Abstract
The Cdk2 inhibitor, p27Kip1, is degraded in a phosphorylation- and ubiquitylation-dependent manner at the G1–S transition of the cell cycle. Degradation of p27Kip1 requires import into the nucleus for phosphorylation by Cdk2. Phosphorylated p27Kip1 is thought to be subsequently re-exported and degraded in the cytosol. Using two-hybrid screens, we now show that p27Kip1 interacts with a nuclear pore-associated protein, mNPAP60, map the interaction to the 310 helix of p27 and identify a point mutant in p27Kip1 that is deficient for interaction (R90G). In vivo and in vitro, the loss-of-interaction mutant is poorly transported into the nucleus, while ubiquitylation of p27R90G occurs normally. In vivo, co-expression of cyclin E and Cdk2 rescues the import defect. However, mutant p27Kip1 accumulates in a phosphorylated form in the nucleus and is not efficiently degraded, arguing that at least one step in the degradation of phosphorylated p27Kip1 requires an interaction with the nuclear pore. Our results identify a novel component involved in p27Kip1 degradation and suggest that degradation of p27Kip1 is tightly linked to its intracellular transport.
Keywords: degradation/intracellular transport/mNPAP60/p27Kip1
Introduction
The p27Kip1 protein has a dual role in the G1 phase of the cell cycle: it inhibits Cdk2 complexes in resting cells and during the G1 phase of the cell cycle, and promotes assembly and nuclear import of cyclin D–Cdk4 complexes (for review, see Sherr and Roberts, 1999). At the end of the G1 phase, p27Kip1 is degraded. Degradation of p27Kip1 is sensitive to inhibitors of proteasome function, suggesting that degradation occurs via the ubiquitin pathway (Pagano et al., 1995). Ubiquitylated forms of p27Kip1 have been detected in vivo and degradation and ubiquitylation have been reconstituted in vitro using partially purified systems (Montagnoli et al., 1999; Nguyen et al., 1999; Shirane et al., 1999).
Two mechanisms are known that ensure the correct timing of p27Kip1 degradation. First, p27Kip1 is a substrate of cyclin E–Cdk2 kinase and is phosphorylated by Cdk2 mainly, but not exclusively, at Thr187 (Müller et al., 1997; Sheaff et al., 1997; Vlach et al., 1997). In vivo, degradation of p27Kip1 requires active Cdk2 kinase and mutation of Thr187 inhibits degradation; in vitro, phosphorylation of Thr187 is required for ubiquitylation and subsequent degradation of p27Kip1 (Montagnoli et al., 1999; Tsvetkov et al., 1999). Together, the findings show that active cyclin–Cdk2 complexes during the G1 phase are required for elimination of p27Kip1.
Secondly, skp2 has been identified as a candidate component of the E3 ligase complex that ubiquitylates p27Kip1 (Carrano et al., 1999). In vivo, skp2 function is required for p27Kip1 degradation (Carrano et al., 1999). Ectopic expression of skp2 in quiescent cells stimulates entry into S phase of the cell cycle and promotes degradation of p27Kip1, providing evidence that the amount of skp2 is rate limiting for degradation of p27Kip1 (Sutterlüty et al., 1999). In normal cells, expression of skp2 is restricted to the S phase (Zhang et al., 1995; Lisztwan et al., 1998). Although the mechanisms underlying this restriction are not known, the findings suggest that regulation of skp2 expression is a second mechanism that contributes to the correct timing of p27Kip1 degradation.
In order to exert its function during G1 and to be degraded at the end of G1, p27Kip1 needs to be imported into the nucleus (Reynisdottir and Massague, 1997; Tomoda et al., 1999). Phosphorylation of p27Kip1 by Cdk2 is restricted to the cell nucleus due to the nuclear localization of CAK (Darbon et al., 1994), which may account for the requirement for nuclear import in degradation. A nuclear localization signal (NLS) has been assumed to be responsible for transport of p27Kip1 into the nucleus, since deletion of the C-terminus of p27Kip1 including the NLS abolishes import (Reynisdottir and Massague, 1997; Tomoda et al., 1999). Recently, a component of the signalosome, Jab1, has been found to interact with p27Kip1 and promote its degradation. Jab1 interacts with p27Kip1 and promotes its export from the nucleus, suggesting that degradation of phosphorylated p27Kip1 occurs in the cytosol (Tomoda et al., 1999). The signals mediating nuclear export of p27Kip1 are unknown.
Using two-hybrid cloning we have identified an interaction between p27Kip1 and a nuclear pore-associated protein and provide evidence that this interaction is required for nuclear import of p27Kip1 and for degradation of phosphorylated p27Kip1 after nuclear import. Our findings identify a new component involved in p27Kip1 metabolism, support the notion that degradation of phosphorylated p27Kip1 requires export from the nucleus and have implications for the mechanisms by which intracellular transport of p27Kip1 is regulated.
Results
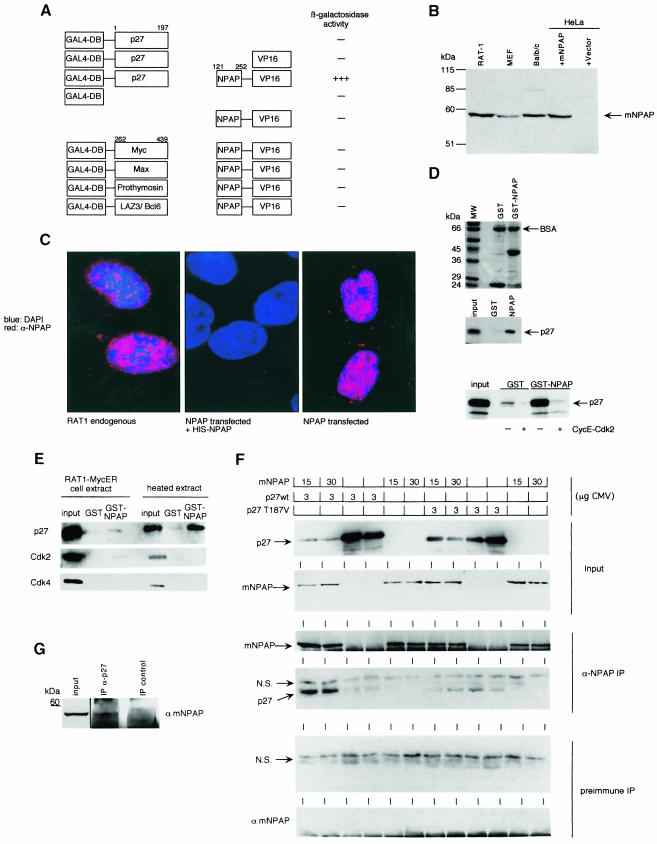
In order to identify novel proteins that interact with p27, we screened a mouse embryo cDNA library using full-length mouse p27 as bait. Of 500 positive clones, all except two coded for known cyclins (not shown); the remaining two were identical and encoded a novel p27-interacting protein (Figure 1A). The longest cDNAs that were isolated from a Balb/c-3T3 library encode a protein of 466 amino acids; the fragment recovered in the two-hybrid screen corresponds to amino acids 121–252 of the full-length protein. Western blotting using affinity-purified antibodies raised against this protein reveals an endogenous protein in a number of different cell lines; the endogenous protein comigrates with a protein expressed from a cDNA clone by transient transfection, demonstrating that a full-length cDNA was isolated (Figure 1B). Immunofluorescence experiments using these antibodies detected the endogenous protein in a speckled pattern at the nuclear periphery highly reminiscent of nuclear pore proteins or nuclear transport factors; mNPAP60 expressed by transient transfection showed a very similar distribution (Figure 1C). Database searches revealed that a rat cDNA homologous to the clones we had isolated had been described previously; the rat cDNA encodes a nucleoporin termed NPAP60 (Fan et al., 1997). We therefore refer to our clone as m(ouse)NPAP60. Both clones differ by a small sequence divergence in the N-terminus and by the presence of a putative RAN-binding domain in the C-terminus of the clone we have recovered, suggesting that the rat clone may possibly represent a shorter splice variant (see Supplementary figure 1, available at The EMBO Journal Online). Weak similarities to other nucleoporins were found, mainly within the putative RAN-binding domain.
Fig. 1. Identification of mNPAP60 as a p27-interacting protein. (A) Summary of yeast two-hybrid interaction data. Plasmids expressing the indicated chimeras were transformed into yeast strains and β-galactosidase activity was determined by a semi-quantitative filter assay. (B) Western blot documenting the expression of endogenous mNPAP60 in rodent fibroblast cell lines (left lanes). The right lanes contain extracts from HeLa cells transfected with vector alone or with an expression vector encoding mNPAP60. The antibody raised against mouse NPAP does not cross-react with human NPAP. (C) Immunofluorescence of cultured RAT1 cells using an affinity-purified antiserum raised against mNPAP60 (left). The right panel shows the localization of mNPAP60 expressed by transient transfection in HeLa cells. The middle panel shows a control after pre-incubation of the antiserum with a GST–mNPAP60 fusion protein. The pictures are a false-colour superimposition of the 4′,6-diamidino-2-phenylindole-stained nuclei (coloured in blue) and anti-mNPAP60 fluorescence (coloured in red). (D) In vitro binding of 35S-labelled p27 to GST–mNPAP60(121–252). The upper panel shows Coomassie-stained gels of the input into the in vitro binding reactions together with a molecular weight marker. BSA was added to reduce background binding. The middle panel shows a fluorography demonstrating specific binding of p27 to GST–mNPAP60. The lower panel shows reduced binding of p27 to GST–mNPAP60 after pre-incubation of p27 with cyclin E–Cdk2 complexes purified from baculovirus. The input lanes correspond to 10% of the total amount of p27 loaded on to the GST beads. (E) In vitro binding reactions from cell lysates. Detergent lysates from RAT1-MycER cells were prepared with or without heat treatment and incubated with GST–mNPAP60 as above. Western blots of the input lanes (10% of loading) and the recovered beads are shown. (F) Co-immunoprecipitation of p27 with anti-mNPAP60 antibodies. HeLa cells were transfected with the expression plasmids indicated, lysates prepared and immunoprecipitated with either antiserum against mNPAP60 or preimmune serum. Shown are Western blots probed with the antibodies indicated. N.S. reflects a non-specific band present in all immunoprecipitates. mNPAP60 migrates just above the IgG heavy chain on SDS gels; since the same antibody is used for both immunoprecipitation and Western blotting, the heavy chain is visible below the mNPAP60 bands in the immunoprecipitations. (G) Co-immunoprecipitation of endogenous mNPAP60 with anti-p27, but not with control antibodies from lysates of NIH 3T3 cells. The input represents 5% of cell extract used for the precipitation.
A number of controls established that mNPAP60 interacts specifically with p27 in the yeast two-hybrid assay (Figure 1A). In order to verify that p27 and mNPAP60 interact outside the context of two-hybrid experiments, we generated and purified a glutathione S-transferase (GST)–mNPAP60 chimeric protein and incubated it with 35S-labelled p27 translated in a reticulocyte lysate (Figure 1D). In these experiments, p27 interacted efficiently with GST–mNPAP60, but not with equal amounts of GST alone. In similar experiments, no specific interaction of mNPAP60 was seen with cyclin D or E or with p21Waf1 (data not shown). p27 present in lysates of RAT1-MycER cells under conditions where it is bound to Cdk2 and Cdk4 (as judged by immunoprecipitation and gel filtration; not shown) did not interact with GST–mNPAP60. In contrast, p27 extracted from such lysates by a brief heat treatment specifically bound to GST–mNPAP60, suggesting that binding of p27 to Cdk2 and Cdk4 complexes prevents interaction with mNPAP60 in vitro (Figure 1E). Consistent with this notion, pre-incubation of p27 with cyclin E–Cdk2 complexes purified from insect cells infected with recombinant baculovirus abolished the interaction with GST–mNPAP60 (Figure 1D).
An interaction between mNPAP60 and p27 was also detected by immunoprecipitation from cellular lysates (Figure 1F). HeLa cells were transfected with CMV-based expression vectors encoding p27 and mNPAP60 and detergent lysates were precipitated with either anti-mNPAP60 antibodies or preimmune antiserum. In these experiments, p27 was detected in anti-mNPAP60 immunoprecipitates when both proteins were co-expressed. No p27 was detected in precipitates using preimmune serum; also, p27 was not detected in anti-mNPAP60 immunoprecipitates from cells in which mNPAP60 had not been expressed. We noticed that expression of large amounts of mNPAP60 diminished expression of p27; addition of the proteasome inhibitor LLNL partly reversed this effect (not shown). Close inspection of the gels suggested that mNPAP60 associated preferentially with a slower- migrating, phosphorylated form of p27 (not shown). Therefore, we repeated the experiment with a mutant of p27 that lacks the Cdk2 phosphorylation site (T187V). In contrast to the two-hybrid situation (see below), this mutation diminished the association of p27 with mNPAP60 (Figure 1F). Potentially, therefore, proteins that specifically recognize the phosphorylated form of p27 stabilize the p27–mNPAP60 complex in vivo.
The antibodies we have raised do not detect the human mNPAP60 protein. To determine whether endogenous mNPAP interacts with p27, lysates of NIH 3T3 cells were immunoprecipitated with anti-p27 and control antibodies. In the precipitates, mNPAP60 could be detected in anti-p27, but not in control precipitates, demonstrating that the endogenous proteins associate with each other (Figure 1G).
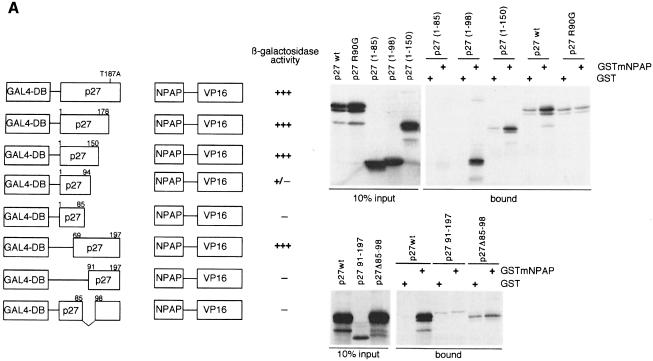
To map the interaction surface between p27 and mNPAP60, progressive N- and C-terminal deletions of p27 were tested in the two-hybrid assay and by in vitro pull-down experiments (Figure 2A). Both assays gave consistent results, which map the N-terminal border of the interaction domain between amino acids 69 and 91 of p27 and the C-terminal border between amino acids 85 and 94. An internal deletion mutant of p27 spanning amino acids 85–98 failed to interact with mNPAP60 both in vitro and in two-hybrid assays (Figure 2A). Thus, the interaction surface centres on the 310 helix of p27, overlapping the cdk2-binding domain of the protein. This is consistent with the in vitro observations demonstrating that cdk2 and mNPAP compete for binding to p27.
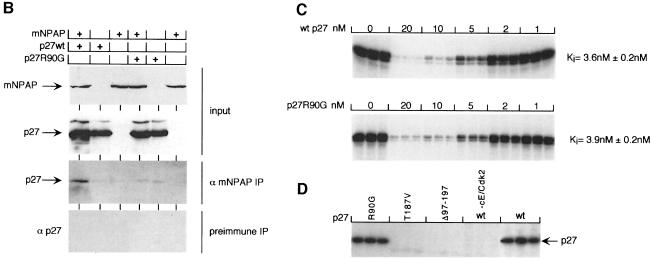
Fig. 2. Isolation of a mNPAP60 loss-of-interaction mutant of p27. (A) Summary of two-hybrid data (left) and in vitro interaction assays (right) documenting the interaction of C-terminal, N-terminal and internal deletion mutants of p27 with mNPAP60. The assays were carried out as described in the legend to Figure 1. p27Δ91–197 lacks three of five methionines and is, therefore, labelled more weakly in these experiments. The right panel also documents the reduced interaction of the p27R90G mutant with GST–mNPAP60 in vitro. (B) Western blot documenting co-immunoprecipitation of p27wt, but not of p27R90G after co-expression with mNPAP60 in HeLa cells. Assays were carried out as described in the legend to Figure 1. (C) Inhibition of cyclin E–Cdk2 kinase activity by increasing amounts of p27. His-tagged recombinant p27wt and p27R90G proteins were purified and incubated with cyclin E–Cdk2 complexes isolated from baculovirus lysates. Histone H1 was used as a substrate. Triplicate assays are shown and were used to calculate the Ki values shown on the right. (D) The proteins indicated were purified and equal amounts subjected to phosphorylation by immunoprecipitated cyclin E–Cdk2 kinase. Shown is an autoradiogram of triplicate assays. The control designated ‘-cE/Cdk2’ contains p27wt protein and a mock precipitate using no antibody.
In order to identify a mutant of p27 that specifically disrupts interaction with mNPAP60, a loss-of-interaction screen was performed in yeast. p27 was mutagenized using mismatch PCR in the presence of manganese ions and transformed in Escherichia coli. A total of 950 colonies were picked and the plasmids isolated were tested in a two-hybrid reaction with mNPAP60; 179 candidate negative colonies were selected, the plasmid isolated and re-transformed. From these, 22 plasmids were isolated, which encoded p27 proteins that failed to interact with mNPAP60. The plasmids were retested in a two-hybrid assay using cyclin D1 as prey and the p27 open reading frames of these plasmids were sequenced. Three plasmids were recovered that encoded mutant p27 proteins that interacted with cyclin D1 but failed to interact with mNPAP60 (see Supplementary figure 2). All three carried a single point mutation at position 90 (R90G). The R90G mutation was not present in any of the other plasmids recovered.
In this screen, the mutations we recovered were exclusively A to G transitions (not shown). Therefore, the specificity of the arginine (AGG) to glycine (GGG) change is almost certainly due to the mutagenesis protocol we used. In wild-type p27, the arginine residue that is affected by the mutation forms a salt bridge with a glutamic acid residue at position 85; thus, it is very likely that this mutation at position 90 will affect the stability of the 310 helix (Russo et al., 1996). For all subsequent experiments, this mutation was re-introduced in a wild-type p27 plasmid and the plasmid was sequenced to ensure that it carries this single mutation. The localization of the mutation relative to other mapped interactions in the p27 sequence and within the p27 crystal structure is shown in Supplementary figure 2.
To test whether the mutation indeed specifically disrupts the interaction with mNPAP60, we used in vitro pull-down experiments as before: in these experiments, p27R90G failed to interact with GST–mNPAP60 (Figure 2A). Also, p27R90G expressed by transient transfection in HeLa cells together with mNPAP60 failed to be precipitated by antibodies directed against mNPAP, in contrast to wild-type p27 (Figure 2B). We concluded that the R90G mutation disrupts association of p27 with mNPAP60 both in vitro and in vivo.
To test whether the mutation affects interaction with cyclin E–Cdk2 complexes, p27 and p27R90G were expressed in E.coli as his-tagged recombinant proteins and incubated with cyclin E–Cdk2 complexes isolated from baculovirus-infected cells. In these assays, both proteins were indistinguishable. They inhibited cyclin E–Cdk2 kinase activity with identical Ki values and cyclin E–Cdk2 efficiently phosphorylated both proteins in vitro (Figure 2C and D). This result is in agreement with data obtained for other mutations in the 310 helix (Hashimoto et al., 1998).
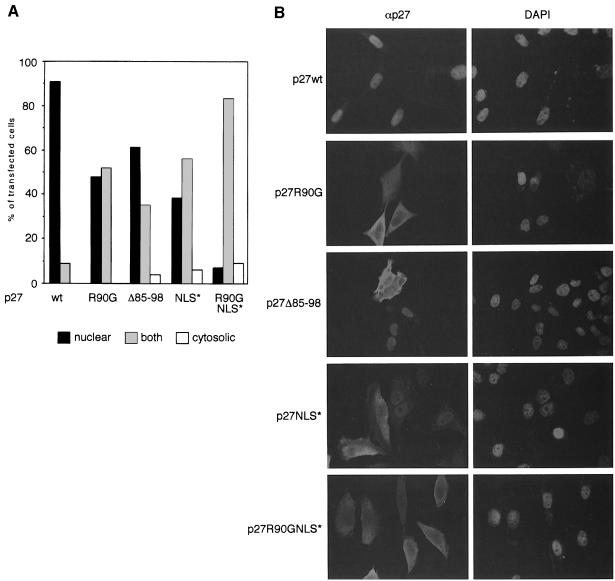
To investigate whether mNPAP60 is involved in the intracellular transport of p27, we expressed wild-type p27 and p27R90G by transient transfection in a number of cell lines and obtained similar results in HeLa, MCF7 and RAT1 cells. The results obtained in HeLa cells are summarized in Figure 3A. Examples of individual transfections are shown in Figure 3B. Using equal amounts of expression plasmid, a significant proportion of cells expressing p27R90G, but not p27wt, showed strong cytosolic staining in addition to nuclear staining. Similarly, the internal deletion mutant p27Δ85–98 mislocalized to the cytosol in a large fraction of the cells.
Fig. 3. Localization of p27 mutants in HeLa cells. (A) Quantitation of the results of several independent transfections into HeLa cells. (B) Examples of individual transfections.
To determine the relevance of the NLS of p27 for nuclear transport in detail, the signal was disrupted by the replacement of three basic amino acids that constitute part of the NLS (R152A K153E R154A). This mutant showed a partial relocalization to the cytosol. A double mutant of p27 that carries both a mutation of the NLS and the R90G mutation mislocalizes to the cytosol in almost all of the transfected cells. The consistent mislocalization of both the R90G and the p27Δ85–98 mutations suggests a role for the mNPAP60–p27 interaction in the nuclear import of p27.
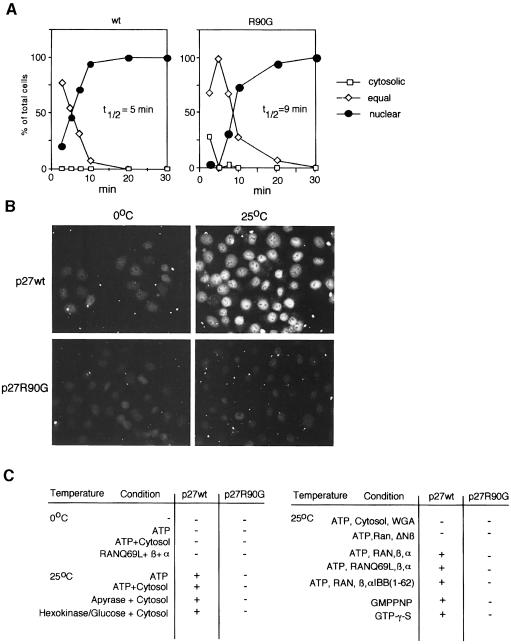
To confirm this suggestion, recombinant p27wt and p27R90G proteins were fluorescently labelled and equal amounts of protein were injected into either the cytosol or the nucleus of HeLa cells (Figure 4A). When injected into the cytosol, wild-type p27 was rapidly imported into the cell nucleus; half-maximal import was observed 5 min after injection. In contrast, p27R90G was transported more slowly into the nucleus; in this assay the rate of import was ∼2-fold lower than that of wild-type p27. The data support the notion that the mNPAP60–p27 interaction has a functional role in nuclear import. When injected into the nucleus of HeLa cells, neither p27wt nor p27R90G were exported within the time frame of the experiment to a detectable degree; instead, the observable fluorescence decayed over the next 30 min. The data suggest that both proteins are retained in the nucleus. We could not, therefore, establish from these experiments whether mNPAP60 has a role in the nuclear export of p27 (see below).
Fig. 4. Characterization of the nuclear import of p27. (A) Recombinant p27wt and p27R90G proteins were fluorescently labelled and injected into the cytosol of exponentially growing HeLa cells. The time course of import is shown; between 50 and 150 cells were injected and evaluated for each time point. (B) Example of in vitro import reactions using fluorescently labelled p27 proteins and permeabilized HeLa cells. (C) Summary of results of in vitro import reactions.
To characterize the mechanism of import in more detail, we tested both p27wt and p27R90G using in vitro nuclear import assays. An example of the results is shown in Figure 4B, and the results are summarized in Figure 4C. Wild-type p27 was imported efficiently into the nucleus in these assays. Import was strictly dependent on temperature and was abolished at 0°C; import was also abolished by addition of either wheat-germ agglutinin or a dominant-negative fragment of importin-β known to disrupt nucleoporin function (Kutay et al., 1997). Thus, nuclear import of p27 relies on a functional interaction with the nuclear pore and does not occur by free diffusion. This is expected, as recombinant p27 forms a dimer with an apparent molecular weight of 70 kDa in gel filtration assays (D.Müller and M.Eilers, unpublished data). Depletion of ATP, depletion of cytosol, addition of the importin-β-binding domain of importin-α (Klebe et al., 1995), addition of non-hydrolysable analogues of GTP or addition of a dominant-negative mutant of Ran (Weis et al., 1996) did not inhibit import. In parallel experiments, these treatments inhibited import of an NLS–bovine serum albumin (BSA) conjugate, as expected (not shown). Thus, nuclear import of p27 does not depend on the recognition of the NLS by importin-α or an energy-dependent mechanism. Import of p27R90G was strongly diminished relative to wild-type p27 under all conditions tested. We concluded that the in vitro nuclear import of p27 occurs via a process of facilitated diffusion across the pore and that interaction of p27 with mNPAP60 is required for this process.
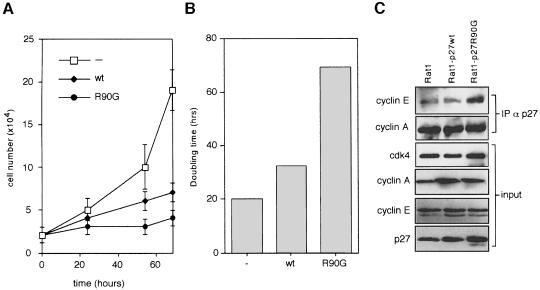
Upon expression from retroviral vectors, p27 inhibits proliferation of RAT1 cells (Vlach et al., 1996; Müller et al., 1997). To determine whether the interaction with mNPAP60 affects this function of p27 in vivo, we constructed retroviral vectors that express either wild-type or mutant p27 together with a puromycin resistance gene. RAT1 cells were infected with retroviral supernatants and pools of resistant cells selected; these pools were plated at low density to determine the rate of proliferation of these cells. Surprisingly, p27R90G consistently affected the rate of proliferation more strongly than wild-type p27 in repeated experiments (Figure 5A and B).
Fig. 5. Inhibition of cell proliferation by p27wt and p27R90G. RAT1 cells were infected with recombinant retroviruses expressing either p27wt or p27R90G. (A) The rate of cell proliferation of resistant cell pools relative to a pool of cells infected with a control (pbabe-puro) virus. (B) Average doubling time calculated from logarithmic plots of two independent infections. (C) Western blots documenting the expression of the proteins indicated and the amounts of cyclin A/p27 and cyclin E/p27 in exponentially growing cells infected with the viruses indicated.
To explain this finding, we considered the possibility that ectopic expression of p27R90G might indirectly cause mislocalization of cyclins that lack an NLS such as cyclin A, but immunofluorescence experiments showed that this was not the case (not shown). However, in exponentially growing cells expressing p27R90G, levels of p27 were elevated relative to p27wt and elevated levels of p27R90G bound to cyclin E compared with either uninfected cells or cells infected with a virus expressing p27wt, indicating a defect in cyclin E-mediated elimination of p27 (Figure 5C).
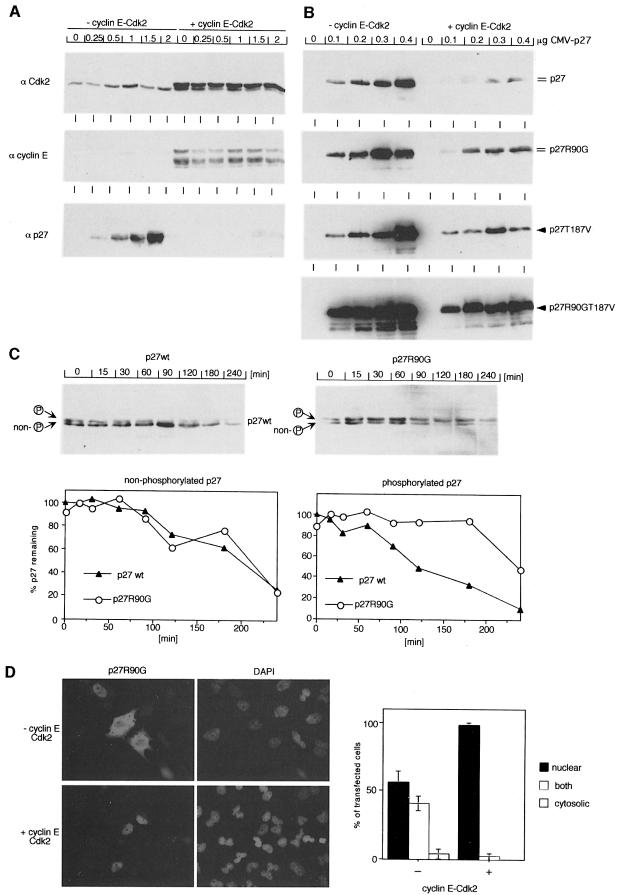
To confirm this suggestion, we co-expressed cyclin E and Cdk2 with different forms of p27 by transient transfection of HeLa cells (Figure 6A and B). In these assays, expression of cyclin E and Cdk2 induced efficient elimination of wild-type p27. This effect on p27 was fully reversible by addition of LLNL, an inhibitor of proteasomal degradation (not shown). In contrast, p27R90G was significantly more stable than wild-type p27; indeed, mutation at R90 stabilized p27 to a similar degree to a mutation of the major Cdk2 phosphorylation site, Thr187. Combining both mutations showed a strong synergy and yielded a mutant of p27 that is resistant to cyclin E-mediated elimination (Figure 6B).
Fig. 6. Cyclin E–Cdk2-induced degradation requires interaction of p27 with mNPAP60. HeLa cells were transfected with the indicated expression plasmids and analysed by Western blotting. (A) Control experiment documenting expression of cyclin E, Cdk2 and p27wt after transfection of the indicated amounts of plasmid; 5 µg of CMV–cyclin E and CMV–Cdk2 were used. (B) Repeat of the experiment using the indicated alleles of p27. Double lines indicate the presence of a phosphorylated form of p27; single arrows indicate absence of phosphorylated p27. (C) Cycloheximide-chase experiment documenting enhanced stability of phosphorylated p27R90G relative to p27wt. Transfections were carried out as in (B). The lower panels show a quantitation of the results and the upper panels show Western blots from a representative experiment. (D) p27R90G accumulates in the nucleus upon expression of cyclin E and Cdk2. Shown are immunofluorescence pictures of HeLa cells transfected with the expression vectors indicated together with a quantitation of the experiment.
To show that the enhanced levels of p27R90G observed after expression of cyclin E–Cdk2 were due to a decreased degradation of the protein, cycloheximide-chase experiments were performed. Cycloheximide was added to a number of dishes 48 h after transfection, cells were harvested after different time points and the amount of remaining p27 was determined by Western blotting (Figure 6C). In these experiments, p27R90G was significantly more stable than p27wt. In particular, mutation at R90 stabilized the phosphorylated form of p27 relative to p27wt; in contrast, the mutation had little effect on the stability of non-phosphorylated p27, which is also degraded in these assays (albeit at a slower rate). From several such experiments, we calculated t1/2s of 60 min and of 240 min for the phosphorylated form of p27wt and p27R90G, respectively, the double mutant (T187VR90G) was degraded with a t1/2 of 250 min (not shown). Taken together, the data show that mutation of R90 inhibits degradation of phosphorylated p27.
Since the p27R90G that accumulated upon co-expression of cyclin E and Cdk2 was phosphorylated (see arrows) and since cyclin E–Cdk2 complexes are only active in the cell nucleus (due to nuclear localization of CAK), the findings show that under these conditions interaction with mNPAP60 is required at a step after nuclear import. Indeed, the remaining fraction of p27R90G accumulated in the nucleus when co-expressed with cyclin E–Cdk2 (Figure 6D). We suggest that the NLSs present in cyclin E mediate efficient nuclear import of a ternary cyclin E–Cdk2–p27R90G complex in these assays.
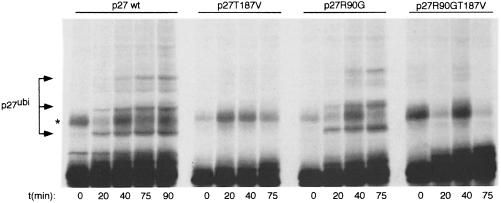
The failure of p27R90G to be correctly degraded might be a consequence of its defects in intracellular transport or may reflect a direct role of mNPAP60 in ubiquitylation of p27. To discriminate between the two possibilities, we performed in vitro ubiquitylation/degradation assays (Figure 7). Consistent with previous reports, wild-type p27, but not p27T187V, was efficiently ubiquitylated in extracts from S phase HeLa cells, confirming that phosphorylation by cyclin E–Cdk2 is required for ubiquitylation (Montagnoli et al., 1999; Nguyen et al., 1999). In contrast, mutation of Arg90 had no effect in these assays; as expected, the double mutant (p27R90G T187V) was not ubiquitylated. The findings show that interaction with mNPAP60 is not required for ubiquitylation of p27 in vitro.
Fig. 7. In vitro ubiquitylation of p27 does not require interaction with mNPAP60. Shown are fluorograms of in vitro ubiquitylation reactions using p27 synthesized in a reticulocyte lysate and an S phase extract from HeLa cells. The panel shows time-courses of ubiquitylation reactions using the indicated alleles of p27. Controls established that the bands indicated are generated in a ubiquitin- and ATP-dependent manner (not shown). The * indicates a background band that is present in reactions in the absence of ubiquitin and HeLa extracts.
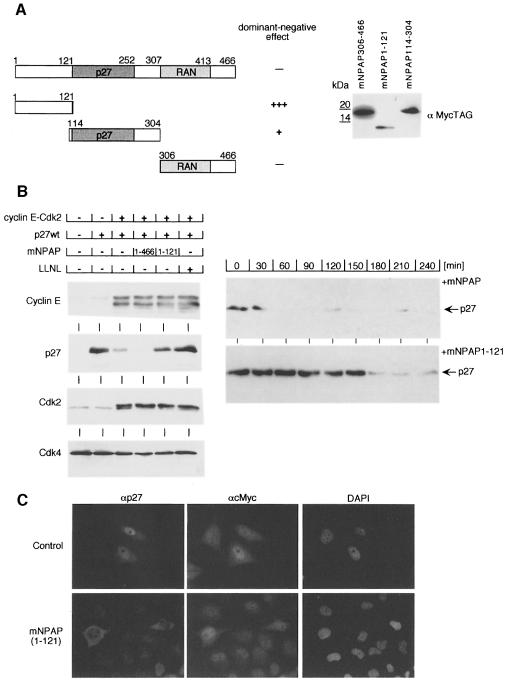
To provide an independent confirmation that interaction of p27 with mNPAP60 is required for efficient degradation, we sought to determine whether fragments of the protein act as dominant-negative alleles that inhibit cyclin E–Cdk2-mediated degradation of p27. The protein was arbitrarily divided into an N-terminus, a central p27-interacting domain and the C-terminus that includes the putative RAN-binding domain (Figure 8A). The individual fragments were tagged and co-expressed with cyclin E–Cdk2 and wild-type p27 to test whether degradation of p27 is affected. Western blots using an anti-mNPAP antibody documented that all fragments that are detectable by the mNPAP antibody were expressed in comparable amounts (see Supplementary figure 3); expression of the remaining fragments were detectable using an anti-MycTag antibody (Figure 8B).
Fig. 8. The N-terminus of mNPAP60 acts as a dominant-negative inhibitor of cyclin E–Cdk2-mediated degradation of p27. (A) Diagram showing the constructs used and their effect on cyclin E–Cdk2-mediated degradation of p27wt (left). The right panel documents the expression of individual fragments of mNPAP60 using α-MYCtag antibodies. Assays were performed essentially as in Figure 6A. (B) Left panels show Western blots documenting expression of p27wt, cyclin E and Cdk2 after transient transfection into HeLa cells together with the expression plasmids indicated. LLNL was added to the cells 8 h before harvesting. Cdk4 was used as a control for equal loading. The right panels document the altered stability of p27wt in the presence of full-length mNPAP or in the presence of the N-terminal (1–121) fragment using a cycloheximide-chase experiment. (C) Immunofluorescence pictures documenting the subcellular localization of p27wt and Myc in the absence and presence of co-transfected mNPAP60(1–121) in HeLa cells.
In these experiments, expression of full-length mNPAP60 further diminished expression of p27. In contrast, expression of the N-terminal fragment of mNPAP60 inhibited cyclin E–Cdk2-mediated degradation of p27; expression of the p27-binding domain gave a weak inhibition. Neither protein affected the expression of either cyclin E or Cdk2. Cycloheximide-chase experiments showed that the enhanced levels of p27 were due to enhanced stability of the protein in the presence of the N-terminal domain (Figure 8B). Expression of the putative RAN-binding domain gave inconsistent results (Figure 8A). In cells expressing the N-terminal fragment of mNPAP60, p27 mislocalized to the cytosol in transfected cells (Figure 8C). In contrast, the nuclear localization of Myc was not affected in these cells (Figure 8C).
These results provide independent confirmation that mNPAP60 specifically affects intracellular transport of p27 and suggest that the N-terminus of mNPAP60 interacts with additional proteins involved in either transport or degradation of p27.
Discussion
In this work, we describe an interaction of p27 with a nuclear pore-associated protein, mNPAP60 (Fan et al., 1997; Trichet et al., 1999). From the phenotype of a loss-of-interaction mutant of p27 and the phenotype of cells overexpressing a dominant-negative allele of mNPAP60 we conclude that the interaction between p27 and mNPAP60 is required for correct intracellular transport and degradation of p27.
Based on the phenotype of progressive C-terminal deletion mutants of p27, its nuclear import was thought to depend exclusively on the integrity of a bipartite nuclear import signal located around amino acid 153 (Reynisdottir and Massague, 1997). However, a triple point mutant that disrupts the NLS only partially abolishes nuclear import of p27 in vivo. In a direct comparison, this phenotype differs strikingly from a C-terminal deletion mutant that removes amino acids 91–197 (D.Müller, unpublished). Therefore, deletion of the conserved C-terminus removes additional elements that directly or indirectly influence the apparent nuclear localization of p27.
Mutation of R90 abolishes the residual nuclear import of p27 that carries a disrupted NLS, arguing that it is mediated by interaction with mNPAP60. A similar use of two distinct nuclear transport pathways by a single protein has been observed for MAP kinase, where monomeric and dimeric forms of the protein are imported via distinct transport routes (Adachi et al., 1999). Similarly, several observations support a model in which p27 bound to Cdks is transported via an NLS-dependent mechanism whereas free p27 (or bound to other partner proteins) is preferentially transported via association with mNPAP60. For example, Cdk2 (and potentially Cdk4) competes for binding of p27 to mNPAP60 in vitro most likely because the 310 helix is part of the Cdk2-interacting surface of p27 (Russo et al., 1996). In vivo, the import defect of the R90G mutant is most pronounced under conditions where p27 is in excess of cyclin–Cdk complexes (see, for example, Figure 6C). The model would predict that the NLS is masked in free p27, potentially due to dimerization of the protein; this is consistent with the finding that recombinant p27R90G is not imported via an NLS-dependent mechanism in vitro.
In vivo, p27R90G is a more potent inhibitor of proliferation than wild-type p27. Initially, we thought that the defect in nuclear import accounted for this phenotype. For example, p27R90G might associate in the cytosol with cyclins that lack a nuclear import signal such as cyclin A. Indeed, transiently transfected cells that express large amounts of p27R90G showed a moderate mislocalization of cyclin A to the cytosol. However, this phenotype required high levels of p27 and was not observed in retrovirally infected cells (D.Müller, unpublished data).
Instead, several observations suggest that a defect in cyclin E–Cdk2-mediated elimination of p27 accounts for the more potent inhibition of cell proliferation by p27R90G. First, elevated levels of cyclin E are found associated with p27R90G relative to p27wt in retrovirally infected cells. Secondly, mutation at R90 renders p27 more resistant to cyclin E–Cdk2-induced degradation in transient transfection assays; the mutation synergizes with a mutation in the major Cdk2 phosphorylation site. p27R90G accumulates in the nucleus when co-expressed with cyclin E (which contains an NLS), providing evidence that the interaction between p27 and mNPAP60 is required for degradation of p27 at a step after import into the nucleus.
This requirement does not reflect a direct role for mNPAP60 in ubiquitylation of p27, since p27R90G was efficiently ubiquitylated in vitro. The phenotype of the mutant is, therefore, compatible with two models: first, essential components involved in p27 degradation may be localized at the nuclear pore. In this view, interaction of p27 with mNPAP60 is required to target p27 efficiently to components of the degradation pathway. For example, it has been shown that proteasomes accumulate at the nuclear periphery (Enenkel et al., 1998; Wilkinson et al., 1998).
Alternatively, the phenotype of the mutant reflects a requirement for export of phosphorylated p27 from the nucleus for degradation. Very similar models have been proposed for p53, which is exported by association with mdm2 for degradation in the cytosol (Freedman and Levine, 1998). Indeed, the enhanced inhibition of proliferation by p27R90G is reminiscent of p53 in the presence of p19ARF (Tao and Levine, 1999) or of inhibitors of nuclear export (Freedman and Levine, 1998), which induce the accumulation of non-degradable, biologically active p53 in the cell nucleus. Potentially, therefore, there is a general requirement for nuclear export in degradation of negative regulators of cell proliferation (Scheffner, 1999).
Nuclear p27 interacts with Jab1, a component of the signalosome (Tomoda et al., 1999). Ectopic expression of Jab1 has been suggested to promote cell proliferation by facilitating nuclear export and degradation of p27. Jab1 and mNPAP60 may, therefore, act either sequentially in a single pathway or in parallel pathways directing export of p27 from the nucleus. In our experiments, ectopic expression of Jab1 reduced protein levels of both nuclear and cytosolic p27 to almost undetectable levels, precluding an analysis of Jab1-mediated nuclear export by transient transfection experiments (D.Müller, unpublished data). A detailed analysis of the individual events following phosphorylation of p27 in the nucleus using in vitro export assays will be required to resolve these open issues.
Materials and methods
DNA manipulation
For two-hybrid cloning, the entire coding region of mouse p27 was amplified and inserted into pGBT10 (Clontech). A total of 105 independent transformants of a mouse embryonal cDNA library (kind gift of S.Hollenberg) were screened. The loss-of-interaction screen was performed according to Hateboer et al. (1995). Full-length mNPAP60 clones were isolated from a λZAP Balb/c-3T3 cDNA library, rescued into pBluescript by phage excision and recloned into pcDNA3 for expression. Details of the generation and sequence of individual p27 mutants will be provided upon request; the sequence of all mutants was verified by sequencing. For retroviral expression, p27 cDNAs were inserted into pbabe-puro (Morgenstern and Land, 1990). The sequence of mouse mNPAP has been submitted to the GenBank and EMBL databases (accession No. AF251799).
Cell culture
HeLa, RAT1, MCF7 and NIH 3T3 cells were cultured in Dulbecco’s modified Eagle’s medium without phenol red supplemented with 10% heat inactivated fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Where indicated, 5 µg/ml puromycin was added for selection and 2.5 µg/ml for sustained expression of viral genes. Transient transfection into HeLa cells was performed by a calcium phosphate precipitation protocol as described earlier (Desbarats et al., 1996). Retroviral supernatants were generated by transient transfections of BOSC23 or PhoenixECO cells (Pear et al., 1993; Grignani et al., 1998) and used to infect RAT1 cells as described (Bouchard et al., 1999). LLNL (Sigma) was used at a final concentration of 100 µM and cells were incubated for 8 h in the presence of the chemical.
Protein analysis
In vitro cyclin E–Cdk2 kinase assays and heat treatment of p27 have been described previously (Müller et al., 1997). In vitro ubiquitylation assays were carried out as described (Montagnoli et al., 1999). Polyclonal antibodies against cdk2 (M2), cMyc (N262), cdk4 (C22) and monoclonal antibody against human cyclin E (HE-12) were obtained from Santa Cruz Biotechnology. Monoclonal antibody anti-p27(K25020) was obtained from Transduction Laboratories. A polyclonal antiserum against mNPAP60 was raised by immunizing rabbits with a his-tagged fragment of mNPAP60 (amino acids 121–252) expressed in E.coli. For in vitro pull-down experiments, a fragment encoding amino acids 121–252 of mNPAP was expressed as a fusion protein with GST. Immunofluorescence microscopy was performed as described previously (Peukert et al., 1997) except that cell samples were analysed on a Leica DMIRB microscope and pictures were taken with a Kappa CF 8/1 FMC CCD camera and electronically processed. A minimum of 300 transfected cells was counted for each mutant of p27.
Recombinant proteins, microinjection and import assays
HeLa cells were microinjected using a Zeiss Axiovert 10 microscope and an Eppendorf microinjector 5242/Micromanipulator 5171 unit. p27 and p27R90G were expressed as his-tagged proteins in E.coli as described previously (Steiner et al., 1995) and coupled to Cy3 or Cy2, respectively, using the Fluorolink kit obtained from Amersham. Labelled proteins were injected at a concentration of 1 mg/ml into 50–150 cells per time point. Cells were fixed in 3.5% formaldehyde/phosphate-buffered saline at the indicated time after injection and subcellular localization of the injected protein was analysed by fluorescence microscopy. In vitro nuclear import assays were performed according to Huber et al. (1998).
Supplementary data
Supplementary data to this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Antje Maier and Antje Grzeschiczek for excellent technical assistance, Reinhard Lührmann for help and support with in vitro transport assays, Bruce Clurman for communication of results before publication, Jun Kato for the gift of Jab1 cDNA, Heike Krebber for stimulating discussions, and the members of the laboratory for critically reading the manuscript. D.M. acknowledges a predoctoral fellowship from the Studienstiftung des Deutschen Volkes, A.D. an AIDS fellowship from the German Cancer Research Center. M.E. acknowledges support from the Deutsche Forschungsgemeinschaft and the Human Frontiers of Science Organization.
References
- Adachi M., Fukuda,M. and Nishida,E. (1999) Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J., 18, 5347–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C. et al. (1999) Direct induction of cyclin D2 by Myc contributes to cell cycle induction and sequestration of p27. EMBO J., 18, 5321–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano A.C., Eytan,E., Hershko,A. and Pagano,M. (1999) Skp2 is required for ubiquitin-mediated degradation of the Cdk inhibitor p27. Nature Cell Biol., 1, 193–199. [DOI] [PubMed] [Google Scholar]
- Darbon J.M., Devault,A., Taviaux,S., Fesquet,D., Martinez,A.M., Galas,S., Cavadore,J.C., Doree,M. and Blanchard,J.M. (1994) Cloning, expression and subcellular localization of the human homolog of p40MO15 catalytic subunit of cdk-activating kinase. Oncogene, 9, 3127–3138. [PubMed] [Google Scholar]
- Desbarats L., Gaubatz,S. and Eilers,M. (1996) Discrimination between different E-box binding proteins at an endogenous target gene of Myc. Genes Dev., 10, 447–460. [DOI] [PubMed] [Google Scholar]
- Enenkel C., Lehmann,A. and Kloetzel,P.M. (1998) Subcellular distribution of proteasomes implicates a major location of protein degradation in the nuclear envelope–ER network in yeast. EMBO J., 17, 6144–6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F., Liu,C.P., Korobova,O., Heyting,C., Offenberg,H.H., Trump,G. and Arnheim,N. (1997) cDNA cloning and characterization of Npap60: a novel rat nuclear pore-associated protein with an unusual subcellular localization during male germ cell differentiation. Genomics, 40, 444–453. [DOI] [PubMed] [Google Scholar]
- Freedman D.A. and Levine,A.J. (1998) Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell. Biol., 18, 7288–7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F., Kinsella,T., Mencarelli,A., Valtieri,M., Riganelli,D., Lanfrancone,L., Peschle,C., Nolan,G.P. and Pelicci,P.G. (1998) High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res., 58, 14–19. [PubMed] [Google Scholar]
- Hashimoto Y., Kohri,K., Kaneko,Y., Morisaki,H., Kato,T., Ikeda,K. and Nakanishi,M. (1998) Critical role for the 310 helix region of p57 (Kip2) in cyclin-dependent kinase 2 inhibition and growth suppression. J. Biol. Chem., 273, 16544–16550. [DOI] [PubMed] [Google Scholar]
- Hateboer G., Gennissen,A., Ramos,Y.F., Kerkhoven,R.M., Sonntag-Buck,V., Stunnenberg,H.G. and Bernards,R. (1995) BS69, a novel adenovirus E1A-associated protein that inhibits E1A transactivation. EMBO J., 14, 3159–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J., Cronshagen,U., Kadokura,M., Marshallsay,C., Wada,T., Sekine,M. and Luhrmann,R. (1998) Snurportin1, an m3G-cap-specific nuclear import receptor with a novel domain structure. EMBO J., 17, 4114–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe C., Bischoff,F.R., Ponstingl,H. and Wittinghofer,A. (1995) Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry, 34, 639–647. [DOI] [PubMed] [Google Scholar]
- Kutay U., Izaurralde,E., Bischoff,F.R., Mattaj,I.W. and Gorlich,D. (1997) Dominant-negative mutants of importin-β block multiple pathways of import and export through the nuclear pore complex. EMBO J., 16, 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisztwan J., Marti,A., Sutterlüty,H., Gstaiger,M., Wirbelauer,C. and Krek,W. (1998) Association of human CUL-1 and ubiquitin-conjugating enzyme CDC34 with the F-box protein p45 (SKP2): evidence for evolutionary conservation in the subunit composition of the CDC34–SCF pathway. EMBO J., 17, 368–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnoli A., Fiore,F., Eytan,E., Carrano,A.C., Draetta,G.F., Hershko,A. and Pagano,M. (1999) Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev., 13, 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J.P. and Land,H. (1990) Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res., 18, 3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D., Bouchard,C., Rudolph,B., Steiner,P., Stuckmann,I., Saffrich,R., Ansorge,W., Huttner,W. and Eilers,M. (1997) Cdk2-dependent phosphorylation of p27 facilitates its Myc-induced release from cyclin E/cdk2 complexes. Oncogene, 15, 2561–2576. [DOI] [PubMed] [Google Scholar]
- Nguyen H., Gitig,D.M. and Koff,A. (1999) Cell-free degradation of p27 (kip1), a G1 cyclin-dependent kinase inhibitor, is dependent on CDK2 activity and the proteasome. Mol. Cell. Biol., 19, 1190–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Tam,S.W., Theodoras,A.M., Beer Romero,P., Del Sal,G., Chau,V., Yew,P.R., Draetta,G.F. and Rolfe,M. (1995) Role of the ubiquitin–proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science, 269, 682–685. [DOI] [PubMed] [Google Scholar]
- Pear W.S., Nolan,G.P., Scott,M.L. and Baltimore,D. (1993) Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl Acad. Sci. USA, 90, 8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peukert K., Staller,P., Schneider,A., Carmichael,G., Hanel,F. and Eilers,M. (1997) An alternative pathway for gene regulation by Myc. EMBO J., 16, 5672–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynisdottir I. and Massague,J. (1997) The subcellular locations of p15 (Ink4b) and p27 (Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev., 11, 492–503. [DOI] [PubMed] [Google Scholar]
- Russo A.A., Jeffrey,P.D., Patten,A.K., Massague,J. and Pavletich,N.P. (1996) Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A–Cdk2 complex. Nature, 382, 325–331. [DOI] [PubMed] [Google Scholar]
- Scheffner M. (1999) Moving protein heads for breakdown. Nature, 398, 103–104. [DOI] [PubMed] [Google Scholar]
- Sheaff R.J., Groudine,M., Gordon,M., Roberts,J.M. and Clurman,B.E. (1997) Cyclin E–CDK2 is a regulator of p27Kip1. Genes Dev., 11, 1464–1478. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. and Roberts,J.M. (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev., 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Shirane M., Harumiya,Y., Ishida,N., Hirai,A., Miyamoto,C., Hatakeyama,S., Nakayama,K. and Kitagawa,M. (1999) Down-regulation of p27 (Kip1) by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J. Biol. Chem., 274, 13886–13893. [DOI] [PubMed] [Google Scholar]
- Steiner P., Philipp,A., Lukas,J., Godden-Kent,D., Pagano,M., Mittnacht,S., Bartek,J. and Eilers,M. (1995) Identification of a Myc-dependent step during the formation of active G1 cyclin/cdk complexes. EMBO J., 14, 4814–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterlüty H., Chatelain,E., Marti,A., Wirbelauer,C., Senften,M., Müller,U. and Krek,W. (1999) p45skp2 promotes p27kip1 degradation and induces S phase in quiescent cells. Nature Cell Biol., 1, 207–214. [DOI] [PubMed] [Google Scholar]
- Tao W. and Levine,A.J. (1999) P19 (ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc. Natl Acad. Sci. USA, 96, 6937–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda K., Kubota,Y. and Kato,J. (1999) Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature, 398, 160–165. [DOI] [PubMed] [Google Scholar]
- Trichet V., Shkolny,D., Dunham,I., Beare,D. and McDermid,H.E. (1999) Mapping and complex expression pattern of the human NPAP60L nucleoporin gene. Cytogenet. Cell Genet., 85, 221–226. [DOI] [PubMed] [Google Scholar]
- Tsvetkov L.M., Yeh,K.H., Lee,S.J., Sun,H. and Zhang,H. (1999) p27 (Kip1) ubiquitination and degradation is regulated by the SCF (Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol., 9, 661–664. [DOI] [PubMed] [Google Scholar]
- Vlach J., Hennecke,S., Alevizopoulos,K., Conti,D. and Amati,B. (1996) Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J., 15, 6595–6604. [PMC free article] [PubMed] [Google Scholar]
- Vlach J., Hennecke,S. and Amati,B. (1997) Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J., 16, 5334–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis K., Ryder,U. and Lamond,A.I. (1996) The conserved amino-terminal domain of hSRP1α is essential for nuclear protein import. EMBO J., 15, 1818–1825. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson C.R., Wallace,M., Morphew,M., Perry,P., Allshire,R., Javerzat,J.P., McIntosh,J.R. and Gordon,C. (1998) Localization of the 26S proteasome during mitosis and meiosis in fission yeast. EMBO J., 17, 6465–6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kobayashi,R., Galaktionov,K. and Beach,D. (1995) p19Skp1 and p45Skp2 are essential elements of the cyclin A–CDK2 S phase kinase. Cell, 82, 915–925. [DOI] [PubMed] [Google Scholar]