Abstract
Purpose: This technical note documents a method that the authors developed for combining a signal to synchronize a patient-monitoring device with a second physiological signal for inclusion into list-mode acquisition. Our specific application requires synchronizing an external patient motion-tracking system with a medical imaging system by multiplexing the tracking input with the ECG input. The authors believe that their methodology can be adapted for use in a variety of medical imaging modalities including single photon emission computed tomography (SPECT) and positron emission tomography (PET).
Methods: The authors insert a unique pulse sequence into a single physiological input channel. This sequence is then recorded in the list-mode acquisition along with the R-wave pulse used for ECG gating. The specific form of our pulse sequence allows for recognition of the time point being synchronized even when portions of the pulse sequence are lost due to collisions with R-wave pulses. This was achieved by altering our software used in binning the list-mode data to recognize even a portion of our pulse sequence. Limitations on heart rates at which our pulse sequence could be reliably detected were investigated by simulating the mixing of the two signals as a function of heart rate and time point during the cardiac cycle at which our pulse sequence is mixed with the cardiac signal.
Results: The authors have successfully achieved accurate temporal synchronization of our motion-tracking system with acquisition of SPECT projections used in 17 recent clinical research cases. In our simulation analysis the authors determined that synchronization to enable compensation for body and respiratory motion could be achieved for heart rates up to 125 beats-per-minute (bpm).
Conclusions: Synchronization of list-mode acquisition with external patient monitoring devices such as those employed in motion-tracking can reliably be achieved using a simple method that can be implemented using minimal external hardware and software modification through a single input channel, while still recording cardiac gating signals.
Keywords: motion correction, event synchronization, list-mode acquisition
INTRODUCTION
During the course of emission imaging, patients undergo both physiological motion1 and gross body-motion.2, 3 These can result in reduced image quality and artifacts that can impact diagnostic accuracy. A number of researchers have developed list-mode based correction techniques. Examples include correction methods for patient respiration motion in single photon emission computed tomography (SPECT) (Refs. 4, 5, 6, 7, 8) and other forms of patient motion during SPECT.9, 10, 11, 12 List-mode correction techniques have also been reported for both animal motion in preclinical positron emission tomography (PET) (Ref. 13) and patient head motion in neuro-PET.14 Additionally, in some imaging protocols the input of the ECG R-wave pulses allow acquisitions to be gated in order to capture heart motion to better assess cardiac function.15 In all of those various methods that incorporate motion tracking and/or ECG gating there is a basic need to synchronize external gating/tracking systems with the imaging system. In our SPECT research we often incorporate cardiac gating along with corrections for both respiratory and gross body motion. We have utilized a visual tracking system (VTS) for estimating signals related to both respiratory and gross body motion through stereo-imaging of retro-reflective markers mounted on stretchy bands wrapped about the patient's chest and abdomen.16, 17, 18, 19, 20 Our correction methods for both respiratory and body motion have been previously reported.21, 22, 23 To capture external events from several asynchronous sources some imaging vendors provide several physiological input channels. Other vendors may only provide a single physiological channel thereby complicating the insertion of inputs from two or more sources. Such is our case in which we can only easily access one physiological input channel to input both our cardiac gating signal and our motion tracking timing signal. In the past this has prevented us from performing respiratory and body motion corrections along with ECG gated acquisitions. A solution to our problem which we have formulated, implemented, and tested in clinical acquisitions is the subject of this technical note. What we believe is novel about our method is that for our synchronization timing mark we use a short duration pulse sequence. This sequence is designed to tolerate a potential “collision” of the individual pulses in the sequence with the asynchronous R-wave output whose input channel to list-mode acquisition it shares. This approach can be modified to work for other applications; thus we believe our method might be useful for other research groups that might need to input several signals onto one physiological channel.
METHODS
Description of the problem
As previously stated, we developed a VTS that can be used to correct patient respiratory and body motion in SPECT myocardial perfusion imaging (MPI).16, 17, 18, 19, 20 Our approach consists of near-infrared MX motion capture cameras, a MX Ultranet hardware controller, and software running on a Windows workstation purchased from Vicon Motion Systems Ltd., Oxford, UK. We use the VTS to track multiple retro-reflective markers placed on a patient's chest and abdomen at a marker-tracking rate of 30 Hz. Motion tracking by this system needs to be temporally synchronized with the list-mode acquisition of SPECT studies acquired by our BrightView XCT Imaging SPECT/CT System (Philips Healthcare, Cleveland, OH). In the past we have performed motion tracking solely in the absence of cardiac gating. We decided to find a way to input into the BrightView XCT (BVXCT) both the ECG signal and a pulse sequence generated by the MX Ultranet controller marking the start of VTS acquisition. With a start signal recorded in the list-mode file of the BVXCT, one-millisecond VTS temporal synchronization accuracy can be established. Our problem was how to achieve this synchronization while also acquiring the ECG through only one physiological input channel.
Novel second input signal
Utilizing a second signal to indicate the VTS-start we are confronted with one fundamental implementation problem. The issue is that the two signals sharing the single input channel are asynchronous. Our solution must handle the case in which any VTS signal arrives during the window in which an ECG R-wave signal is already asserted (i.e., 150 ms pulse length). This will result in an obscured VTS signal. We refer to this condition as a “collision case” and the window as a “collision window.” There are also timing cases that, while not obscuring the VTS signal, might confound interpretation of the cardiac gating for one cardiac interval. These cases would obscure the arrival of the ECG R-wave during the window in which any VTS signal is already asserted (i.e., 50 ms pulse length). The special case of the simultaneous arrival of both an ECG R-wave and a VTS signal may or may not confound interpretation depending on the list-mode processing implementation but again at worst should only confound one gating interval. Confounding one interval of cardiac gating is not an issue in our imaging protocol but might need to be considered in other applications of this method as will be explained further.
Our basic approach for injecting the start of VTS signal is to use a short pulse sequence that can be inserted into the physiological channel such that it will be interpreted by the BVXCT as an ECG R-wave. Postprocessing will then detect these pseudo-R-wave events in the list-mode file to determine if it is a start mark. Our initial concept was to use a reduced pulse interval. This interval needed to have a sufficient duration such that it would be detected as an ECG pulse (i.e., it does not result in an imaging system ECG alarm). We empirically determined that a continuous stream of 50 ms active-low TTL pulses with a pulse rate greater than 115 ms would not trigger a BVXCT cardiac alarm. Based on this finding we decided to use pulse width of 50 ms and a pulse interval of 125 ms or greater. We verified that synthesized active-low TTL pulse streams with those limits would be entered as individual R-wave events in a list-mode file without triggering an alarm. The shorter pulse width is useful for distinguishing pulses on an oscilloscope trace but is not absolutely necessary for our implementation. Having separate pulse widths in order to encode information is known as pulse-width modulation (PWM). PWM might be useful if imaging system vendors were to enhance the capability of a single physiological channel, as we will recommend in Sec. 4.
Having determined our basic pulse timing, we then focused on the aforementioned design goal of tolerating “collision window” cases. We believe that patients would not undergo cardiac stress imaging if they were presenting with heart rates much greater than 110 bpm (a R-wave on average of once every 545 ms) so our design goal was that our short pulse sequence should be able to tolerate a collision for a patient with a rapid heart rate up to 120 bpm (i.e., heartbeat once every 500 ms). We realized that if the interval between pulses were different yet known that we could accurately determine the arrival time of the VTS start. To accomplish start detection we believe that accurate interval timing between two consecutive pulses in the four-pulse sequence is needed (i.e., one known pulse interval would survive any “collision”). For a four-pulse sequence we then selected three different pulse intervals following the first pulse. We experimented with different pulse widths and then somewhat arbitrarily selected intervals of 125, 150, and 175 ms. To summarize our current sequence specification: (1) four active-low TTL pulses, (2) each pulse is 50 ms wide, (3) the first pulse occurs at 25 s after VTS start, (4) the second pulse is 175 ms after the start of the first pulse, (5) the third pulse is 150 ms after the start of the second pulse, and (6) the final pulse is 125 ms following the start of the third pulse.
Our method alters the typical clinical system interface configuration by routing the cardiac trigger monitor (CTM) (Ivy Biomedical Systems Inc., Branford, CT) coaxial output into a simple electronic device of our design instead of directly into the BVXCT. We refer to our device as an input channel combiner (ICC). Into this ICC we also route a second TTL input, which carries the just described four-pulse sequence generated 25 s after VTS start. Simply, the ICC performs a logical AND of the two TTL input signals and outputs a combined TTL signal into the aforementioned BVXCT BNC input connector as shown in Fig. 1.
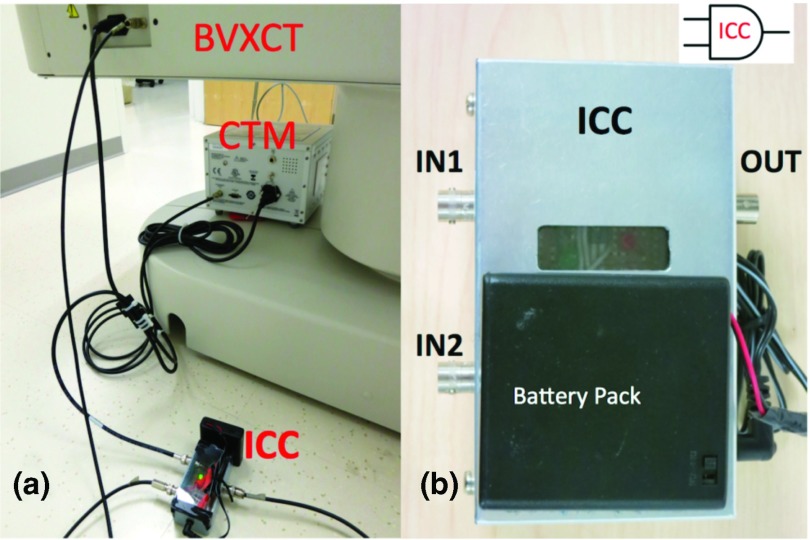
Figure 1.
(a) Input channel combiner (ICC), cardiac trigger monitor (CTM), side of BrightView XCT (BVXCT) imaging bed with channel input BNC connector. [VTS is off-photo.] (b) Closeup of ICC with input coaxial BNCs and the output coaxial BNC. The ICC performs a logical AND of two “active low” TTL signals (cartoon AND-gate upper right).
The “truth” or state table for the ICC using TTL active-low logic is displayed in Table 1. Essentially this table illustrates that the BVXCT input would be asserted (active-low) whenever either ICC input is low.
Table 1.
ICC “truth” or state table.
| VTS out (ICC in 1) | CTM out (ICC in 2) | BVXCT in (ICC out) |
|---|---|---|
| H | H | H |
| xx | L | L |
| L | xx | L |
Note: H = High or ∼5 V (not asserted), L = low or ∼0 V (asserted), xx = either H or L (i.e., so-called “do not care”) for TTL active-low logic.
We add that to generate the four-pulse sequence we take advantage of the capability of the VTS to generate up to four separate single-pulses according to user configurable parameters. This allows us to generate the aforementioned pulse timing. It was necessary for us to combine these separate pulses in an external device that is conceptually similar to the described ICC but implements an active-low TTL quad-AND of the individual pulses to produce the combined pulse sequence.
Postprocessing of list-mode file
Following acquisition, output files from the VTS and list-mode are processed using software from various sources combined into one comprehensive software package written in C. The first subroutine is a significantly modified version of software purchased from Philips Healthcare (Cleveland, OH) to bin the list-mode data into projection data for conventional reconstruction. Within the list-mode data stream there is an event (or in some intervals multiple events) recorded every millisecond. During this rebinning process, all the ECG R-wave pulses recorded in the list are detected and stored in a timing file. After all list-mode events have been processed and separated into files according to category (timing, control, and photon events) subsequent processing occurs. By then the photon events have been collected in 100 ms increments for each projection. The timing file can be processed for both gating marks and the unique pulse sequence (i.e., recorded as “pseudo-R-wave” events). At this point a synchronization file is generated for use by a second subroutine that aligns the SPECT data with the VTS motion data recorded at a rate of 30 Hz in a comma-separated values (csv) text file. After file processing the data streams are synchronized and can then be used for subsequent motion estimation and compensation prior to and during reconstruction, respectively.
Pulse sequence simulation
We validated our four-pulse sequence design through simulation. We wrote a simple simulation program using IDL (Exelis Visual Information Solutions, Inc., Boulder, CO). The program simulates two signals: a repetitive CTM output trigger pulse for a specified heart rate; the specified four-pulse sequence previously described. The two signals are logically combined consistent with a TTL AND-gate. The output is then analyzed to ensure that VTS start can be detected. For each case the two signals are combined at every possible interval (at one-millisecond resolution) thereby simulating various timing interactions. These variations would include all possible collision and noncollision cases for the specified heart rate. Any simulated interaction in which the VTS start cannot be detected is reported. We illustrate a nonobscured timing case in Fig. 2 and a “collision case” in which VTS start timing can still be recovered in Fig. 3.
Figure 2.
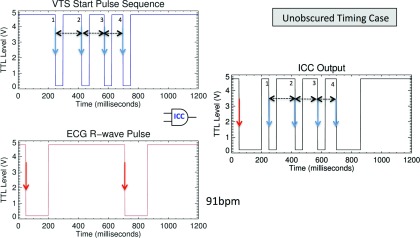
Illustration of a case in which the VTS start pulse is not obscured despite the proximate arrival of the R-wave signal with the fourth VTS start pulse. (Left) Shown are the input VTS start pulse sequence at the TOP and the ECG R-wave pulse at the bottom. (Right) Output to the BVXCT. In this case all four start pulses would be recognized because pulse-width 1-2 (175 ms), pulse-width 2-3 (150 ms), and pulse-width 3-4 (125 ms) are known and occur within the expected window. The ECG R-wave pulse arrives 9 ms after last start pulse and therefore would not obscure the fourth VTS timing mark.
Figure 3.
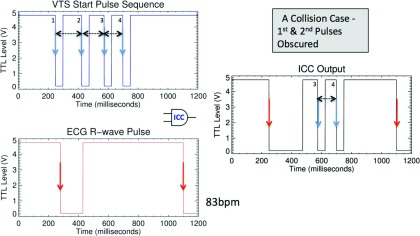
Illustration of the case of “collision” between R-wave signal and VTS start pulse. (Left) Shown are the input VTS start pulse sequence at the top and the ECG R-wave signal at the bottom. (Right) Output to the BVXCT. In this case pulses 3 and 4 would be received and pulse-width 3-4 (125 ms) is known and occurs within the expected window. Start would be detected even though pulse width 1-2 and pulse width 2-3 were obscured by the R-wave signal.
Clinical application of the method
Under IRB approval24 and with patient consent, all of our research acquisitions are obtained during the stress portion of physician-ordered clinical SPECT MPI. Immediately following the clinical stress imaging a second nongated research study is performed and the patient is asked to move. Both acquisitions require VTS and SPECT synchronization for accurate motion and respiratory correction. For our method a research acquisition requires three additional steps beyond typical clinical SPECT MPI acquisition:
-
1.
Placement of the retro-reflective markers on the patient's chest and abdomen.
-
2.
Use of list-mode acquisition.
-
3.
The addition of the aforementioned ICC device and reconfiguration of coaxial inputs.
The VTS is started shortly after the start of the imaging protocol. The VTS pulse pattern starts precisely 25 s after VTS start. This start interval can be adjusted but for our research this initial 25 s delay period was selected so that the start pattern would occur prior to actual emission imaging (i.e., during bed positioning). Our system list-mode acquisition includes the recording of timing marks, camera, and bed motion, etc., which occur prior to the start of recording emission events. This makes the insertion of our pattern possible without conflicting with R-wave trigger marks. Other systems that start list-mode recording only upon start of emission events there is the likelihood of a conflict that might corrupt one cardiac event. We speculate that for those systems there is a possibility that the start pattern described herein would result in a shortened R-wave interval that would then be rejected by the window employed for acceptance. This would mean that those emissions for that one interval would not contribute to imaging. Over an entire imaging study this one lost interval might be acceptable.
RESULTS
We verified basic system list-mode timing accuracy of one millisecond by examining if there were any variations in list-mode determined timing from a known external timing source (the CTM). The imaging system provides a list-mode event every millisecond. The CTM can output a simulated heart rate. We setup test studies with CTM configured for various heart rates (40, 60, 90, and 120 bpm). In all of those studies the standard deviation for heart rate was 0 ms. Simulation results confirmed that with a R-wave interval greater than 475 ms (i.e., heart rate less than 126 bpm) one of the known VTS start pulse intervals would always be detected for any of the possible collision arrival times throughout a 450 ms pulse sequence window (i.e., from start of first pulse to start of fourth pulse). The simulation program examines 610 cases for any given ECG interval. The number of timing cases cover an overlap window from 155 ms prior to the first pulse in the sequence through 5 ms following the fourth pulse in the sequence in one millisecond steps (i.e., 610 ms overlap period). Each output case from the combination of the two asynchronous signals is determined as a variable ECG window is slid in one millisecond steps over the entire 450 ms pulse sequence window. This simulated overlap period will cover all of the possible collision cases. The simulation results show that for any ECG interval of 476 ms or greater that at least one VTS start interval can be reliably detected by the analysis software.
Since February 2013 we have incorporated our synchronization method into our research protocol. From that time we have successfully synchronized data streams for 17 clinical research cases. For these cases the research patients’ heart rates ranged from 66 to 100 bpm. A majority of these cases involved a collision window obscuring at least one of the four pulses as will be explained. Keeping in mind the simulation example illustrated in Figure 3 in which only one of the known pulse intervals (the 125 ms or 3rd interval between pulse 3 and 4) was recovered, we examined the pulse intervals for all 17 clinical cases. All three pulse intervals were captured in five of the cases (i.e., no “collision”). In nine of the collision cases two intervals were detected (interval 1 and 2 in seven cases; interval 2 and 3 in two cases). In three collision cases only one pulse interval was recorded (interval 2 in one case and interval 3 in two cases). The results of these 12 collision cases in the 17 clinical cases reinforce the simulation results that for typical clinical heart rates, synchronization can be determined.
DISCUSSION
As referenced earlier, we are aware of a previous method for the synchronization of respiratory motion data with list-mode data;25 however, we believe that our implementation is novel because it allows the input channel to be shared and it handles the so-called “collision window” cases.
While some medical imaging systems do provide additional physiological channels, we believe there may be instances in which there may be a need to combine asynchronous signals on a single physiological channel. In such instances we believe our method could be a useful.
Additionally, our method eases postprocessing of two input data streams because only one short four-pulse sequence must be processed; thereafter all subsequent list-mode input would only be ECG timing events. This simplifies the postprocessing of timing signals.
In our specific implementation we discovered without vendor assistance that pulse-widths less than 150 ms, as configured by the vendor for ECG R-wave detection in clinical use, would also be interpreted as R-wave events. As we have discussed, in our implementation we determined that 50 ms active-low TTL pulses were equivalent to 150 ms pulses. This permits us to speculate that it might be possible for us to reduce both the CTM pulse-width as well as the VTS start sequence pulse-widths. This would reduce but not eliminate the overlap of any simultaneous active-low TTL signals thereby somewhat reducing the collision window and possibly allow support for higher heart rates. We intend to discuss this with our vendor and so may modify our pulse timing in the future.
We offer that it would also be possible for vendors of medical imaging system to use pulse-width modulation (PWM) and thus enable their channel input processing to differentiate between different pulse widths in a single channel. By differentiating among list-mode events based on pulse widths, postprocessing would not be needed to discriminate events. We speculate that it might be possible to extend this concept to more than two event classes. Ideally, if there were a standard among vendors for encoding interface pulse sequences, methods for combining channel input could be even more widely shared.
We now state a few caveats. No timing drift analysis of VTS and our SPECT system between synchronization at the start and the end of SPECT acquisition was performed in our work. Our results are based on list-mode processing for one system (BVXCT) and one motion tracking system (Vicon). While we hope that our method may be useful to others, list-mode implementations by other vendors and integration with other motion tracking systems might produce different results.
CONCLUSIONS
Patient motion is inevitable during several imaging modalities. Patient motion is known to degrade diagnostic accuracy. Our overall research goal is to develop and improve methods for motion tracking and correcting patient motion when it occurs. One of our recent method improvements that we have just described involved the synchronization of our VTS with our acquisition list-mode events using a shared BVXCT input channel.
The distinct and we believe novel aspects of our method are:
-
1.
Use of a short pulse sequence to insert the second signal (VTS start) into a physiological input channel configured to receive ECG timing up to ∼120 bpm. The sequence can be as short as four pulses.
-
2.
Variation of each pulse interval within the pulse sequence so that in the event of collision of two asynchronous signals that synchronization can still be determined based on a known pulse interval pattern that can be detected in postprocessing of a list-mode file.
We have shown in our current implementation and early usage that even in collision cases synchronization can be determined. While we have only achieved results using our specific devices, we believe this method could be adapted to work reliably in other systems.
ACKNOWLEDGMENTS
Our research was supported in part by the National Institutes of Health under Grant No. R01-EB01457 and a grant from Philips Healthcare. The authors acknowledge the cooperation of Philips Healthcare during our methods development. The contents of this paper are solely the responsibility of the authors and represent neither the views of the NIH nor Philips Healthcare.
References
- Ter-Pogossian M. M., Bergmann S. R., and Sobel B. E., “Influence of cardiac and respiratory motion on tomographic reconstructions of the heart: Implications for quantitative nuclear cardiology,” J. Comput. Assist. Tomogr. 6(6), 1148–1155 (1982). 10.1097/00004728-198212000-00016 [DOI] [PubMed] [Google Scholar]
- Botvinick E. H. et al. , “A quantitative assessment of patient motion and its effect on myocardial perfusion SPECT images,” J. Nucl. Med. 34(2), 303–310 (1993). [PubMed] [Google Scholar]
- Cooper J. A., Neumann P. H., and McCandless B. K., “Effect of patient motion on tomographic myocardial perfusion imaging,” J. Nucl. Med. 33(8), 1566–1571 (1992). [PubMed] [Google Scholar]
- Livieratos L. et al. , “Rigid-body tranformation of list-mode projection data for repiratory motion correction in cardiac PET,” Phys. Med. Biol. 50(14), 3313–3322 (2005). 10.1088/0031-9155/50/14/008 [DOI] [PubMed] [Google Scholar]
- Kovalski G. et al. , “Correction of heart motion due to respiration in clinical myocardial perfusion SPECT scans using respiratory gating,” J. Nucl. Med. 48(4), 630–636 (2007). 10.2967/jnumed.106.037390 [DOI] [PubMed] [Google Scholar]
- Lamare F. et al. , “List-mode-based reconstruction for respiratory motion correction in PET using non-rigid body transformations,” Phys. Med. Biol. 52(17), 5187–5204 (2007). 10.1088/0031-9155/52/17/006 [DOI] [PubMed] [Google Scholar]
- Bruyant P. P. et al. , “Compensation of respiratory motion in cardiac SPECT with list-mode acquisition and an external respiration-tracking device,” J. Nucl. Cardiol. 11(4), S34 (2004). 10.1016/j.nuclcard.2004.06.111 [DOI] [Google Scholar]
- Dey J. et al. , “Estimation and correction of cardiac respiratory motion in SPECT in the presence of limited-angle effects due to irregular respiration,” Med. Phys. 37(12), 6453–6465 (2010). 10.1118/1.3517836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B. et al. , “Estimation of the rigid-body motion from three-dimensional images using a generalized center-of-mass points approach,” IEEE Trans. Nucl. Sci. 53(5), 2712–2718 (2006). 10.1109/TNS.2006.882747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B. et al. , “Use of the three-dimensional Gaussian interpolation in the projector/backprojector pair of iterative reconstruction for compensation of known rigid-body motion in SPECT,” IEEE Trans. Med. Imaging 25(7), 838–844 (2006). 10.1109/TMI.2006.871397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S. et al. , “Body deformation correction for SPECT imaging,” IEEE Trans. Nucl. Sci. 57(1, Part 1), 214–224 (2010). 10.1109/TNS.2009.2031114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher H., Modersitzki J., and Fischer B., “Combined reconstruction and motion correction in SPECT imaging,” IEEE Trans. Nucl. Sci. 56(5, Part 1), 73–80 (2009). 10.1109/TNS.2008.2007907 [DOI] [Google Scholar]
- Kyme A. Z. et al. , “Optimised motion tracking for positron emission tomography studies of brain function in awake rats,” PLoS ONE 6(7), e21727 (2011). 10.1371/journal.pone.0021727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield P. M. et al. , “The design and implementation of a motion correction scheme for neurological PET,” Phys. Med. Biol. 48(8), 959–978 (2003). 10.1088/0031-9155/48/8/301 [DOI] [PubMed] [Google Scholar]
- Bailey D. L. and Kalemis A., “Externally triggered gating of nuclear medicine acquisitions: A useful method for partioning data,” Phys. Med. Biol. 50, N55–N62 (2005). 10.1088/0031-9155/50/7/N02 [DOI] [PubMed] [Google Scholar]
- Beach R. D. et al. , “Feasibility of stereo-infrared tracking to monitor patient motion during cardiac SPECT imaging,” IEEE Trans. Nucl. Sci. 51(5, Part 2), 2693–2698 (2004). 10.1109/TNS.2004.835786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyant P. P. et al. , “A robust visual tracking system for motion detection in SPECT: Hardware solutions,” IEEE Trans. Nucl. Sci. 52(5, Part 1), 1288–1294 (2005). 10.1109/TNS.2005.858208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach R. D. et al. , “An adaptive approach to decomposing patient-motion tracking data acquired during cardiac SPECT imaging,” IEEE Trans. Nucl. Sci. 54(1, Part 1), 130–137 (2007). 10.1109/TNS.2006.887471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara J. E. et al. , “An assessment of a low-cost visual tracking system (VTS) to detect and compensate for patient motion during SPECT,” IEEE Trans. Nucl. Sci. 55(3, Part 1), 992–998 (2008). 10.1109/TNS.2008.915688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara J. E. et al. , “A flexible multi-camera visual-tracking system for detecting and correcting motion-induced artifacts in cardiac SPECT slices,” Med. Phys. 36(5), 1913–1923 (2009). 10.1118/1.3117592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey J. and King M. A., “Theoretical and numerical study of an MLEM and OSEM reconstruction algorithms for motion correction in emission tomography,” IEEE Trans. Nucl. Sci. 56(5, Part 1), 2739–2749 (2009). 10.1109/TNS.2009.2021765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra Mukherjee J. et al. , “Estimation of rigid-body and respiratory motion of the heart from marker-tracking data for SPECT motion correction,” IEEE Trans. Nucl. Sci. 56(1, Part 1), 147–155 (2009). 10.1109/TNS.2008.2010319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra Mukherjee J. et al. , “Quantitative study of rigid-body and respiratory motion of patients undergoing stress and rest cardiac SPECT imaging,” IEEE Trans. Nucl. Sci. 57(3), 1105–1115 (2010). 10.1109/TNS.2010.2043852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMMS, IRB Docket No. H-10943, Institutional Review Board (IRB) , University of Massachusetts Medical School, 2012.
- Bruyant P. P. et al. , “A method for synchronizing an external respiratory signal with a list-mode PET acquisition,” Med. Phys. 34(11), 4472–4475 (2007). 10.1118/1.2791036 [DOI] [PubMed] [Google Scholar]