Abstract
Study design
A retrospective review of prospectively collected data in an academic institution.
Objective
To evaluate the safety and efficacy of a new type of titanium mesh cage (TMC) in single-level, anterior cervical corpectomy and fusion (ACCF).
Methods
Fifty-eight patients consecutive with cervical spondylotic myelopathy (CSM) from cervical degenerative spondylosis and isolated ossification of the posterior longitudinal ligament were treated with a single-level ACCF using either a new type of TMC (28 patients, group A) or the traditional TMC (30 patients, group B). We evaluated the patients for TMC subsidence, cervical lordosis (C2–C7 Cobb and Cobb of fused segments) and fusion status for a minimum of 30 months postoperatively based on spine radiographs. In addition, neurologic outcomes were evaluated using the Japanese Orthopedic Association (JOA) scores. Neck pain was evaluated using a 10-point visual analog scale (VAS).
Results
The loss of height of the fused segments was less for group A than for group B (0.8 ± 0.3 vs. 2.8 ± 0.4 mm) (p < 0.01); also, there was a lower rate of severe subsidence (≥3 mm) in group A (4 %, 1/28) than in group B (17 %, 5/30) (p < 0.01). There were no differences in the C2–C7 Cobb and Cobb of fused segments between the groups preoperatively or at final follow-up (p > 0.05), but the Cobb of fused segments immediately postoperative were significantly less for group B than for group A (p < 0.01). All patients, however, had successful fusion (100 %, each). Both groups had marked improvement in the JOA score after operation (p < 0.01), with no significant differences in the JOA recovery ratio (p > 0.05). The postoperative VAS neck pain scores for group A were significantly less than that for group B (p < 0.05); severe subsidence was correlated with neck pain.
Conclusions
The new type of TMC provides comparable clinical results and fusion rates with the traditional TMC for patients undergoing single-level corpectomy. The new design TMC decreases postoperative subsidence (compared to the traditional TMC); the unique design of the new type of TMC matches the vertebral endplate morphology which appears to decrease the severity of subsidence-related neck pain in follow-up.
Keywords: Cervical, Corpectomy, Fusion, Titanium mesh cage
Introduction
Anterior reconstruction using a titanium mesh cage (TMC) with anterior cervical plating has been used widely after corpectomy because of its excellent clinical outcomes; and because this technique avoids the need for a bone graft harvest and the complications at the donor site [1–6]. TMC subsidence, however, was observed frequently in the early postoperative period and led to subsidence-related complications, including neck pain, neurologic deterioration, and instrument failure [7–11].
Although a variety of factors such as sex, age, resection of the posterior longitudinal ligament, type of plate and endplate preparation, and bone mineral density have already been associated with TMC subsidence in the literature [12–16], the differences in the orientation of the contact face of the TMC with the vertebral endplates may be important in preventing TMC subsidence [9]. For this reason, we designed a new type TMC which fully matches the vertebral endplate morphology and evaluated this new TMC in 28 patients.
The purpose of this study was to assess the safety and efficacy of the new type TMC in single-level, anterior cervical corpectomy and fusion (ACCF) by comparing the clinical and radiographic results between the patients with the new type TMC and those with the traditional TMC. Both groups were combined with cervical plate fixation.
Materials and methods
Description of the new type TMC
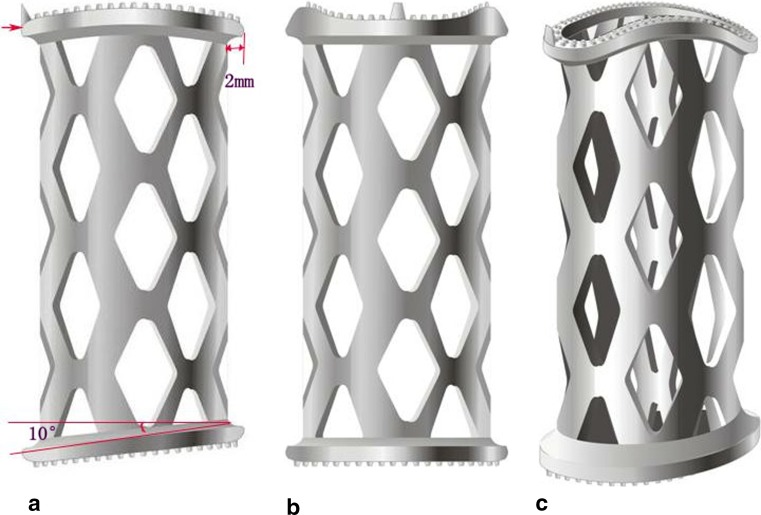
The new type TMC is composed of the hollow reticular cylindrical body which is similar as that of the traditional TMC but incorporates two endcaps. Different from the regular shape of the traditional TMC at both ends, the superior endcap of the new type TMC is curved and its inferior endcap tilts backward and upward with an angle of 10° which conforms to the shape of the adjacent vertebral endplates. To increase the contact surface of the new type TMC with the vertebral endplates, the ring border of its endcaps exceeds the edge of cylindrical body by 2 mm. Moreover, several sizes can be chosen according to the width of the channel after corpectomy. The analog pictures are shown in Fig. 1a–c (Patent no.: ZL 200720070668.9).
Fig. 1.
a The lateral figure of the analog picture of the new type TMC, Large red arrow reflects its curved superior endcap. The other side reflects its inferior endcap which tilts backward and upward with an angle of 10°. Short red line reflects a width of 2 mm of the ring border of its endcaps exceeding the edge of cylindrical body. b and c shows the anteroposterior and oblique figure of the analog pictures of the new type TMC
Patient population
From June 2009 to January 2010, a total of 58 consecutive patients with cervical spondylotic myelopathy (CSM) underwent single-level ACCF using either a new type TMC (designed by us) or the traditional TMC (DePuy, USA). All the patients were treated consecutively and alternately with the new type TMC (group A) or the traditional TMC (group B) according to the sequence of admission. There were 28 patients in group A which included 20 men and 8 women (mean age 61 years; range 47–73 years), and there were 30 in group B which included 20 men and 10 women (mean age 59 years; range 46–76 years). The preoperative diagnoses included cervical degenerative spondylosis (CDS) and isolated ossification of the posterior longitudinal ligament (OPLL). Patients with a non-degenerative pathology, such as infection, tumor, fracture/dislocation, ankylosing spondylitis, and rheumatoid spondylitis, which may lead to osteoporosis, were excluded from the study. Three patients in each group developed neurologic deterioration after low-energy trauma. Indications for single-level ACCF included: (1) lesions extending posteriorly to the vertebra and involving only one vertebra, and (2) neurologic deterioration caused by progression of disease or minor trauma not responding to conservative treatment. The level of corpectomy and fusion was as follows: In group A, C4 in seven cases, C5 in 12 cases, and C6 in nine cases; in group B, C4 in eight cases, C5 in 12 cases, and C6 in ten cases. There were no demographic differences between the two groups (Table 1). All procedures were performed by the senior author (C.D.Y.). All data were collected and reviewed by an independent observer who was not directly involved in the care of the study patients.
Table 1.
Demographic data of the patients
| Group A | Group B | p | |
|---|---|---|---|
| No. of patients | 28 | 30 | |
| Mean age (years) | 60.6 | 59.3 | 0.53 |
| Sex (n) | |||
| Male | 8 | 10 | |
| Female | 20 | 20 | 0.70 |
| Cervical segment of corpectomy | C4 7 C5 12 C6 9 |
C4 8 C5 12 C6 10 |
0.23 |
| Diagnosis | |||
| CDS | 15 | 17 | |
| OPLL | 13 | 13 | 0.81 |
| Type of plate (n) | |||
| Coddman | 4 | 6 | |
| Slimlock | 24 | 24 | 0.82 |
Operative technique
Under general endotracheal anesthesia, a right-sided, Smith–Robinson approach was used to expose the cervical spine. The operative vertebral level was identified radiographically. After necessary discectomies, the central three-fifths of the anterior vertebral body was then excised as far as the posterior longitudinal ligament (PLL). The PLL was removed completely in all cases. All cartilaginous endplates were removed down to the level of the bleeding, subchondral bone with curettes. For patients in group A, it was necessary to decompress the intervertebral spaces bilaterally more than those in group B to easily accommodate the larger endcaps of the new type TMC. A traditional TMC or a new type TMC packed with local bone autograft was then inserted into the decompressive groove under a 2–3 mm intervertebral distraction. Two different types of end caps were employed separately in all patients (the former has a better degree of match than the latter, Fig. 2a, b vs. c, d). Finally, a semi-constrained, cervical plate (Codman or Slimlock, DePuy) was used to bridge the affected segments. All patients were placed in a soft cervical collar for 2 months.
Fig. 2.

a, b Intraoperative fluoroscopy and operative picture in one patient of adopting the new type TMC showed a close contact between the endcaps and the endplates. c, d Intraoperative fluoroscopy and operative pictures in another patient of adopting the traditional TMC showed a poor matching between the endcaps and the endplates
Outcome assessment
We followed the patients a minimum of 30 months postoperatively with radiographs to assess TMC subsidence, cervical sagittal alignment, and fusion status. TMC subsidence was defined as loss of height of the fusion segments on lateral radiographs at 1, 3, 6, 9, and 12 months postoperatively, compared with that at day 1 after the procedure. The height of the fused segments was measured at the midportion of the adjacent superior and inferior endplates [9]. Cervical sagittal alignment was evaluated according to the degrees of C2–C7 Cobb angle [17] and Cobb of fused segments (Fig. 3a, b). Solid fusion was achieved when mature bony trabeculae bridged across the TMC and the adjacent upper and lower endplates. If the fusion was questionable, sagittal reconstructive computed tomography was performed. Neurologic status was assessed using the Japanese Orthopedic Association (JOA) scoring system and the recovery rate. The recovery rate was calculated as follows: (postoperative JOA score–preoperative JOA score)/(17-preoperative JOA score) × 100 % [18]. Neck pain was graded using a 10-point visual analog scale (VAS) [19]. The data were collected and reviewed by an independent, experienced, academic spine surgeon and one radiologist.
Fig. 3.
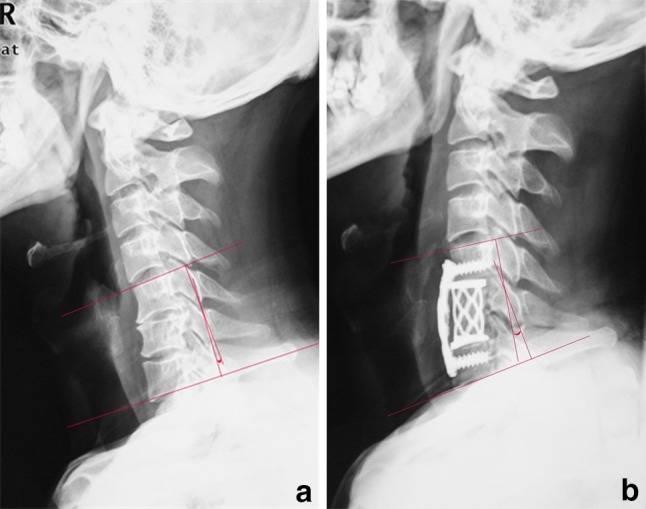
a, b Pre- and postoperative cervical lateral radiographs in a typical case of adopting the new type TMC showed a significantly improved lordosis of the fused segments (Cobb angle, ranged from −3° to 11°) and a close contact between the endcaps and the endplates
Ethical approval
The institutional committee for medical ethics approved the design of the study.
All patients consented to participate in the study.
Statistical analysis
Statistical analyses were performed using SPSS (Mac Version 17.0) (SPSS Inc., Chicago, IL, USA). Student’s t test was used to evaluate mean data, and Pearson’s Chi-squared test was used for categorical data. A p value <0.05 was considered statistically significant. Continuous data are presented as mean ± standard deviation.
Results
No patients were lost to follow-up in this study. Mean duration of follow-up for the 58 patients was 32 ± 2 months for group A and 33 ± 3 months for group B (p = 0.72); the range of follow-up was 30–36 months. The mean loss of height of the fused segments was less in group A than group B (0.8 ± 0.3 vs. 2.8 ± 0.4 mm; p < 0.01) Cases of TMC subsidence were divided into a mild group (<3 mm) and a severe group (≥3 mm), There was a lower rate of severe subsidence in group A (4 %, 1/28) than in the group B (17 %, 5/30) (p < 0.01). The mesh cages subsided into the caudal bodies predominately at the posterior rim of the TMC. Most subsidences occurred 1–4 months postoperatively before bone fusion was achieved; the mean time to bone fusion was 3.8 months in this study. All patients had bone fusion at the final follow-up.
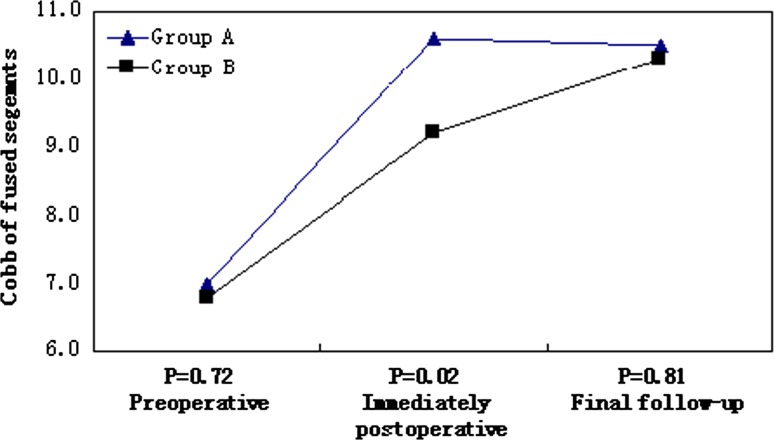
The average preoperative JOA score for group A was 8.3 ± 2.1 (range 6–11) and increased to 13.6 ± 1.8 (range 10–16) at the final follow-up (p < 0.01); the recovery rate of the JOA score was 60 % ± 9 % (Table 2). Similar values were present in group B; the average preoperative JOA score was 8.0 ± 1.9 (range 6–11) and increased to 13.7 ± 2.2 (range 11–16) at the last follow-up (p < 0.01), the recovery rate of the JOA score was 56 % ± 8 %. The preoperative and postoperative JOA scores and the recovery rates did not differ between the two groups (p ≥ 0.56 each).The Cobb of fused segments immediately postoperatively were significantly less in group B than in group A (p < 0.01), but there were no differences between the groups preoperatively or at final follow-up (Fig. 4). The mean preoperative VAS score did not differ for group A and group B (8.6 ± 1.2 vs. 8.4 ± 1.5; p = 0.68). In contrast, the mean postoperative VAS score was greater for group B vs. group A (4.3 ± 1.8 vs. 3.0 ± 1.7; p < 0.05) (Table 2). Also severe subsidence correlated with neck pain (p < 0.05). No cerebrospinal fluid leak, wound infection, or wound hematoma occurred in either group. Screw extrusion occurred in one case in group B with severe TMC subsidence, but no revisional surgery was needed, because bone fusion had been observed after the prolonged neck immobilization.
Table 2.
Clinical outcome and radiographic data
| Group A | Group B | p | |
|---|---|---|---|
| Loss of height of fused segments (mm) | 0.8 ± 0.3 | 2.8 ± 0.4 | <0.01 |
| Rate of severe subsidence (≥3 mm) (%) | 4 | 17 | <0.01 |
| JOA scores | |||
| Preoperative | 8.3 ± 2.1 | 8.0 ± 1.9 | 0.68 |
| Final follow-up | 13.6 ± 1.8 | 13.7 ± 2.2 | |
| p | <0.01 | <0.01 | |
| JOA recovery ratio (%) | 60 ± 8 | 56 ± 9 | 0.56 |
| Cervical pain (VAS) | |||
| Preoperative | 8.6 ± 1.2 | 8.4 ± 1.5 | 0.03 |
| Final follow-up | 3.0 ± 1.7 | 4.3 ± 1.8 | |
| p | <0.01 | <0.01 | |
| Follow-up periods (mo) | 32 ± 2 | 33 ± 3 | 0.72 |
Fig. 4.
Cobb of fused segments at each time point
Discussion
ACCF with TMC is an established operative treatment for degenerative cervical pathologies and offers several advantages, including direct decompression of neural tissue, immediate stability of the anterior column, restoration and maintenance of the intervertebral disk height, enlargement of a stenotic neural foramen, and avoidance of the morbidity at the harvest site of a bone graft [20–23]. TMC subsidence after ACCF, however, the most common implant-related complication, occurs in up to 80 % of patients in the early postoperative period (<6 months) [7–11, 24, 25]. Although the clinical relevance of TMC subsidence remains controversial, theoretically, the acquired advantages of a TMC scaffold could be offset by subsidence due to the loss of intervertebral height, which can lead to buckling of the cervical yellow ligament, foraminal stenosis, and consequent re-compression of spinal cord and nerve roots.
The reported causes of TMC subsidence have included increased patient age and osteopenia, progressive resection of the endplate, the sharp edge of the cage, the removal of the PLL, intervertebral overdistraction, or improper trimming of the cage [12–16]. Several technical measures have also been proposed to reduce or avoid postoperative TMC subsidence, such as avoiding the use of TMC in patients with osteoporosis, appropriate removal of the endplate, avoiding excessive distraction, the use of titanium mesh endcaps, or additional posterior fusion and instrumentation [9, 26]. Although these measures decrease the incidence of TMC subsidence to a certain extent, the shortcomings inherent to the traditional TMC are related to its regular shape at both ends, which fails to match well with the irregular shape of the endplate. The tension band effect in which the majority of the load is transferred from the anterior elements (the plate and anterior portion of the cage) to the posterior elements (the facets and posterior portion of the cage) can occur during activities of neck flexion and extension due to the relatively immobile plate and the mobile posterior elements. This tension band effect results in a piston-type action [27] which can be greatly amplified due to this lack of appropriate matching of the TMC to the endplates of the vertebra, increasing the probability of cage subsidence predominately at the posterior rim of TMC.
Our study suggested that TMC subsidences occurred mainly before bone fusion (<4 months after operation); importantly, the subsidence was less with the group of new type TMC (average, 0.8 mm vs. 2.5 mm). This observation suggests that the new type TMC provides greater stability than the traditional TMC; a similar effect was also shown by our previous biomechanical study using three-dimensional finite element analysis [28]. Our results suggest that the unique shape and a larger diameter endcap allows for an expansion of the contact surface which not only distributes axial stress but also load the periphery of the endplates where there is thicker cortical bone. Interestingly, due to the greater subsidence that occurred with the traditional TMC at the posterior portion of the caudal end plate after operation, there was an improved lordosis of the fused segments at the final follow-up compared to that of the immediate postoperative radiograph in group B. The improved Cobb angles of the fused segments in group B at follow-up are because there was less decrease in the anterior height, which determines the overall height of the segment.
Our study also found that postoperative neck pain was more serious in group B using the traditional TMC; also, subsidence was strongly correlated with neck pain. Although the exact correlation between TMC subsidence and the neck pain is still unclear, theoretically, graft subsidence of the traditional TMC may divert the bulk of the load from the anterior element (plate and anterior portion of graft) to the posterior elements (facet and posterior portion of graft), which is probably what causes the neck pain. Because of the greater loss of height of the fused segments, re-compression of the spinal cord and the nerve roots by the buckling of the ligamentum flavum and stenosis of the neural foramen may also be responsible in part for the neck pain; however, there were no significant differences in neurologic outcomes, and no patients required revisional surgery due to the re-compression of nerve tissues.
Conclusions
According to the findings of our study, the new type of TMC provides comparative clinical results and fusion rates with the traditional TMC for the patients with single-level corpectomy. Moreover, the new type of TMC decreased postoperative subsidence compared to the traditional TMC due to its unique design which matches more closely the vertebral endplate morphology. This new type TMC will also mitigate the severity of subsidence-related neck pain.
Conflict of interest
None.
Footnotes
Y. Fengbin and M. Jinhao contributed equally to this work.
Contributor Information
Yu Fengbin, Phone: +86-057-23269681, FAX: +86-057-23269999, Email: yufengbin1977@sina.com.
Chen Deyu, Phone: +86-021-81886806, FAX: +86-021-63520020, Email: chendyspine@sina.com.
References
- 1.Chagas H, Domingues F, Aversa A, et al. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64(Suppl 1):30–35. doi: 10.1016/j.surneu.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Cheng NS, Lau PY, Sun LK, et al. Fusion rate of anterior cervical plating after corpectomy. J Orthop Surg (Hong Kong) 2005;13:223–227. doi: 10.1177/230949900501300302. [DOI] [PubMed] [Google Scholar]
- 3.Liu JK, Apfelbaum RI, Schmidt MH. Surgical management of cervical spinal metastasis: anterior reconstruction and stabilization techniques. Neurosurg Clin N Am. 2004;15:413–424. doi: 10.1016/j.nec.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Chun HJ, Oh SH, Yi HJ, et al. Efficacy and durability of the titanium mesh cage spacer combined with transarticular screw fixation for atlantoaxial instability in rheumatoid arthritis patients. Spine. 2009;34:2384–2388. doi: 10.1097/BRS.0b013e3181b04f1d. [DOI] [PubMed] [Google Scholar]
- 5.Sevki K, Mehmet T, Ufuk T, et al. Results of surgical treatment for degenerative cervical myelopathy: anterior cervical corpectomy and stabilization. Spine. 2004;29:2493–2500. doi: 10.1097/01.brs.0000145412.93407.c3. [DOI] [PubMed] [Google Scholar]
- 6.Epstein NE. Reoperation rates for acute graft extrusion and pseudarthrosis after one-level anterior corpectomy and fusion with and without plate instrumentation: etiology and corrective management. Surg Neurol. 2001;56:73–780. doi: 10.1016/S0090-3019(01)00523-7. [DOI] [PubMed] [Google Scholar]
- 7.Nakase H, Park YS, Kimura H, et al. Complications and long-term follow-up results in titanium mesh cage reconstruction after cervical corpectomy. J Spinal Disord Tech. 2006;19:353–357. doi: 10.1097/01.bsd.0000210113.09521.aa. [DOI] [PubMed] [Google Scholar]
- 8.Das K, Couldwell WT, Sava G, et al. Use of cylindrical titanium mesh and locking plates in anterior cervical fusion. Technical note. J Neurosurg. 2001;94:174–178. doi: 10.3171/spi.2001.94.1.0174. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Chen D, Guo Y, et al. Subsidence of titanium mesh cage: a study based on 300 cases. J Spinal Disord. 2008;21:489–492. doi: 10.1097/BSD.0b013e318158de22. [DOI] [PubMed] [Google Scholar]
- 10.Kanayama M, Hashimoto T, Shigenobu K, et al. Pitfalls of anterior cervical fusion using titanium mesh and local autograft. J Spinal Disord Tech. 2003;16:513–518. doi: 10.1097/00024720-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Daubs MD. Early failures following cervical corpectomy reconstruction with titanium mesh cages and anterior plating. Spine. 2005;30:1402–1406. doi: 10.1097/01.brs.0000166526.78058.3c. [DOI] [PubMed] [Google Scholar]
- 12.Yan D, Wang Z, Deng S, et al. Anterior corpectomy and reconstruction with titanium mesh cage and dynamic cervical plate forcervical spondylotic myelopathy in elderly osteoporosis patients. Arch Orthop Trauma Surg. 2011;131:1369–1374. doi: 10.1007/s00402-011-1317-2. [DOI] [PubMed] [Google Scholar]
- 13.Hee HT, Madj ME, Holt RT, et al. Complications of multilevel cervical corpectomies and reconstruction with titanium cages and anterior plating. J Spinal Disord. 2003;6:1–8. doi: 10.1097/00024720-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Lim TH, Kwon H, Jeon CH, et al. Effect of endplate conditions and bone mineral density on the compressive strength of the graft-endplate interface in anterior cervical spine fusion. Spine. 2001;26:951–956. doi: 10.1097/00007632-200104150-00021. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Sung JK. Anterior cervical stabilization using a semi-constrained cervical plate and titanium mesh cage for single level corpectomy. Journal of Clinical Neuroscience. 2008;15:1227–1234. doi: 10.1016/j.jocn.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Ying Z, Xinwei W, Jing Z, et al. Cervical corpectomy with preserved posterior vertebral wall for cervical spondylotic myelopathy: a randomized control clinical study. Spine. 2007;32:1482–1487. doi: 10.1097/BRS.0b013e318068b30a. [DOI] [PubMed] [Google Scholar]
- 17.Meyer SA, Wu JC, Mummaneni PV. Laminoplasty outcomes: is there a difference between patients with degenerative stenosis and those with ossification of the posterior longitudinal ligament? Neurosurg Focus. 2011;30:E9. doi: 10.3171/2011.1.FOCUS10279. [DOI] [PubMed] [Google Scholar]
- 18.Hirabayashi K, Watanabe K, Wakano K, et al. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine. 1983;8:693–699. doi: 10.1097/00007632-198310000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Hwang SL, Lee KS, Su YF, et al. Anterior corpectomy with iliac bone fusion or discectomy with interbody titanium cage fusion for multilevel cervical degenerated disc disease. J Spinal Disord Tech. 2007;20:565–570. doi: 10.1097/BSD.0b013e318036b463. [DOI] [PubMed] [Google Scholar]
- 20.Kepler CK, Rawlins BA. Mesh cage reconstruction with autologous cancellous graft in anterior cervical discectomy and fusion. J Spinal Disord Tech. 2010;23:328–332. doi: 10.1097/BSD.0b013e3181aed73c. [DOI] [PubMed] [Google Scholar]
- 21.Eck KR, Bridwell KH, Ungacta FF, et al. Analysis of titanium mesh cages in adults with minimum two-year follow-up. Spine. 2000;25:2407–2415. doi: 10.1097/00007632-200009150-00023. [DOI] [PubMed] [Google Scholar]
- 22.Riew KD, Rhee JM. The use of titanium mesh cages in the cervical spine. Clin Orthop Relat Res. 2002;394:47–54. doi: 10.1097/00003086-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Chuang HC, Cho DY, Chang CS, et al. Efficacy and safety of the use of titanium mesh cages and anterior cervical plates for interbody fusion after anterior cervical corpectomy. Surg Neurol. 2006;65:464–471. doi: 10.1016/j.surneu.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Bilbao G, Duart M, Aurrecoechea JJ, et al. Surgical results and complications in a series of 71 consecutive cervical spondylotic corpectomies. Acta Neurochir (Wien) 2010;152:1155–1163. doi: 10.1007/s00701-010-0660-3. [DOI] [PubMed] [Google Scholar]
- 25.Kim HW, Ryu JI, Bak KH. The safety and efficacy of cadaveric allografts and titanium cage as a fusion substitutes in pyogenic osteomyelitis. J Korean Neurosurg Soc. 2011;50:348–356. doi: 10.3340/jkns.2011.50.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acosta FL, Jr, Aryan HE, Chou D, et al. Long-term biomechanical stability and clinical improvement after extended multilevel corpectomy and circumferential reconstruction of the cervical spine using titanium mesh cages. J Spinal Disord Tech. 2008;21:165–174. doi: 10.1097/BSD.0b013e3180654205. [DOI] [PubMed] [Google Scholar]
- 27.DiAngelo DJ, Foley KT, Vossel KA, et al. Anterior cervical plating reverses load transfer through multilevel strut-grafts. Spine. 2000;25:783–795. doi: 10.1097/00007632-200004010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Chen D, Yang L, et al. Three-dimensional finite elements study of a new titanium mesh cage for bone grafting. J Spinal Surg. 2010;l8:290–294. [Google Scholar]