Abstract
Purpose
Adjacent segment disease (ASD) is an increasing problematic complication following lumbar fusion surgeries. ASD requires appropriate treatment, although there are only few reports on surgery for ASD. This study aimed to clarify surgical outcomes of posterior lumbar interbody fusion (PLIF) for ASD.
Methods
Medical charts of 18 patients who underwent the second (repeat) PLIF for ASD were retrospectively investigated (average follow-up, 40 [27–66] months). Modified Japanese Orthopaedic Association (JOA) score and Whitecloud classification were used as outcome measures.
Results
Mean modified JOA score improved from 7.7 just before repeat PLIF to 11.4 at maximum recovery and declined to 10.2 at final follow-up. Mean recovery rate of modified JOA score was 52.9 % at maximum recovery and 31.6 % at final follow-up. According to Whitecloud classification, 17 patients (94 %) were excellent or good and only 1 was fair at maximum recovery, whereas 10 (56 %) were excellent or good, 6 were fair, and 2 were poor at final follow-up. Eight patients (44 %) deteriorated again because of recurrent ASD. Two poor patients underwent a third PLIF.
Conclusion
PLIF is effective for ASD after PLIF in the short term, although it tends to lead to a high incidence of recurrent ASD.
Keywords: Posterior lumbar interbody fusion (PLIF), Adjacent segment disease (ASD), Additional surgery, Lumbar fusion surgery
Introduction
Adjacent segment disease (ASD) is one of the problematic complications following lumbar spinal fusion, although whether ASD is the result of the normal progression of degenerative changes or biomechanical alteration caused by fusion remains controversial. Within 5 years of lumbar fusion surgery, the clinical incidence of symptomatic ASD is reportedly 5.2–18.5 % [1] and the incidence of additional surgery for symptomatic ASD is reportedly 3.0–11.0 % [2, 3]. A prospective randomized study reported that fusion accelerates degenerative changes at the adjacent segment of fused spine compared with naturally occurring changes [4]. Spinal fusion alters the biomechanics of spinal motion and increases intradiscal pressure or the load on facet joints of the adjacent motion segment of the fused spine [1]. Accordingly, we assume that lumbar fusion surgery would lead to ASD rather than the normal progression of degenerative changes.
The surgical approach to symptomatic ASD remains controversial. Some cases have undergone decompression surgery, whereas others have undergone adjunctive fusion surgery [5–9]. Till date, no study has compared operative procedures for ASD [9]. In principle, we apply the lumbar decompression and fusion procedure for symptomatic ASD, particularly instrumented posterior lumbar interbody fusion (PLIF). Fusion is necessary to eliminate abnormal mechanical changes in the adjacent segment induced by previous lumbar fusion and destabilization caused by an additional decompression procedure itself. Among lumbar fusion techniques, the instrumented PLIF procedure achieves the decompression of traversing nerves and the cauda equina as well as the exiting nerve root of the affected segment and results in the highest fusion rate and excellent clinical results [10–12].
Although many reports have recorded the incidence, reoperation rate, and risk factors of ASD [2, 3, 6, 12–16], few studies have dealt with surgical outcomes of symptomatic ASD [5, 7, 8, 16]. In addition, no report has discussed surgical outcomes of PLIF for ASD. This study aimed to elucidate surgical outcomes of additional PLIF for symptomatic ASD.
Materials and methods
This was a retrospective study of 18 patients who underwent a second (repeat) single-segment L3/4 PLIF for symptomatic ASD (following single-segment L4/5 PLIF) between 2005 and 2009. Study subjects were 6 men and 12 women (mean age at repeat PLIF, 71 [50–83] years; average follow-up period, 40 [27–66] months). Symptomatic ASD was defined as that when the patient showed relief from symptoms after the initial PLIF, then developed neurological symptoms compatible with lesions in the adjacent motion segment as confirmed by magnetic resonance imaging (MRI), and showed at least partial relief from symptoms following the repeat procedure. Bone union was achieved in all of study subjects at L4/5 segment prior to development of newly neurological symptoms. Diagnoses prior to the initial PLIF were degenerative spondylolisthesis (n = 14) and recurrence of lumbar disc herniation (n = 4). The condition of all the patients had deteriorated because of cranial adjacent motion segment degeneration, and they underwent additional surgery for radicular pain or neurological claudication after the failure of conservative treatment. Diagnoses prior to repeat PLIF were spinal canal stenosis (n = 11), degenerative spondylolisthesis (n = 4), and disc herniation (n = 3). The average symptom relief duration following the initial PLIF was 37 (3–144) months, and the average duration between the initial PLIF and repeat PLIF was 48 (7–153) months. Seven patients deteriorated within 1 year following the initial PLIF, whereas seven underwent repeat PLIF within 2 years of the initial PLIF.
Our PLIF procedure was performed using Brantigan I/F cages (DePuy Spine, Inc., Raynham, MA, USA) filled with local bone graft, and posterior instrumentation was performed with pedicle screws. Autografting was performed using local lamina and spinous process bone. After complete removal of intervertebral disc material and cartilaginous endplates, morselized bone was packed and two cages were inserted into the intervertebral space with strut bone block grafts lateral to the cages. Posterolateral fusion (PLF) was not added.
The clinical status of lumbar lesions was assessed by the modified Japanese Orthopaedic Association (JOA) score (Table 1) and Whitecloud classification (Table 2) [8]. The modified JOA score comprises subjective symptoms (low back pain, 3 points; leg pain, 3 points; and ambulatory ability, 3 points) and clinical symptoms (straight-leg-raising test, 2 points; sensory abnormality, 2 points; and motor weakness, 2 points), giving a total score of 15 points for the normal spine. The clinical status was recorded just prior to repeat PLIF, at the time of the maximum recovery and at the final follow-up. If further lumbar surgery was performed after repeat PLIF, the clinical status at the final follow-up was recorded just prior to that operation rather than at the final follow-up.
Table 1.
Modified Japanese Orthopaedic Association (JOA) score
| Subjective symptoms (0–9 pts) | Pts |
|---|---|
| Low back pain | |
| None | 3 |
| Occasionally mild | 2 |
| Always mild or occasionally severe | 1 |
| Always severe | 0 |
| Leg pain or numbness | |
| None | 3 |
| Occasionally mild | 2 |
| Always mild or occasionally severe | 1 |
| Always severe | 0 |
| Ambulant ability | |
| No restriction | 3 |
| Able to walk for more than 500 m, but feel some pain or numbness | 2 |
| Unable to walk for more than 500 m | 1 |
| Unable to walk for more than 100 m | 0 |
| Clinical symptoms (0–6 pts) | Pts |
|---|---|
| Straight-leg-raising test | |
| >80 | 2 |
| >30, <80 | 1 |
| <30 | 0 |
| Sensory abnormality | |
| Normal | 2 |
| Slight hypesthesia or hypalgesia | 1 |
| Severe hypesthesia or hypalgesia | 0 |
| Motor weakness | |
| MMT 5 | 2 |
| MMT 4 | 1 |
| MMT < 3 | 0 |
The total score is 15 points
Pts points, MMT manual muscle test
Table 2.
Whitecloud classification
| Category | Criteria |
|---|---|
| Excellent | No symptoms, except for occasional back pain; no medications required; and return to work |
| Good | Marked improvement in symptoms, occasional pain, the need for occasional pain medication, minimal functional limitations, and return to work, although not at the same job activity |
| Fair | Some improvement, the need for pain medications, functional restrictions |
| Poor | No change in symptoms or worsening of the patient’s condition |
To evaluate the risk factors of recurrent ASD, the radiological lumbar status was evaluated. The L2/3 disc degeneration was graded by MRI according to Pfirrmann classification [17] before repeat PLIF, and lumbar lordosis angle of T12/S1 and L3/5 was measured by sagittal section on the computed tomography just after repeat PLIF. Statistical analysis was performed using JMP 10 (SAS Institute Inc., Cary, NC, USA). A p < 0.05 was considered to be statistically significant.
Results
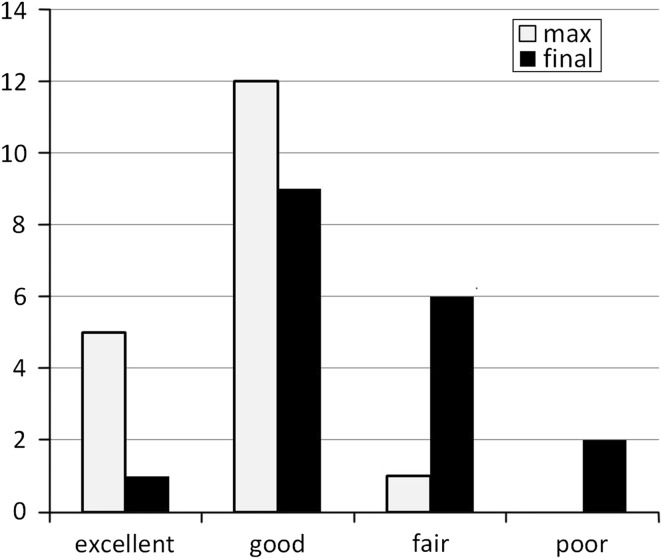
No immediate postoperative neurological complications were observed, although an incidental dural tear was observed in one patient. Successful bone union was achieved in all the 18 patients at the final follow-up. The mean modified JOA score had improved from 7.7 (4–11) just prior to repeat PLIF to 11.4 (7–15) at the time of the maximum recovery and had slightly declined to 10.2 (5–13) at the final follow-up. The duration between repeat PLIF and the latest date of the maximum recovery was 30 (3–60) months. The recovery rate of the modified JOA score was 52.9 % (14–100 %) at the time of the maximum recovery and 31.6 % (−25 to 75 %) at the final follow-up. According to Whitecloud classification, 5 patients were excellent, 12 were good, 1 was fair, and none was poor at the maximum recovery date, whereas only 1 patient was excellent, 9 were good, 6 were fair, and 2 were poor at the final follow-up (Fig. 1). Eight of the 18 patients (44 %) deteriorated because of recurrent ASD, which was confirmed by plain radiography and MRI. Of these 8 patients, 2 underwent a third PLIF with pedicle screws, 3 patients improved conservatively with medication or epidural block, and the condition of 3 was maintained by medication or they refused further surgery. The mean duration between repeat PLIF and the onset of deterioration was 24 (11–39) months. Although one of the patients who underwent the third PLIF was classified as good according to Whitecloud classification, the other suffered from severe leg numbness and urge incontinence secondary to epidural hematoma.
Fig. 1.
Surgical outcomes for repeat posterior lumbar interbody fusion (PLIF) according to Whitecloud classification
The mean age of the patients with recurrent ASD was 71 (58–80) (3 males and 5 females), and that without recurrent ASD was 71 (50–83) (3 males and 7 females). There was no statistical difference in age (p = 0.92, t test) and gender ratio (p = 0.74, Chi-test) between two groups. The mean lordosis angle of T12/S1 was −33 (−40 to −23) in patients with recurrent ASD, whereas that was −35 (−53 to −5) without recurrent ASD. The mean lordosis angle of L3/5 was −16 (−26 to 4) in patients with recurrent ASD, whereas that was −24 (−35 to −9) without recurrent ASD. There was no statistical difference in the lordosis angle of T12/S1 (p = 0.77, t test) and L3/5 (p = 0.09, t test) between two groups. In evaluation of magnetic resonance classification of lumbar intervertebral disc degeneration by Pfirrmann, there were 2 Grade III, 3 Grade IV and 2 Grade V in patients with recurrent ASD, whereas there were 1 Grade III, 5 Grade IV, and 1 Grade V without recurrent ASD.
Discussion
Since the initiation of PLIF for lumbar disc herniation by Cloward, this procedure has been reinforced by instrumentation such as pedicle screws and intervertebral spacers and applied for various clinical conditions, particularly lumbar instability. Many authors have reported excellent clinical outcomes as well as high fusion rates after PLIF [10, 11], indicating that PLIF is an appropriate procedure for treating an abnormal mobile segment. Although transforaminal lumbar interbody fusion (TLIF) is also reported to produce high fusion rate and excellent clinical outcomes [18, 19], the additional fusion needs enough rigidity because of more stress by the longer lever arm. PLIF has more advantage in achieving solid fusion by removing intervertebral material and cartilaginous endplates through bilateral wide annulotomy and harvesting more amount of local autograft. Accordingly, we employed PLIF for ASD, which is assumed to be triggered by abnormal mechanical stresses following the initial PLIF.
Short-term clinical results of repeat PLIF in ASD were equivalent to those of the initial PLIF. Seventeen patients (94 %) were classified as either excellent or good at the time of the maximum recovery. However, 8 patients (44 %) had deteriorated over an average duration of 2 years. In addition, at the final follow-up, only 10 patients (56 %) were classified as either excellent or good, whereas 2 patients (11 %) required a third PLIF for recurrent ASD. The deterioration rate for repeat PLIF (44 %) is obviously higher than that for the initial PLIF, which is reportedly 5.2–18.5 % over 5 years [1], and this problem was triggered by recurrent ASD. Biomechanical studies have demonstrated increased intradiscal pressure at the adjacent segment in double-level fusion than in single-level fusion [1], and this is one reason why repeat PLIF leads to a higher incidence of ASD than the initial PLIF. In addition, we suggest another reason. The rate of additional surgery for ASD following double-level PLIF was reportedly 5–10 % [20, 21], which is lower than that following repeat PLIF for ASD (11 %) in the present study. Deyo et al. [22] reported in their study of 31,543 patients with surgery for lumbar stenosis that previous spinal surgery was the strongest risk factor for repeat surgery and that the hazard ratio for this was 1.58. These results suggest that patients undergoing repeat PLIF for ASD would incur more risk factors for additional surgery than those undergoing single- or double-level PLIF as the initial surgery. In most of our subjects, L2/3 disc degeneration was Grade IV or V. Pre-existing lumbar disc degeneration in adjacent segment was reported to be a risk factor for ASD [1, 13, 15]. Furthermore, mean age at repeat PLIF was 71. Age was also reported to be a major risk factor for ASD [1, 2, 14]. These factors might lead to high recurrence rate of ASD in our study.
Outcomes for repeat PLIF are not inferior to those of other surgical procedures for ASD, although no study has compared this surgical procedure with others till date [9]. Some surgeons have reported good to excellent results for 57.7–64.2 % of patients undergoing only decompression for ASD [7, 16]. In contrast, in another study, 50 % of the decompression surgery group required repeat surgery for restenosis at an average interval of 10 months [23]. In one systemic review, the extension of fusion after decompression was reported to be associated with better outcomes than decompression alone [1]. Other studies have reported that 36.0–76.9 % of patients undergoing PLF for ASD showed good to excellent results [5, 8, 16], although 12.8 % of them again developed ASD and 5.1 % required additional fusion surgery for recurrent ASD within 5 years [5]. In the present study, 44 % of the patients undergoing PLIF for ASD developed recurrent ASD and 11 % required additional surgery. These reports may suggest that PLIF for ASD is inferior to PLF for ASD in terms of the incidence of recurrent ASD. Although the rigidity achieved by interbody fusion may increase the risk of ASD [1], the outcome measures, follow-up period, primary fusion procedure and patients’ age differ among the abovementioned studies. Particularly subjects in our study group were 10 years older than that in PLF study group, whereas age is a major risk factor supported by multiple studies [1, 2, 14]. Therefore, a prospective comparative study of PLIF for ASD and PLF for ASD would be appropriate.
The treatment of ASD confronts a dilemma because decompression alone seems to be inferior to fusion surgery sometimes requiring additional fusion surgery, whereas fusion surgery brings better outcomes after operation sometimes followed by recurrent ASD. To solve this dilemma, firstly, indication of lumbar fusion surgery at initial operation should be rigorously selected, which will reduce the occurrence of ASD itself most surely. Secondly, surgeries such as less invasive surgery [24] or motion preservation surgery [25] should be imported to avoid iatrogenic biomechanical alteration, although it is not clear what kind of less invasive surgery has clinical impact to reduce symptomatic ASD so far.
In conclusion, repeat PLIF is effective for ASD in the short term, although it tends to lead to a high incidence of recurrent ASD. Because lumbar fusion surgeries have been increasingly performed in recent years, spinal surgeons must address the issue of the increased incidence of ASD. A comparative study of the treatment of ASD, including decompression alone, PLF, PLIF, motion preservation surgery and even conservative treatment is necessary to determine the optimal surgical procedure and to tackle the vicious circle of ASD.
Conflict of interest
None.
References
- 1.Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine. 2004;29:1938–1944. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 2.Cho KS, Kang SG, Yoo DS, Huh PW, Kim DS, Lee SB. Risk factors and surgical treatment for symptomatic adjacent segment degeneration after lumbar spine fusion. J Korean Neurosurg Soc. 2009;46:425–430. doi: 10.3340/jkns.2009.46.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sears WR, Sergides IG, Kazemi N, Smith M, White GJ, Osburg B. Incidence and prevalence of surgery at segments adjacent to a previous posterior lumbar arthrodesis. Spine J. 2011;11:11–20. doi: 10.1016/j.spinee.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Ekman P, Moller H, Shalabi A, Yu YX, Hedlund R. A prospective randomized study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J. 2009;18:1175–1186. doi: 10.1007/s00586-009-0947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen WJ, Lai PL, Niu CC, Chen LH, Fu TS, Wong CB. Surgical treatment of adjacent instability after lumbar spine fusion. Spine. 2001;26:E519–E524. doi: 10.1097/00007632-200111150-00024. [DOI] [PubMed] [Google Scholar]
- 6.Gillet P. The fate of the adjacent motion segment after lumbar fusion. J Spinal Disord Tech. 2003;16:338–345. doi: 10.1097/00024720-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine. 1996;21:970–981. doi: 10.1097/00007632-199604150-00013. [DOI] [PubMed] [Google Scholar]
- 8.Whitecloud TS, Davis JM, Olive PM. Operative treatment of the degenerated segment adjacent to a lumbar fusion. Spine. 1994;19:531–536. doi: 10.1097/00007632-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chou D, Dekutoski M, Hermsmeyer J, Norvell DC. The treatment of lumbar adjacent segment pathology after a previous lumbar surgery: a systematic review. Spine. 2012;37:S180–S188. doi: 10.1097/BRS.0b013e31826d613d. [DOI] [PubMed] [Google Scholar]
- 10.Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system. Spine. 2000;25:1437–1446. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 11.Kasis AG, Marshman LAG, Krishna M, Bhatia CK. Significantly improved outcomes with a less invasive posterior lumbar interbody fusion incorporating total facetectomy. Spine. 2009;34:572–577. doi: 10.1097/BRS.0b013e3181973e35. [DOI] [PubMed] [Google Scholar]
- 12.Okuda S, Iwasaki M, Miyauchi A, Aono H, Morita M, Yamamoto T. Risk factors for adjacent segment degeneration after PLIF. Spine. 2004;29:1535–1540. doi: 10.1097/01.BRS.0000131417.93637.9D. [DOI] [PubMed] [Google Scholar]
- 13.Anandjiwala J, Seo JY, Ha KY, Oh IS, Shin DC. Adjacent segment degeneration after instrumented posterolateral lumbar fusion: a prospective cohort study with a minimum five-year follow-up. Eur Spine J. 2011;20:1951–1960. doi: 10.1007/s00586-011-1917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen BL, Wei FX, Ueyama K, Xie DH, Sannnohe A, Lui SY. Adjacent segment degeneration after single-segment PLIF: the risk factor for degeneration and its impact on clinical outcomes. Eur Spine J. 2011;20:1946–1950. doi: 10.1007/s00586-011-1888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CS, Hwang CJ, Lee SW, Ahn YJ, Kim YT, Lee DH, Lee MY. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18:1637–1643. doi: 10.1007/s00586-009-1060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips FM, Carlson GD, Bohlman HH, Hughes SS. Results of surgery for spinal stenosis adjacent to previous lumbar fusion. J Spinal Disord. 2000;13:432–437. doi: 10.1097/00002517-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Pfirrmann CWA, Metzdorf A, Zanetti M, Jodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 18.Mura PP, Costaglioli M, Piredda M, Caboni S, Casula S. TLIF for symptomatic disc degeneration: a retrospective study of 100 patients. Eur Spine J. 2011;20(suppl 1):S57–S60. doi: 10.1007/s00586-011-1761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logroscino CA, Proietti L, Pola E, Scaramuzzo L, Tamburrelli FC. A minimally invasive posterior lumbar interbody fusion for degenerative lumbar spine instabilities. Eur Spine J. 2011;20(suppl 1):S41–S45. doi: 10.1007/s00586-011-1762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hioki A, Miyamoto K, Kodama H, Hosoe H, Hishimoto H, Sakaeda H, Simizu K. Two-level posterior lumbar interbody fusion for degenerative disc disease: improved clinical outcome with restoration of lumbar lordosis. Spine J. 2005;5:600–607. doi: 10.1016/j.spinee.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Sakaura H, Yamashita T, Miwa T, Suzuki S, Ohzono K, Ohwada T. Clinical outcome of two-level posterior lumbar interbody fusion for two-level degenerative lumbar spondylolisthesis. J Spine Res. 2011;3:741. [Google Scholar]
- 22.Deyo RA, Martin BI, Kreuter W, Jarvik JG, Angier H, Mirza SK. Revision surgery following operation for lumbar stenosis. J Bone Joint Surg Am. 2011;93:1979–1986. doi: 10.2106/JBJS.J.01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoji H, Yamazaki A, Katsumi K, Ohashi M, Suhara Y, Sato Y (2011) Operative treatment of adjacent segment disease after lumbar spinal fusion–comparison between decompression and fusion. In: Proceedings for annual meeting of Japanese spinal instrumentation society, 141
- 24.Fan SW, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19:316–324. doi: 10.1007/s00586-009-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morishita Y, Ohta H, Naito M, Matsumoto Y, Huang G, Tatsumi M, Takemitsu Y, Kida H. Kinematic evaluation of the adjacent segments after lumbar instrumented surgery: a comparison between rigid fusion and dynamic non-fusion stabilization. Eur Spine J. 2011;20:1480–1485. doi: 10.1007/s00586-011-1701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]