium. Dev. Biol., 183, 208–221. [DOI] [PubMed] [Google Scholar]
- Matsukuma S. and Durston,A.J. (1979) Chemotactic cell sorting in Dictyostelium discoideum. J. Embryol. Exp. Morphol., 50, 243–251. [PubMed] [Google Scholar]
- Mee J.D., Tortolo,D.M. and Coukell,M.B. (1986) Chemotaxis-associated properties of separated prestalk and prespore cells of Dictyostelium discoideum. Biochem. Cell Biol., 64, 722–732. [Google Scholar]
- Meima M.E. and Schaap,P. (1999) Fingerprinting of adenylyl cyclase activities during Dictyostelium development indicates a dominant role for adenylyl cyclase B in terminal differentiation. Dev. Biol., 212, 182–190. [DOI] [PubMed] [Google Scholar]
- Pakula A.A. and Sauer,R.T. (1989) Genetic analysis of protein stability and function. Annu. Rev. Genet., 23, 289–310. [DOI] [PubMed] [Google Scholar]
- Parent C.A. and Devreotes,P.N. (1995) Isolation of inactive and G protein-resistant adenylyl cyclase mutants using random mutagenesis. J. Biol. Chem., 270, 22693–22696. [DOI] [PubMed] [Google Scholar]
- Parent C.A. and Devreotes,P.N. (1996a) Constitutively active adenylyl cyclase mutant requires neither G proteins nor cytosolic regulators. J. Biol. Chem., 271, 18333–18336. [DOI] [PubMed] [Google Scholar]
- Parent C.A. and Devreotes,P.N. (1996b) Molecular genetics of signal transduction in Dictyostelium. Annu. Rev. Biochem., 65, 411–440. [DOI] [PubMed] [Google Scholar]
- Pitt G.S., Milona,N., Borleis,J., Lin,K.C., Reed,R.R. and Devreotes,P.N. (1992) Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell, 69, 305–315. [DOI] [PubMed] [Google Scholar]
- Rietdorf J., Siegert,F. and Weijer,C.J. (1996) Analysis of optical density wave propagation and cell movement during mound formation in Dictyostelium discoideum. Dev. Biol., 177, 427–438. [DOI] [PubMed] [Google Scholar]
- Rietdorf J., Siegert,F. and Weijer,C.J. (1998) Induction of optical density waves and chemotactic cell movement in Dictyostelium discoideum by microinjection of cAMP pulses. Dev. Biol., 204, 525–536. [DOI] [PubMed] [Google Scholar]
- Rubin J. and Robertson,A. (1975) The tip of Dictyostelium discoideum pseudoplasmodium as an organizer. J. Embryol. Exp. Morphol., 33, 227–241. [PubMed] [Google Scholar]
- Siegert F. and Weijer,C. (1989) Digital image processing of optical density wave propagation in Dictyostelium discoideum and analysis of the effects of caffeine and ammonia. J. Cell Sci., 93, 325–335. [Google Scholar]
- Siegert F. and Weijer,C.J. (1991) Analysis of optical density wave propagation and cell movement in the cellular slime mold Dictyostelium discoideum. Physica D, 49, 224–232. [Google Scholar]
- Siegert F. and Weijer,C.J. (1995) Spiral and concentric waves organize multicellular Dictyostelium mounds. Curr. Biol., 5, 937–943. [DOI] [PubMed] [Google Scholar]
- Simon M.N., Pelegrini,O., Veron,M. and Kay,R.R. (1992) Mutation of protein kinase-A causes heterochronic development of Dictyostelium. Nature, 356, 171–172. [DOI] [PubMed] [Google Scholar]
- Sussman M. (1987) Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol., 28, 9–29. [DOI] [PubMed] [Google Scholar]
- Tesmer J., Sunahara,R., Gilman,G. and Sprang,S. (1997) Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsα.GTPγS. Science, 278, 1907–1916. [DOI] [PubMed] [Google Scholar]
- Tomchik K.J. and Devreotes,P.N. (1981) Adenosine 3′,5′-monophosphate waves in Dictyostelium discoideum: A demonstration by isotope dilution-fluorography technique. Science, 212, 443–446. [DOI] [PubMed] [Google Scholar]
- Traynor D., Kessin,R.H. and Williams,J.G. (1992) Chemotactic sorting to cAMP in the multicellular stages of Dictyostelium development. Proc. Natl Acad. Sci. USA, 89, 8303–8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P.J.M. (1984) A method for studying cAMP-relay in Dictyostelium discoideum—the effect of temperature on cAMP-relay. J. Gen. Microbiol., 130, 2559–2564. [Google Scholar]
- Zhang G.Y., Liu,Y., Ruoho,A.E. and Hurley,J.H. (1997) Structure of the adenylyl cyclase catalytic core. Nature, 386, 247–253. [DOI] [PubMed] [Google Scholar]