Abstract
Aging is a multi-factorial process that ultimately induces a decline in our physiological functioning, causing a decreased health-span, quality of life and independence for older adults. Exercise participation is seen as a way to reduce the impact of aging through maintenance of physiological parameters. Eccentric exercise is a model that can be employed with older adults, due to the muscle’s ability to combine high muscle force production with a low energy cost. There may however be a risk of muscle damage before the muscle is able to adapt. The first part of this review describes the process of aging and how it reduces aerobic capacity, muscle strength and therefore functional mobility. The second part highlights eccentric exercise and the associated muscle damage, in addition to the repeated bout effect. The final section reviews eccentric exercise interventions that have been completed by older adults with a focus on the changes in functional mobility. In conclusion, eccentric endurance exercise is a potential training modality that can be applied to older adults for improving muscle strength, aerobic capacity and functional ability. However, further research is needed to assess the effects on aerobic capacity and the ideal prescription for eccentric endurance exercise.
Keywords: Eccentric exercise, functional ability, muscle strength, aerobic capacity, muscle damage
As life-span is on the increase and the population of older adults (65+) continues to grow, the prevalence of chronic disease increases and health-span decreases. Due to the proportion of older adults expanding, it is important to understand how the aging process influences functional ability of individuals (i.e. ‘the competence of an individual to have the physiological capacity to perform normal everyday activities safely and independently without undue fatigue’) [1] and whether regular exercise is useful as a preventive measure for the decline in functional ability. The capacity to perform activities of daily living (ADL) is essential to a satisfactory health-related quality of life. The etiology of functional decline is complex, however, a primary contributor to this is sarcopenia – the age-related loss of muscle mass and quality [2] – and a less efficient cardiovascular system [3]. It is therefore important to develop interventions that will address functional decline by reducing the decline in both the muscular and cardiovascular systems and improve the quality of life for older adults.
The use of eccentric exercise training is an appealing non-conventional exercise model for older adults. Due to its reduced oxygen requirement lowering the metabolic demand, such an activity has the potential to address the age-related decline in functional ability [4–6]. Eccentric muscle contractions – the muscle lengthens during what is termed a lengthening contraction – occur daily during ADL such as descending the stairs or transitioning from standing to sitting [6]. Training programmes that utilise predominantly repetitive maximal or sub-maximal eccentric muscle contractions to induce skeletal muscle injury are becoming a more common form of exercise that is considered an effective mode of conditioning for improving muscular strength and mobility in older adults [7–9]. This however is not a universal view by all [10, 11], as there is some evidence that older adults have an increased susceptibility to exercise-induced muscle damage and impaired, or delayed recovery from injury [10], raising a potential issue with such an exercise model [6].
The aim of the present review was to examine the evidence of aging and its effects on functional capacity of older adults, and whether or not eccentric exercise is an appropriate form of activity to be used as a rehabilitation tool for improving functional capacity of older adults.
Mechanisms of Aging
Aging is seen as the chronological time something has existed and the result of a process or group of processes occurring in living organisms that with time lead to a loss of adaptability, functional impairment, and eventual death. Early research viewed aging as the consequence of a single gene or a key body system. More recently however, it has been viewed as a complex multi-factorial process that not only involves the natural processes of aging, but also the increased risk of disease – coronary heart disease, diabetes and cancer - with aging [12]. Approximately fifteen theories of aging have been presented by Weinert and Timiras [13] in an attempt to explain how and why aging occurs. Theories were classified into four distinct categories: evolutionary, molecular, cellular and systemic. These theories are not individually explicit and may overlap at various levels of organisation [14]. For example, the alteration of molecular events with aging may lead to cellular alteration, and these, in turn, contribute to organ and systemic failure with evolutionary implications for reproduction and survival [13]. Sarcopenia is one of the major consequences of such aging mechanisms, leading to a loss in muscle mass and strength [15], in addition to cardiovascular dysfunction reducing aerobic capacity [16]. Such consequences contribute to the geriatric syndrome of frailty, thereby severely limiting the function, quality of life, and life expectancy of older adults [16].
Age-related decline in aerobic capacity
Maximal oxygen uptake (V̇O2max) is a measure of aerobic capacity – i.e. functional capacity of the cardiorespiratory system [17]. Such a measurement indicates an individual’s ability to transport substances essential for metabolism and provide energy to the working muscles [17]. The aging-related decline in V̇O2max is associated with changes in lung capacity (respiratory function), cardiac output (Q̇) and oxygen extraction from the blood (arterio-venous oxygen difference, a-v O2 difference) (cardiovascular function), leading to an estimated decline in V̇O2max of up to 20% per decade in older, sedentary adults [3, 18].
Aging related changes in respiratory function that contribute to the reduced V̇O2max include an increased compliance of the chest wall and lung parenchyma [19]. These structural changes cause an enlargement of the terminal air space, increased residual volume, decreased tidal volume and a decline in arterial oxygenation. Although changes in respiratory function have been discussed, it’s changes in the cardiovascular systems that are more frequently discussed regarding the age-related decline in aerobic capacity [20–22]. Cardiac output (Q̇), is a central mechanism responsible for the cardiovascular system to deliver oxygen and nutrients to the exercising muscles. The higher the volume of blood pumped to the periphery, the greater potential for oxygen transport and uptake [23, 24]. Fagard and colleagues [25] identify an estimated decline of 0.23 L·min−1 per decade in Q̇ at rest. The decline in Q̇ is much greater during peak exercise with an estimated decline of up to 1.7 L·min−1 [24, 25]. Normalization of Q̇ for differences in body surface area and loss of muscle mass with aging does not eliminate the effects of aging on V̇O2max [24, 26]. One study has however suggested that peak cardiac output does not decline with age, because of the age-related increase in peak exercise end-diastolic volume and stroke volume [27]. Maximal heart rate rather than stroke volume has been shown to be more related to the decline in Q̇ with age during maximal exercise [24, 28]. Although stroke volume remains similar with age, the mechanism by which it is increased during maximal exercise differs between young and old [24]. Young adults had a 10% decrease in end-diastolic volume index, which increased their stroke volume by an increase in ejection fraction [24]. In contrast, older adults increased their stroke volume primarily through cardiac dilation with an increase in end-diastolic volume index, with only a third of an increase in ejection fraction in comparison to the young adults [24]. Contributing factors linked to the aging-related decline in cardiac output include disease, physical inactivity, intrinsic structural and functional changes [29], and a reduced responsiveness to β-adrenergic stimulation [21, 24].
Arterio-venous oxygen difference (a-v O2 difference) –amount of oxygen extracted by active muscles – is a peripheral mechanism that contributes to the age-related decline in V̇O2max [23, 29]. Relative to young adults, maximal a-v O2 difference of older adults is reduced by approximately 10% [28, 30]. The decline in a-v O2 difference with age is attributed to a diminished extraction of oxygen through a decline in muscle oxidative capacity, capillary density, and a smaller ratio of skeletal muscle to total body mass [18, 26, 31]. A review on the age-related decline in V̇O2max suggests there is only a minor age-related decline in maximal cardiac output, and therefore peripheral factors such as a decrease in a-v O2 difference may better account for the age-related decline in V̇O2max [21].
The effects of regular exercise on the age-related decline in aerobic capacity has been widely investigated, with conclusions that endurance training for varying periods of time can elicit increases in V̇O2max in older men and women [32–35]. It has been demonstrated that older adults can elicit the same 10–30% increase in V̇O2max with endurance training as young adults [34–36]. Puggard [34] demonstrated a 7% increase in V̇O2max of older adults (65 – 85 years) following 8-months of a class based exercise programme consisting of endurance, strength, flexibility and balance exercises. An earlier study reported that the age-related decline in V̇O2max between 25- and 65-years was 40% lower for endurance trained men in comparison to their sedentary peers [18]. An increased a-v O2 difference is the most common mechanism responsible for an improved V̇O2max following an exercise intervention in older adults [35, 37], and indicative of an improved ability for the muscle to extract oxygen from the blood due to an increased muscle mass, mitochondria content and capillary density [35].
Age-related decline in skeletal muscle strength
It is well established the human aging process is associated with a significant decline in neuromuscular function and muscle mass [38–40]. The age-related loss of muscle mass and fibre atrophy – particularly among type II muscle fibres – is referred to as ‘sarcopenia’ [38]. Doherty [41] has viewed sarcopenia to encompass the effects of altered central and peripheral nervous system innervations, altered hormonal status, inflammatory effects, and altered caloric and protein intake.
The age-related decline in muscle mass can be as much as 40% between the ages of 25–80-years contributing to an overall strength loss [41]. When assessing the relationship between age and m. quadriceps femoris cross-sectional area, using ultrasound scanning, older women had a 33% lower muscle mass than younger controls [42]. Additionally, older men had a lower muscle thickness than their younger counterparts, particularly in the lower body; however, it was relatively well-maintained in the upper body with age [43]. A limitation to the use of ultrasound for muscle thickness is that it cannot differentiate between muscle and non-muscle tissue, including intramuscular fat [43]. And, as intramuscular fat is higher in older adults [44], using ultrasound would overestimate the amount of muscle tissue because intramuscular fat would be included in the measurement [43]. A decrease in muscle cross-sectional area is indicative of a decline in muscle fibre size, mechanisms responsible for this are closely linked to a reduced activity level and an imbalance between the rate of muscle protein synthesis and the breakdown of muscle protein, which leads to an overall loss in muscle mass [45, 46]. This can be the result of a lower availability of growth hormones and testosterone limiting the ability to increase muscle size with age [38].
Although loss of strength with aging is largely due to a loss of muscle mass and a decline in type II muscle fibres [47], strength loss is often greater than the decline in muscle mass [43]. Other contributing factors are the result of neuromuscular dysfunction including a decrease in excitation-contraction coupling resulting from a decrease in myosin concentration [48], and reduced calcium sensitivity and uptake by the sarcoplasmic reticulum [49]. The loss of muscle fibres has also been attributed to the loss of α-motor neurones [41, 50]. As muscle fibres become disused, motor units are disused and denervation occurs [38, 45, 51]. Decreased electromyography (EMG) recordings during muscle contractions have demonstrated up to a 25% decrease in motor units present in aged muscle, relative to young muscles [50, 52]. Fewer motor neurons increases the size of remaining motor units, with each motor neuron innervating a greater number of muscle fibres, contributing to the decline in muscle strength [53, 54].
Exercise, specifically resistance training has been shown to improve muscle strength of older adults counteracting the effects of sarcopenia [55–57]. An 11% increase in mid-thigh cross-sectional area was reported following 12-weeks of resistance training 3 d·wk−1 at 80% 1-repetition maximum (1-RM) in older men [58]. This could be due to individual fibre hypertrophy, as resistance training increases muscle protein synthesis, number of myofibrils, actin and myosin filaments, sarcoplasm and connective tissue [46, 59]. Resistance training also increases the number of motor units recruited to perform a given task, allowing them to act in synchronization, increasing the ability to generate a force [60]. Endurance based exercise, such as walking and cycling, are less commonly used to improve muscle strength.
Functional ability
The combined effects of aging on the physiological systems (aerobic capacity and muscle strength) not only have social and economic consequences but, most importantly, cause a decline in functional ability for the aged individual [1]. Additional years are more likely spent in ill health. A reduced functional ability means elderly adults encounter a decline in quality of life leading to greater dependence on friends and family, as the completion of daily tasks e.g. standing up and sitting down, crossing the road, become more challenging [61, 62]. This is because the age-related decrease in maximal strength and maximal oxygen uptake requires older adults to work at a higher percentage of their maximum for the completion of ADL [63].
The decline in functional ability with age is not only associated with a decline in aerobic capacity and the ability of muscles to produce a maximal force; but also a deterioration in the sensory system [64]. As we age, the ability to provide sensory feedback (i.e. visual, vestibular and somatosensory) to generate a motor response becomes impaired [65]. And the decline in motor neurons increases innervation ratio and motor unit size. Therefore, a decline in the sensory feedback mechanisms, and increased motor unit size results in poor sub-maximal force control through an increased mechanical output and summation of motor unit forces. This decreases muscle steadiness, and increases the risk of falls [66, 67].
Eccentric exercise for older adults
Characteristics of eccentric muscle contractions
Muscle movement can occur through three types of contraction: 1) the muscle is activated and shortened (concentric); 2) the muscle is activated and lengthened (eccentric); and 3) muscle is activated and maintained at the same length (isometric) [10]. The concentric (CON) muscle contractions produce body movement such as locomotion or prehensility, were as eccentric (ECC) muscle contractions generate antigravity and braking movements [5]. Of these three types of contraction, ECC contractions are considerably more damaging to muscle [68] and produce a greater muscle force than CON and isometric muscle contractions [69]. Although such an activity can produce a greater force, ECC exercise is characterised by a lower metabolic demand than CON exercise [4,70, 71]. Predominately ECC muscle exercise can be performed by walking or running downhill. Such an activity, allows the quadriceps muscles to work eccentrically when exerting a braking force to maintain or slow the pace. When the metabolic demand of such an activity is compared to predominately CON work, in the form of level walking/running, at the same absolute speed, oxygen consumption is lower on a downhill gradient [4, 71]. Navalta et al [71] reported older adults to have a 3 mL·kg·min−1 reduction in V̇O2 walking on a -10% gradient in comparison to 0%gradient. Similar findings were reported by Gault et al [4], with a 25% lower oxygen demand represented by a lower stroke volume and therefore cardiac output during a self-selected treadmill walk by older adults, in addition to reduced systolic blood pressure and arterio-venous O2 difference. Additional studies have investigated the effects of ECC exercise in the form of ECC cycle ergometry [72, 73]. Bigland-Ritchie and Woods [74] reported the oxygen demand for ECC cycling to be only one-sixth to one-seventh of the oxygen demand required for CON cycling at the same workload. It is due to this lower metabolic demand that makes exercise training with repetitive sub-maximal ECC muscle contractions an appealing non-conventional exercise model for older adults [4, 73].
Unaccustomed ECC muscle contractions cause exercise-induced muscle damage with symptoms of delayed onset muscle soreness (DOMS) [75]. Some protocols of ECC contractions can result in more severe initial and secondary injury to muscle fibers of old compared with young adults [10, 76], however, this may promote muscle repair and improve maximal strength of key functional muscles in the elderly [77].There are a number of ways in which exercise-induced muscle damaged can be established. A direct method is to assess the level of disorganisation of skeletal muscle sarcomeres with z-line disruption, lesions of the sarcoplasmic reticulum and transverse tubule system [78]. Indirect measurements of damage include assessing functional performance i.e. maximal force production and serum levels of skeletal muscle enzymes or proteins such as creatine kinase [79–81]. The decline in muscle performance however, only occurs following the initial bout of eccentric contractions, as repetitive bouts of eccentric work lead to adaptations making the muscle more resistant to future damage known as the ‘repeated bout effect’ [82, 83]. Using mouse models, it is well documented that when the muscles of old rodents are damaged from exercise-induced muscle damage, function does not return for up to two-months [76, 84]. There are limited human models utilising older adults. But it is shown exercise-induced muscle damage, and its associated problems, peak between 12 – 72-hours after the damaging bout of ECC exercise [78]. An initial bout of downhill treadmill walking (−10%) at self-selected walking speed elicited a 15% decline in maximal voluntary isometric force (MVIF) of older adults (mean: 67 ± 4 yr) 48-hours later [85], a similar decrement in performance to that found in young adults following downhill running [86]. Biopsies from the human m. vastus lateralis immediately after a bout of ECC cycling showed disorganisation of sarcomeres, with a higher percentage of disorganisation in older (59 – 63-yrs) compared to younger adults (20 – 30-yrs) [87]. An additional study assessed m. vastus lateralis biopsies following the final bout of a 9-week resistance training programme [88]. It was shown that older women (65 – 75-yrs) had up to 17% sarcomere disorganisation, with only 2–5% in young women (20 – 30-yrs). The type of activity completed by Roth and colleagues [88] was maximal single joint ECC muscle contractions were as Gault et al [85] and Manfredi et al [87] had participants complete sub-maximal contractions across a range of joints, which may produce a lower level of damage and greater ability for older adults to adapt.
Mechanisms of muscle damage
Mechanisms underlying a reduced muscle force from ECC muscle damage have been widely reviewed [78, 79, 89–91]. These reviews include the popping-sarcomere theory, changes in the excitation-contraction (E-C) coupling and cytoskeletal proteins. The popping-sarcomere theory, results in damaged sarcomeres. Sarcomere inhomogeneity leads to unstable sarcomeres on the descending limb of the force-length curve [79]. As muscles are stretched on the descending limbs rapidly, weak sarcomeres exceed their yield point and become elongated creating very little or no filament overlap [79]. If such a contraction occurs just once (single contraction) and the muscle relaxes, most overstretched sarcomeres re-integrate and are undamaged. However, after repeated eccentric contractions those sarcomeres that failed to reintegrate, were unable to develop tension in subsequent contractions, therefore, putting extra load on neighbouring sarcomeres and causing them to pop and become disrupted, known as the popping-sarcomere theory [90]. It is thought that the popping of additional sarcomeres leads to an increased disruption and can eventually lead to the tearing of membranes (sarcolemma, T-tubules or sarcoplasmic reticulum, SR) causing a decline in maximal voluntary isometric force [90].
A further explanation for the decline in muscle strength following ECC muscle contractions is failure of the excitation-contraction (E-C) coupling. The E-C coupling is the sequence of events beginning with the release of acetylcholine at the neuromuscular junction and finishing with the release of Ca2+ from the sarcoplasmic reticulum [91]. Calcium released by the SR along the Ca2+ release channels increase the levels of free Ca2+, this binds to troponin initiating the cross-bridge cycle between actin and myosin, and the production of a force [92]. It has been suggested that because ECC contractions overstretch sarcomeres this causes damage to t-tubules and the SR, resulting in an inward leakage of extracellular Ca2+ and an increased resting level of Ca2+ [90]. There is also a decrease in tetanic intracellular Ca2+ following repetitive ECC contractions on single mouse fibres [93]. This would suggest that the signal sent from the t-tubules to the SR Ca2+ release channels have in some way failed, reducing SR Ca2+ release and levels of free Ca2+ to assist cross-bridge initiation [91].
Titin, nebulin, desmin and dystrophin are cytoskeletal proteins that play a role in stabilizing the sarcomeric structure and transmission of forces laterally across the fibre and from fibre to fibre [79]. Lieber and Fridén [94] have shown a loss of the cytoskeletal protein desmin following eccentric damage. This has led to the suggestion that overextended sarcomeres following ECC muscle contractions cause a local rise in intracellular Ca2+, which will activate proteases like calpain that hydrolyse desmin, causing a loss of structural support and disrupt the sarcomere [94].
In summary, ECC contractions cause elongation of sarcomeres, most of which return to normal during relaxation, allowing actin and myosin to re-integrate. Repeated ECC contractions prevent re-integration of actin and myosin and cause damage to the t-tubules, SR and cytoskeletal proteins. A damaged SR and t-tubules result in a reduced ability of the SR to open their Ca2+ release channels, inhibiting force production.
Repeated bout effect
Exercise-induced muscle damage is progressively reduced after repetition of the same ECC exercise, known as the ‘repeated bout effect’. Therefore, subjects who participate in regular exercise that involve predominantly ECC muscle contractions are less susceptible to exercise-induced muscle damage than untrained individuals [95]. Numerous studies have demonstrated the repeated bout effect, but there is little consensus as to the one specific mechanism [96, 97]. Generally, 3 categories have been proposed to explain the repeated bout effect: 1) neural theory; 2) mechanical theory; and 3) cellular theory [98, 99].
During an ECC contraction less motor unit activation is required in comparison with a CON contraction of the same muscle [100]. And, as high threshold motor units are selectively recruited during ECC contractions, muscle damage is the result of high stress on a small number of active fibres [101]. With the selective recruitment of fast-twitch fibres leaving them more susceptible to damage during ECC exercise [101, 102]. Golden and Dudley [103] suggest a change in motor unit recruitment due to selective recruitment. Whereby, less motor unit activation during ECC contractions allows the opportunity for the muscle to learn, and be more efficient with motor unit recruitment during a repeated bout. It has also been suggested that motor unit firing rates become more synchronized following the initial bout of ECC contractions, reducing further myofibrillar stresses during repeated bouts [104]. There has also been a decrease in force per motor unit activation demonstrated [105, 106], supporting Nosaka and Clarkson’s [107] theory that a neural adaptation would distribute the workload over a greater number of active fibres in a repeated bout.
The connective tissue theory/mechanical theory is closely linked to sarcomere elongation and the popping sarcomere [98, 99]. When the sarcomere pops a greater dependence is placed on the passive structure to maintain serial tension, with repetitive ECC contractions causing further sarcomeres to pop. Intermediate filaments (desmin etc.) are responsible for maintaining structural integrity of both serial and parallel sarcomeres [99]. Damage to these following the first bout of ECC exercise result in mechanical failure, contributing to strength loss [108]. The initial bout of eccentric exercise is thought to initiate structural reorganisation of intermediate filaments and prevent further damage from ECC contractions [108]. This protective effect has been attributed to the increased ability of connective tissue to withstand myofibrillar stress [98]. Lapier et al [109] have suggested that tissue repair following repeated ECC contractions in rat muscles is characterised by an increase in intramuscular connective tissue and therefore protecting against further damage through an increase in muscle stiffness [110].
As the initial bout of repeated ECC contractions cause sarcomere disruption, damage to cell membranes occurs (sarcolemma, SR and t-tubules) causing an influx of Ca2+, and a response within the muscle fibre (myofibril or sarcomere) – cellular theory [98, 99]. There are three underlying suggestions for this repeated bout effect: 1) strengthening of cell membranes; 2) removal of a pool of weak fibres or sarcomeres; and 3) addition of sarcomeres in series [98, 99]. Strengthening of the cell membranes is promoted through the initial influx of intracellular Ca2+ from the disrupted sarcolemma and SR [(Clarkson and Tremblay, 1988). This causes a loss of homeostasis and initiates the loss of cytoskeletal proteins (i.e. desmin; cellular necrosis); however, the sarcolemma and SR are suggested to become stronger following the initial bout of ECC exercise preventing further disruption [82]. The initial bout of ECC exercise also causes disruption to a pool of susceptible muscle fibres or sarcomeres [111] through identification and removal of susceptible myofibres or sarcomeres [107]. Finally, it has been proposed that to repair muscle damage following the initial bout of ECC exercise sarcomeres within a myofibril are added in series [112]. The longitudinal addition of sarcomeres reduce sarcomere strain during a repeated bout allowing the continuation of myofilament overlap, reducing sarcomere popping, avoiding disruption and further strength loss [113]. Although Lynn and Morgan [114] support this proposal by showing the addition of serial sarcomeres in vastus intermedius rat muscle following one-week of downhill running, an adequate amount of time needs to be given for sarcomeres to regenerate and an adequate stimulus for disruption of sarcomeres in the first bout of ECC exercise [99].
There is also thought to be an adaptation of the E-C coupling with an increase in Ca2+ release from the SR and an improved Ca2+ sensitivity [82].
In general, there is no one mechanism solely responsible for the repeated bout effect following ECC exercise. And, the initial bout of exercise does not have to cause substantial damage for protection to be given to the muscle.
Eccentric exercise interventions
Eccentric exercise interventions completed by the elderly have been implemented in various forms. The majority of studies have focused on the use of eccentric resistance training to improve functional mobility of older adults [115, 116]. It has been demonstrated that compared to standard CON single joint movement strength training, ECC overload and isokinetic exercises can lead to better strength gains in young and old individuals [117, 118]. Studies that focus on improving the functional ability of the lower body, specifically the knee extensors, are essential in helping maintain independence and reducing the risk of falls in older adults. Twelve-weeks of ECC isokinetic knee extension training, using maximal voluntary contractions (3-days per week, 30-reps), improved maximal CON strength, ECC strength and isokinetic strength by up to 26% in the knee extensors of older men and women (65 – 87-years) [116]. Similar improvements in maximal knee extensor strength were reported by Melo et al [115] with a lower intensity (75–80%), and frequency (2-days per week) of ECC isokinetic training than Symons et al [116]. However, participants in Melo et al [115] gradually completed a larger number of repetitions (48) per session, had smaller subject numbers, no control group was used, and the study focused on heart rate variability in healthy older men from high ECC strength training for 12-weeks. When compared to conventional weight training programmes, that showed no improvement in ECC knee extensor torque, ECC resistance training improved torque by up to 17%, with a corresponding increase in fascicle length [119]. The two training regimens resulted in differential adaptations in muscle architecture and strength [119]. Conventional strength training, that involves lifting and lowering a constant external load, with ‘overload’ only on the lifting phase (CON), improved maximal CON torque only, in addition to an increase in pennation angle [119]. This is representative of a strategy to pack more contractile material along the tendon aponeurosis, and consistent with an increase in parallel sarcomeres [120]. A greater increase in fascicle length was observed following ECC training, and an increase in ECC torque only. The higher loads in the ECC group may have induced a greater stretch on the muscle fibres, serving as a more potent stimulus for the addition of serial sarcomeres compared to conventional strength training [119]. The concept of specificity, in the adaptations to training, is consistent across the literature on an isokinetic dynamometer. Conventional training has a greater effect on CON strength and ECC training elicits a greater improvement in ECC strength, consistent with the type of contraction used within training programmes [117, 121].
A fewer number of studies have investigated the effects of endurance exercise, with predominately ECC muscle contractions, on the functional ability of older adults. This has included exercise in the form of ECC cycling and downhill treadmill walking, with demonstrations of an improved muscle strength, cross-sectional area and functional performance [7, 8, 122–124]. Although ECC resistance training can successfully be applied to the elderly [119, 121], it can result in cardiovascular as well as substantial mechanical stress on single joints. Endurance ergometer training can however be carried out in a closed muscle chain at high-angular velocities, using ECC muscle contractions minimizes peak forces on single joints with benefits for strength, muscle mass and potentially aerobic adaptations [122]. And, because such a training programme has favourable features, such as a lower cardiovascular demand, it can be applied to older adults with co-morbidities, which may have a limited tolerance for conventional exercise programmes [7, 125, 124].
Older adults that completed 12-weeks of ECC cycling improved isometric knee extensor strength more than a conventional resistance training programme [122]. Improvements in functional mobility (timed up-and-go; Berg balance scale) were also reported, for both ECC cycling and conventional strength training participants [122]. Similar increases in isometric knee extensor strength and functional mobility were reported following 12-weeks of level (predominately CON muscle contractions) and downhill (predominately ECC muscle contractions) treadmill walking [7, 8]. Although small, these improvements in isometric strength are seen to be biologically relevant, as it has resulted in larger improvements in sub-maximal performance during the functional mobility tests, specifically for the participants that completed the downhill treadmill walking intervention [7, 8, 122]. However, the mechanisms for adaptation were different between CON and ECC treadmill walking. The increased maximal isometric force following CON treadmill walking was due to an increased neural activation of the m. vastus lateralis, with no such changes identified following ECC treadmill walking [8]. Participants in Mueller et al [122] had a smaller improvement in functional mobility tasks in comparison to those older adults in Gault et al [7] (7% vs. 22%). Older adults in both studies had similar levels of functional mobility at baseline, indicating they were have a good level of physical condition before they participated in the exercise programme. However, Gault et al [7] completed 3-exercise session per week for 30-minutes from the outset, at a subjective, self-selected walking speed. Mueller et al [122] gradually increased their ECC training load on the cycle ergometer, to avoid delayed onset muscle soreness, and progressively increased exercise duration to 20-minutes of ECC cycling on two occasions per week. This lower initial load, duration and training frequency may not have been a sufficient overload, causing a delayed adaptation by Mueller et al [122]. Also, the functional tests completed were predominately walking based, making the walking intervention completed by Gault et al [7] and Gault & Willems [8] an activity more specific for the completion of functional tasks assessed. This study however did not progressively increase ECC loading to avoid muscle damage as participants suffered a 15% decline in maximal voluntary isometric force 48-hours after the initial bout of downhill treadmill walking. It would be interesting to investigate if a similar approach to ECC training was taken to Mueller et al [122], with a gradual increase in load, through a 3-week progression in treadmill gradient, intensity and duration. Can similar improvements in strength and functional mobility be gained with the absence of muscle damage and delayed onset muscle soreness? Also, because training intensities within the treadmill walking interventions [7, 8] were not matched between level and downhill treadmill walking this makes it difficult for comparison. Future investigations should assess the adaptations to a matched intensity programme.
Participants within these previously discussed studies are all healthy older adults in good physical condition [7, 8, 122]. Some further studies have investigated the use of an ECC endurance type training programme in clinical populations. LaStayo et al [126] assessed the impact of an ECC stepper programme on muscle and mobility of older cancer survivors. Similar to Gault et al [7] the intensity was subjective, with the use of rate of perceived exertion as an intensity indicator, and gradually progressed. Following 12-weeks of ECC training, for up to 20-minutes, 3-times per week, participants had an improved quadriceps lean tissue cross-sectional area, knee extension strength, six minute walk distance and a decreased time to safely descend stairs [126]. Although participants had a lower muscular strength in comparison to healthy older adults in Gault and Willems [8] they report similar adaptations in muscle strength to an ECC training programme for muscle strength and functional mobility, including an improved aerobic capacity, as measured by the 6-minute walk test (LaStayo et al 2011). There is limited available research, to our knowledge on the effects of ECC endurance type training on cardiovascular functioning, as most studies focus on muscle function. An additional clinical study investigated the effects of ECC training on strength and physical performance, including aerobic capacity, in older women with impaired glucose tolerance [127]. 12-weeks of ECC ergometer training not only improved aerobic capacity (8%), as measured by the 6-minute walk test; but also had an increase in leg lean soft tissue mass and quadriceps maximal isometric strength [127]. Changes in aerobic of older adults following such exercise interventions are however difficult to interpret, as sub-maximal exercise tests have been utilised, and are not a direct measure of maximal oxygen uptake [126, 127]. Also, all studies that have implemented an endurance based ECC exercise intervention have had participants complete under the minimum requirements for exercise participation to improve aerobic capacity, in accordance with the American College of Sports Medicine (150 minutes per week, moderate intensity [128]). Altogether, these findings suggest that for both healthy older adults, and those with clinical conditions, endurance exercise with predominately ECC muscle contractions, maybe suitable for improving strength and functional capacity. Mechanisms for this may be linked via the specific expression of transcripts encoding factors involved in muscle growth, repair and remodelling [129].
Summary
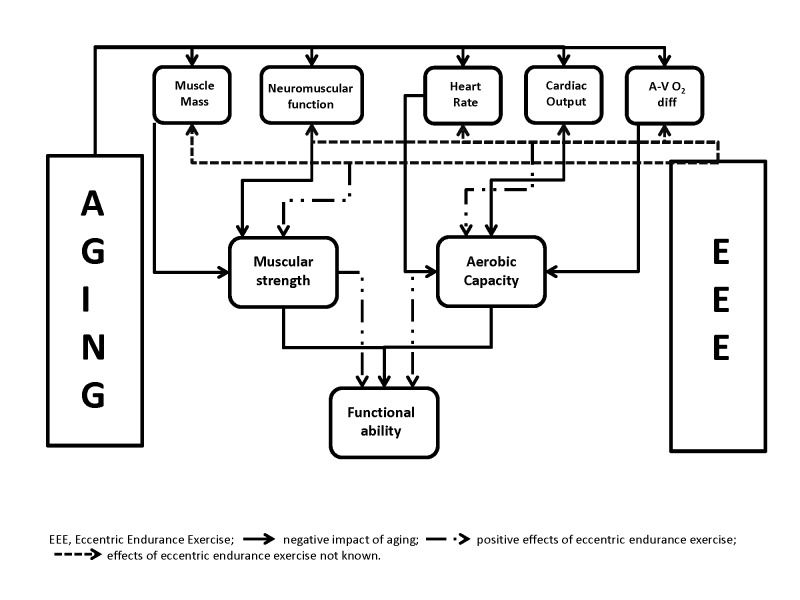
Aging is a multifactorial process, which not only is the consequences of the natural aging processes, but is also enhanced by lifestyle and environmental factors that promote the existence of disease. Sarcopenia is one of the consequences of such aging mechanisms, leading to a loss in muscle mass and strength, in addition to cardiovascular dysfunction reducing aerobic capacity. Such consequences contribute to the geriatric syndrome of frailty, thereby severely limiting the function, quality of life, and life expectancy of older adults with a decline in functional mobility. ECC exercise, characterised by the production of high mechanical forces at a low metabolic demand could be used with elderly adults, with or without clinical conditions, to improve quality of life. Endurance based exercise, involving large muscle groups, with predominantly ECC muscle contractions (cycling, treadmill, stepping) has resulted in an improved maximal strength and aerobic capacity of older adults. It has also been shown to reduce the risk of falls by improving the ability to complete functional tasks such as timed up-and-go, 5-repetition sit-to-stand, stair descent and maximal walking speed. Although a promising exercise modality for older adults, endurance based ECC exercise needs to be further explored to try and understand the underlying physiology for the adaptations to training modalities (Figure 1). Particular attention needs to be taken when interventions avoid muscle damage, can similar adaptations be achieved with and without the presence of delayed onset muscle soreness and a decline in maximal strength? Also there is limited available research on the aerobic adaptations to ECC exercise interventions, as well as the systemic, cellular and molecular events involved in the skeletal muscle and nervous system responses to chronic endurance based ECC exercise.
Figure 1.
Illustration of the relationship between aging, eccentric exercise and functional ability.
References
- [1].Rikli RE, Jones CJ. The development and validation of functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129–161. [Google Scholar]
- [2].Buford TW, MacNeil RG, Clough LG, Dirain M, Sandesara B, Manini TM, Leeuwenburgh C. Active muscle regeneration following eccentric contraction-induced injury is similar between healthy you and older adults. J Appl Physiol. 2013 doi: 10.1152/japplphysiol.01350.2012. Doi: 10.1152/japplphysiol.01350.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shephard R. Maximal oxygen intake and independence in old age. Br J Sports Med. 2009;43:342–346. doi: 10.1136/bjsm.2007.044800. [DOI] [PubMed] [Google Scholar]
- [4].Gault ML, Clements RE, Willems ME. Cardiovascular responses during downhill treadmill walking at self-selected intensity in older adults. J Aging Phys Act. 2013;21:335–347. doi: 10.1123/japa.21.3.335. [DOI] [PubMed] [Google Scholar]
- [5].Isner-Horobeti ME, Dufour SP, Vautravers P, Geny B, Coudeyre E, Richard R. Eccentric exercise training: Modalities, applications and perspectives. Sports Med. 2013 doi: 10.1007/s40279-013-0052-y. Doi: 10.1007/s40279-013-0052-y. [DOI] [PubMed] [Google Scholar]
- [6].Lovering RM, Brooks SV. Eccentric exercise in aging and diseased skeletal muscle: good or bad? J Appl Physiol. 2013 doi: 10.1152/japplphysiol.00174.2013. Doi:10.1152/japplphysiol.00174.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gault ML, Clements RE, Willems ME. Functional mobility of older adults after concentric and eccentric endurance exercise. Eur J Appl Physiol. 2012;112(11):3699–3707. doi: 10.1007/s00421-012-2338-4. [DOI] [PubMed] [Google Scholar]
- [8].Gault ML, Willems ME. Isometric strength and steadiness adaptations of the knee extensor muscle to level and downhill treadmill walking in older adults. Biogerontology. 2012;14(2):197–208. doi: 10.1007/s10522-013-9423-x. [DOI] [PubMed] [Google Scholar]
- [9].Norrbrand L, Fluckey JD, Pozzo M, Tesch PA. Resistance training using eccentric overload induces early adpatations in skeletal muscle size. Eur J Appl Physiol. 2008;103:271–281. doi: 10.1007/s00421-007-0583-8. [DOI] [PubMed] [Google Scholar]
- [10].Close GL, Kayani A, Vasilaki A, McArdle A. Skeletal muscle damage with exercise and aging. Sports Med. 2005;35(5):413–427. doi: 10.2165/00007256-200535050-00004. [DOI] [PubMed] [Google Scholar]
- [11].Mayhew TP, Rothstein JM, Finucane SD, Lamb RL. Muscular adaptation to concentric and eccentric exercise at equal power levels. Med Sci Sports Exerc. 1995;27(6):868–873. [PubMed] [Google Scholar]
- [12].Kowald A, Kirkwood TB. A network theory of ageing: the interactions of defective mitochondria, aberrant proteins, free radicals and scavengers in the ageing process. Mutat Res. 1996;316:209–236. doi: 10.1016/s0921-8734(96)90005-3. [DOI] [PubMed] [Google Scholar]
- [13].Weinert BT, Timiras PS. Invited review: Theories of aging. J Appl Physiol. 2003;95(4):1706–1716. doi: 10.1152/japplphysiol.00288.2003. [DOI] [PubMed] [Google Scholar]
- [14].Franceschi C, Valensin S, Bonafe M, Paolisso G, Yashin AI, Monti D, De Benedictis G. The network and the remodelling theories of aging: historical background and new perspectives. Exp Geronto. 2000;35:879–896. doi: 10.1016/s0531-5565(00)00172-8. [DOI] [PubMed] [Google Scholar]
- [15].Evans WJ. What is sarcopenia? J Gerontol A:Biol Sci Med. 1995;50:5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- [16].Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- [17].Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- [18].Ogawa T, Spina RJ, Martin WH, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- [19].Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Resp J. 1999;13(1):197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- [20].Kaplan DT, Furman MI, Pincus SM, Ryan SM, Lipsitz LA, Goldberger AL. Aging and the complexity of cardiovascular dynamics. Biophys J. 1991;59:945–949. doi: 10.1016/S0006-3495(91)82309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lakatta EG. Changes in cardiovascular function with aging. Eur HeartJ. 1990;11(Suppl C):22–29. doi: 10.1093/eurheartj/11.suppl_c.22. [DOI] [PubMed] [Google Scholar]
- [22].Lakatta EG, Yin FC. Myocardial aging: functional alterations and related cellular mechanisms. Am J Physiol. 1982;242(6):H927–941. doi: 10.1152/ajpheart.1982.242.6.H927. [DOI] [PubMed] [Google Scholar]
- [23].Heath GW, Hagberg JM, Ehsani AA, Holloszy JO. A physiological comparison of young and older endurance athletes. J Appl Physiol. 1981;51(3):634–640. doi: 10.1152/jappl.1981.51.3.634. [DOI] [PubMed] [Google Scholar]
- [24].Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise: Effects of aging and exercise training in healthy men. Circulation. 1994;89:1648–1655. doi: 10.1161/01.cir.89.4.1648. [DOI] [PubMed] [Google Scholar]
- [25].Fagard R, Thijs L, Amery A. Age and the hemodynamic response to posture and exercise. Am J Geriat Cardiol. 1993;2:23–40. [PubMed] [Google Scholar]
- [26].Fleg J, Lakatta E. Role of muscle loss in the age-associated reduction in V̇O2max. J Appl Physiol. 1988;65:494–503. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- [27].Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilation and increased stroke volume compensate for a diminished heart rate. Circulation. 1984;69(2):203–213. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- [28].Hossack KF, Bruce RA. Maximal cardiac function in sedentary normal men and women: Comparison of age-related changes. J Appl Physiol. 1982;53:799–804. doi: 10.1152/jappl.1982.53.4.799. [DOI] [PubMed] [Google Scholar]
- [29].Fleg JL. Alterations in cardiovascular structure and function with advancing age. Am J Cardiol. 1986;57:33C–44C. doi: 10.1016/0002-9149(86)91025-8. [DOI] [PubMed] [Google Scholar]
- [30].Niinimaa V, Shephard RJ. Training and exercise conductance in the elderly. II. The cardiovascular system. J Gerontol. 1978;33:362–367. doi: 10.1093/geronj/33.3.362. [DOI] [PubMed] [Google Scholar]
- [31].Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest. 1974;33(1):79–86. doi: 10.3109/00365517409114201. [DOI] [PubMed] [Google Scholar]
- [32].Blumenthal JA, Emery CF, Madden DJ, George LK, Coleman RE, Riddle MW, McKee DC, Reasoner J, Williams RS. Cardiovascular and behavioural effects of aerobic exercise training in healthy older men and women. J Gerontol. 1989;44:M147–157. doi: 10.1093/geronj/44.5.m147. [DOI] [PubMed] [Google Scholar]
- [33].Kasch FW, Boyer JL, Schmidt PK, Wells RH, Wallace JP, Verity LS, Guy H, Schneider D. Ageing of the cardiovascular system during 33 years of aerobic exercise. Age Ageing. 1999;28(6):531–536. doi: 10.1093/ageing/28.6.531. [DOI] [PubMed] [Google Scholar]
- [34].Puggard L. Effects of training on functional performance in 65, 75 and 85 year-old women: Experiences deriving from community based studies in Odense, Denmark. Scandinavian J Med Sci Sports. 2003;13:70–76. doi: 10.1034/j.1600-0838.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- [35].Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Endurance training in older men and women. I. Cardiovascular responses to exercise. J Appl Physiol. 1984;57(4):1024–1029. doi: 10.1152/jappl.1984.57.4.1024. [DOI] [PubMed] [Google Scholar]
- [36].Hagberg J, Graves J, Limacher M, Woods D, Connonie C, Leggett S, Cononie C, Gruber JJ, Pollock ML. Cardiovascular responses of 70–79 year old men and women to exercise training. J Appl Physiol. 1989;66(6):2589–2594. doi: 10.1152/jappl.1989.66.6.2589. [DOI] [PubMed] [Google Scholar]
- [37].Spina RJ. Cardiovascular adaptations to endurance exercise training in older men and women. Exerc Sport Sci Rev. 1999;27:317–332. [PubMed] [Google Scholar]
- [38].Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34(12):809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- [39].Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit: a brief review. Can J Appl Physiol. 1993;18(4):331–358. doi: 10.1139/h93-029. [DOI] [PubMed] [Google Scholar]
- [40].Grimby G, Saltin B. The ageing muscles. Clin Physiol. 1983;3:209–218. doi: 10.1111/j.1475-097x.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- [41].Doherty TJ. Physiology of aging: Invited review: aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- [42].Young A, Stokes M, Crowe M. Size and strength of the quadriceps muscles of old and young women. Eur J Clin Invest. 1984;14:282–287. doi: 10.1111/j.1365-2362.1984.tb01182.x. [DOI] [PubMed] [Google Scholar]
- [43].Candow DG, Chilibeck PD. Differences in size, strength, and power of upper and lower body muscle groups in young and older men. J Gerontol A:Biol Sci Med Sci. 2005;60(2):148–156. doi: 10.1093/gerona/60.2.148. [DOI] [PubMed] [Google Scholar]
- [44].Overend TJ, Cunningham DA, Paterson DH, Lefcoe MS. Thigh composition in young and elderly men determined by computed tomography. Clin Physiol. 1992;12:629–640. doi: 10.1111/j.1475-097x.1992.tb00366.x. [DOI] [PubMed] [Google Scholar]
- [45].Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A:Biol Sci Med Sci. 2003;56(10):M911–916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- [46].Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol A:Biol Sci Med Sci. 2003;58(10):M918–922. doi: 10.1093/gerona/58.10.m918. [DOI] [PubMed] [Google Scholar]
- [47].Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- [48].Hook P, Sriramoju V, Larsson L. Effects of aging on actin sliding speed on myosin from single skeletal muscle cells of mice, rats, and humans. Am J Physiol Cell Physiol. 2001;280:C782–C788. doi: 10.1152/ajpcell.2001.280.4.C782. [DOI] [PubMed] [Google Scholar]
- [49].Hunter SK, Thompson MW, Ruell PA, Harmer AR, Thom JM, Gwinn TH, Adams RD. Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol. 1999;86(6):1858–1865. doi: 10.1152/jappl.1999.86.6.1858. [DOI] [PubMed] [Google Scholar]
- [50].Brown WF. A method for estimating the number of motor units in thenar muscles and the changes in motor unit count with ageing. J Neurol Neurosurg Psychiat. 1972;35(6):845–852. doi: 10.1136/jnnp.35.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lexell L, Taylor CC, Sjostrom M. What is the cause of ageing atrophy? Total number, size and proportion of different fibre types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurolog Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- [52].McComas AJ, Upton AR, Sica RE. Motorneurone disease and ageing. Lancet. 1973;2:1477–1480. doi: 10.1016/s0140-6736(73)92735-9. [DOI] [PubMed] [Google Scholar]
- [53].Lexell J, Taylor C. Fibre density: a fast and accurate way to estimate human muscle fibre areas. Muscle Nerve. 1991;14(5):476–477. [PubMed] [Google Scholar]
- [54].Stålberg E, Fawcett PR. Macro EMG in healthy subjects of different ages. J Neurol Neurosurg Psychiat. 1982;45(10):670–678. doi: 10.1136/jnnp.45.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenhoff R. Skeletal muscle fibre quality in older men and women. Am J Physiol Cell Physiol. 2000;279(3):C611–618. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- [56].Taaffe DR. Sarcopenia: Exercise as a treatment strategy. Aust FamPhysician. 2006;35(3):130–134. [PubMed] [Google Scholar]
- [57].Taylor AH, Cable NT, Faulkner G, Hillsdon M, Narici M, Van Der Bij AK. Physical activity and older adults: a review of health benefits and the effectiveness of interventions. J Sports Sci. 2004;22:703–725. doi: 10.1080/02640410410001712421. [DOI] [PubMed] [Google Scholar]
- [58].Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- [59].Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- [60].Kamen G, Gabriel DA. Essentials of Electromyography. Human Kinetics; Champagne, IL: 2010. [Google Scholar]
- [61].Abrass IB. The biology and physiology of aging. West J Med. 1990;153(6):641–645. [PMC free article] [PubMed] [Google Scholar]
- [62].Langlois JA, Keyl PM, Guralinik JM, Foley DJ, Marottoli RA, Wallace RB. Characteristics of older pedestrians who have difficulty crossing the street. Am J Public Health. 1997;87(3):393–397. doi: 10.2105/ajph.87.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Asher L, Aresu M, Falaschetti E, Mindell J. Most older pedestrians are unable to cross the road in time: a cross-sectional study. Age Ageing. 2012;41:690–694. doi: 10.1093/ageing/afs076. [DOI] [PubMed] [Google Scholar]
- [64].Hortobágyi T, Tunnel D, Moody J, Beam S, DeVita P. Low- or high-intensity strength training partially restores impaired quadriceps force accuracy and steadiness in aged adults. J Gerontol A:Biol Sci Med Sci. 2001;56(1):B38–47. doi: 10.1093/gerona/56.1.b38. [DOI] [PubMed] [Google Scholar]
- [65].Gauchard GC, Jeandel C, Tessier A, Perrin PP. Beneficial effect of proprioceptive physical activities on balance control in elderly human subjects. Neurosci Lett. 1999;273(2):81–84. doi: 10.1016/s0304-3940(99)00615-1. [DOI] [PubMed] [Google Scholar]
- [66].Carville SF, Perry MC, Rutherford OM, Smith IC, Newham DJ. Steadiness of quadriceps contractions in young and older adults with and without a history of falling. Eur J Appl Physiol. 2007;100(5):527–533. doi: 10.1007/s00421-006-0245-2. [DOI] [PubMed] [Google Scholar]
- [67].Seynnes O, Hue OA, Garrandes F, Colson SS, Bernard PL, Legros P, Fiatarone-Singh MA. Force steadiness in the lower extremities as an independent predictor of functional performance in older women. J Aging Phys Act. 2005;13(4):395–408. doi: 10.1123/japa.13.4.395. [DOI] [PubMed] [Google Scholar]
- [68].Newham DJ, Mills KR, Quigley BM, Edwards RH. Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Lond) 1983;64(1):55–62. doi: 10.1042/cs0640055. [DOI] [PubMed] [Google Scholar]
- [69].Westing SH, Cresswell AG, Thorstensson A. Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur J Appl Physiol Occup Physiol. 1991;62(2):104–108. doi: 10.1007/BF00626764. [DOI] [PubMed] [Google Scholar]
- [70].Dufour SP, Lampert E, Doutreleau S, Lonsdorfer-Wolf E, Billat VL, Piquard F, Richard R. Eccentric cycle exercise: training application of specific circulatory adjustments. Med Sci Sports Exerc. 2004;36(11):1900–1906. doi: 10.1249/01.mss.0000145441.80209.66. [DOI] [PubMed] [Google Scholar]
- [71].Navalta JW, Sedlock DA, Park KS. Physiological responses to downhill walking in older and younger individuals. J Exerc Physiol. 2004;7(6):45–51. [Google Scholar]
- [72].Abbott BC, Bigland B, Ritchie JM. The physiological cost of negative work. J Physiol. 1952;117(3):280–390. doi: 10.1113/jphysiol.1952.sp004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].LaStayo PC, Larsen S, Smith S, Dibble L, Marcus R. The feasibility and efficacy of eccentric exercise with older cancer survivors: A preliminary study. J Feriatr Phys Ther. 2010;33(3):135–140. [PMC free article] [PubMed] [Google Scholar]
- [74].Bigland-Ritchie B, Woods JJ. Integrated electromyogram and oxygen uptake during positive and negative work. J Physiol. 1976;260:267–277. doi: 10.1113/jphysiol.1976.sp011515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Byrne C, Twist C, Eston R. Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med. 2004;34(1):49–69. doi: 10.2165/00007256-200434010-00005. [DOI] [PubMed] [Google Scholar]
- [76].Faulkner JA, Brooks SV, Zebra E. Skeletal muscle weakness and fatigue in old age: underlying mechanisms. Annu Rev Gerontol Geriatr. 1990;10:147–166. doi: 10.1007/978-3-662-38445-9_9. [DOI] [PubMed] [Google Scholar]
- [77].Hameed M, Toft AD, Pedersen BK, Harridge SDR, Goldspink F. Effects of eccentric cycling exercise on IGF-I splice variant expression in the muscles of young and elderly people. J Med Sci Sports. 2008;18:447–452. doi: 10.1111/j.1600-0838.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- [78].Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537(2):333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Allen DG. Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiol Scand. 2001;171(3):311–319. doi: 10.1046/j.1365-201x.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- [80].Clarkson PM, Nosaka K, Braun B. Muscle function after exercise induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24(5):512–520. [PubMed] [Google Scholar]
- [81].Davies RC, Eston RG, Poole DC, Rowland AV, DiMenna F, Wilerson DP, Twist C, Jones AM. Effect of eccentric exercise-induced muscle damage on the dynamic of muscle oxygenation and pulmonary oxygen uptake. J Appl Physiol. 2008;105(5):1413–1421. doi: 10.1152/japplphysiol.90743.2008. [DOI] [PubMed] [Google Scholar]
- [82].Clarkson PM, Tremblay I. Exercise-induced muscle damage repair, and adaptations in humans. J Appl Physiol. 1988;65(1):1–6. doi: 10.1152/jappl.1988.65.1.1. [DOI] [PubMed] [Google Scholar]
- [83].Paddon-Jones D, Muthalib M, Jenkins D. The effects of a repeated bout of eccentric exercise on indices of muscle damage and delayed onset muscle soreness. J Sci Med Sport. 2000;3(1):35–43. doi: 10.1016/s1440-2440(00)80046-8. [DOI] [PubMed] [Google Scholar]
- [84].McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004;18(2):355–357. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- [85].Gault ML, Clements RE, Willems MET. Eccentric contraction-induced muscle injury does not change walking economy in older adults. J Hum Kinet. 2011;27(1):55–65. [Google Scholar]
- [86].Chen TC, Nosaka K, Wu CC. Effects of a 30-min running performed daily after downhill running on recovery of muscle function and running economy. J Sci Med Sport. 2008;11:271–279. doi: 10.1016/j.jsams.2007.02.015. [DOI] [PubMed] [Google Scholar]
- [87].Manfredi TG, Fielding RA, O’Reilly KP, Meredith CN, Young LH, Evans WJ. Plasma creatine kinase activity and exercise-induced muscle damage in older men. Med Sci Sports Exerc. 1991;23:991–1117. [PubMed] [Google Scholar]
- [88].Roth SM, Martel GF, Ivey FM, Lemmer JT, tracy BL, Hurlbut DE, Metter EJ, Hurley BF, Rogers MA. Ultrastructural muscle damage in young vs. older men after high-volume, heavy-resistance strength training. J Appl Physiol. 1999;86:1833–1840. doi: 10.1152/jappl.1999.86.6.1833. [DOI] [PubMed] [Google Scholar]
- [89].Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991;12(3):184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- [90].Morgan DL, Allen DG. Early event in stretch-induced muscle damage. J Appl Physiol. 1999;87(6):2007–2015. doi: 10.1152/jappl.1999.87.6.2007. [DOI] [PubMed] [Google Scholar]
- [91].Warren GL, Ingallis CP, Lowe DA, Armstrong RB. What mechanisms contribute to the strength loss that occurs during and in the recover from skeletal muscle injury? J Orthop Sports Phys Ther. 2002;32(2):58–64. doi: 10.2519/jospt.2002.32.2.58. [DOI] [PubMed] [Google Scholar]
- [92].MacIntosh B, Gardiener P, McComas A. Skeletal muscle: Form and function. Leeds: Human Kinetics; 2006. [Google Scholar]
- [93].Balnave CD, Allen DG. Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J Physiol. 1995;488:25–36. doi: 10.1113/jphysiol.1995.sp020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lieber RL, Fridén J. Mechanisms of muscle injury after eccentric contraction. J Sci Med Sport. 1999;2(3):253–265. doi: 10.1016/s1440-2440(99)80177-7. [DOI] [PubMed] [Google Scholar]
- [95].Gibala MJ, MacDougall JD, Tarnopolsky MA, Stauber WT, Elorriaga A. Changes in human skeletal muscle ultrastructure and force production after acute resistance exercise. J Appl Physiol. 1995;78(2):702–708. doi: 10.1152/jappl.1995.78.2.702. [DOI] [PubMed] [Google Scholar]
- [96].Clarkson PM, Byrnes WC, Gillisson E, Harper E. Adaptation to exercise-induced muscle damage. Clin Sci (Lond) 1987;73(4):383–386. doi: 10.1042/cs0730383. [DOI] [PubMed] [Google Scholar]
- [97].Howatson G, Van Someren K, Hotrobagyi T. Repeated bout effect after maximal eccentric exercise. Int J Sports Med. 2007;28(7):557–563. doi: 10.1055/s-2007-964866. [DOI] [PubMed] [Google Scholar]
- [98].McHugh MP. Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand J Med Sci Sports. 2003;13(2):88–97. doi: 10.1034/j.1600-0838.2003.02477.x. [DOI] [PubMed] [Google Scholar]
- [99].McHugh MP, Connolly DA, Eston RG, Gleim GW. Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Med. 1999;27(3):157–170. doi: 10.2165/00007256-199927030-00002. [DOI] [PubMed] [Google Scholar]
- [100].Potvin JR. Effects of muscle kinematics on surface EMG amplitude during fatiguing dynamic contractions. J Appl Physiol. 1997;82(1):144–151. doi: 10.1152/jappl.1997.82.1.144. [DOI] [PubMed] [Google Scholar]
- [101].Enoka RM. Eccentric contractions require unique activation strategies by the nervous system. J Appl Physiol. 1996;81(6):2339–2346. doi: 10.1152/jappl.1996.81.6.2339. [DOI] [PubMed] [Google Scholar]
- [102].Moritani T, Muramatsu S, Muro M. Activity of motor units during concentric and eccentric contractions. Am J Phys Med. 1988;66:338–350. [PubMed] [Google Scholar]
- [103].Golden CL, Dudley GA. Strength after bouts of eccentric or concentric actions. Med Sci Sports Exerc. 1992;24:926–933. [PubMed] [Google Scholar]
- [104].Pierrynowski MR, Tüdus PM, Plyley MJ. Effects of downhill or uphill training prior to a downhill run. Eur J Appl Physiol. 1987;56:668–672. doi: 10.1007/BF00424808. [DOI] [PubMed] [Google Scholar]
- [105].Hortobagyi T, Barrier J, Beard D, Braspennincx J, Koens P, Devita P, Dempsey L, Lambert J. Greater initial adaptations to submaximal muscle lengthening than maximal shortening. J Appl Physiol. 1996;81:1677–1682. doi: 10.1152/jappl.1996.81.4.1677. [DOI] [PubMed] [Google Scholar]
- [106].Komi PV, Buskirk ER. Effect of eccentric and concentric muscle conditioning on tension and electrical activity of human muscle. Ergonomics. 1972;15:417–434. doi: 10.1080/00140137208924444. [DOI] [PubMed] [Google Scholar]
- [107].Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sport Exerc. 1995;27(9):1263–1269. [PubMed] [Google Scholar]
- [108].Fridén J, Lieber RL. Structural and mechanical basis of exercise-induced injury. Med Sci Sports Exerc. 1992;24(5):521–30. [PubMed] [Google Scholar]
- [109].Lapier TK, Burton HW, Almon R, Cerny F. Alterations in intramuscular connective tissue after limb casting affect contraction-induced muscle injury. J Appl Physiol. 1995;75:1065–1069. doi: 10.1152/jappl.1995.78.3.1065. [DOI] [PubMed] [Google Scholar]
- [110].Kovanen V, Suominen H, Heikkinen E. Mechanical properties of fast and slow skeletal muscle with special reference to collagen and training. J Biomech. 1984;17(10):725–735. doi: 10.1016/0021-9290(84)90103-9. [DOI] [PubMed] [Google Scholar]
- [111].Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- [112].Fridén J, Sjöström M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4:170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- [113].Morgan DL. New insights into the behaviour of muscle during active lengthening. Biophys J. 1990;57:209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Lynn R, Morgan DL. Decline running produces more sarcomeres in rat vastus intermedius muscle fibers than does incline running. J Appl Physiol. 1994;77:1439–1444. doi: 10.1152/jappl.1994.77.3.1439. [DOI] [PubMed] [Google Scholar]
- [115].Melo RC, Quiterio RJ, Takahashi ACM, Silva E, Martins LEB, Catai AM. High eccentric strength training reduces heart rate variability in healthy older men. Br J Sports Med. 2008;42:59–63. doi: 10.1136/bjsm.2007.035246. [DOI] [PubMed] [Google Scholar]
- [116].Symons TB, Vandervoort AA, Rice CL, Overend TJ, Marsh GD, Morley JE. Effects of maximal isometric and isokinetic resistance training on strength and functional mobility in older adults. J Gerontol A Biol Sci Med Sci. 2005;60(6):777–781. doi: 10.1093/gerona/60.6.777. [DOI] [PubMed] [Google Scholar]
- [117].Hortobagyi T, De Vita P. Favourable neuromuscular and cardiovascular responses to 7 days of exercise with an eccentric overload in elderly women. J Gerontol A Biol Sci Med Sci. 2000;55(8):B401–10. doi: 10.1093/gerona/55.8.b401. [DOI] [PubMed] [Google Scholar]
- [118].Roig M, O’Brien K, Kirk G, Murray R, McKinnon P, Shadgan B, Reid WD. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis. Br J Sports Med. 2009;43:556–568. doi: 10.1136/bjsm.2008.051417. [DOI] [PubMed] [Google Scholar]
- [119].Reeves ND, Maganaris CN, Longo S, Narici MV. Differential adaptations to eccentric versus conventional resistance training in older humans. Exp Physiol. 2009;94(7):825–833. doi: 10.1113/expphysiol.2009.046599. [DOI] [PubMed] [Google Scholar]
- [120].Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol. 1993;74:2740–2744. doi: 10.1152/jappl.1993.74.6.2740. [DOI] [PubMed] [Google Scholar]
- [121].Higbie EJ, Cureton KJ, Warren GL, Prior PM. Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J Appl Physiol. 1996;81:2173–2181. doi: 10.1152/jappl.1996.81.5.2173. [DOI] [PubMed] [Google Scholar]
- [122].Mueller M, Breil FA, Vog M, Steiner R, Lippuner K, Popp A, Klossner S, Hoppeler H, Dapp C. Different response to eccentric and concentric training in older men and women. Eur J Appl Physiol. 2009;107(2):145–153. doi: 10.1007/s00421-009-1108-4. [DOI] [PubMed] [Google Scholar]
- [123].LaStayo PC, Reich TE, Urquhart M, Hoppeler H, Lindstedt SL. Chronic eccentric exercise: improvement in muscle strength with little demand for oxygen consumption. Am J Physiol. 1999;276:R611–R615. doi: 10.1152/ajpregu.1999.276.2.R611. [DOI] [PubMed] [Google Scholar]
- [124].Steiner R, Meyer K, Lippuner K, Schmid J-P, Saner H, Hoppeler H. Eccentric endurance training in subjects with coronary artery disease: a novel exercise paradigm in cardiac rehabilitation? Eur J Appl Physiol. 2004;91:572–578. doi: 10.1007/s00421-003-1000-6. [DOI] [PubMed] [Google Scholar]
- [125].LaStayo PC, Woolf JM, Lewek MD, Snyder-Mackler L, Reich T, Linstedt SL. Eccentric muscle contractions: their contribution to injury, prevention, rehabilitation, and sport. J Orthop Sports Phys Ther. 2003;33(10):557–571. doi: 10.2519/jospt.2003.33.10.557. [DOI] [PubMed] [Google Scholar]
- [126].LaStayo P, Marcus RL, Dibble LE, Smith SB, Beck SL. Eccentric exercise versus usual-care with older cancer surviviors: the impact on muscle and mobility- an exploratory pilot study. BMC Geriat. 2011;11 doi: 10.1186/1471-2318-11-5. doi: 10.1186/1471-2318-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Marcus RL, LaStayo PC, Dibble LE, Hill L, McClain DA. Increased strength and physical performance eccentric training in women with impaired glucose tolerance: A Pilot study. J Women Health. 2009;18(2):253–260. doi: 10.1089/jwh.2007.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].American College of Sports Medicine . Guideline for Exercise Testing and Prescription. 6th edition. Philadelphia: Lippincott, Williams and Wilkins; 2000. [Google Scholar]
- [129].Mueller M, Breil FA, Lurman G, Klossner S, Fluck M, Billeter R, Dapp C, Hoppeler H. Different molecular and structural adaptations with eccentric and conventional strength training in elderly men and women. Gerontology. 2011;57(6):528–538. doi: 10.1159/000323267. [DOI] [PubMed] [Google Scholar]