Abstract
Purpose
To understand the relative histopathological effects of PEEK particulate debris when applied within the epidural versus the intervertebral disc space. We hypothesized that due to the avascular nature of the intervertebral disc acting as a barrier to immune cells, the intradiscal response would be less than the epidural response.
Methods
The inflammatory effects of clinically relevant doses (3 mg/5-kg rabbit) and sizes (1.15 µm diameter) of PEEK implant debris were assed when placed dry on epidural and intradiscal tissues in an in vivo rabbit model. The size of the particulate was based on wear particulate analysis of wear debris generated from simulator wear testing of PEEK spinal disc arthroplasty devices. Local and systemic gross histology was evaluated at the 3- and 6-month time points. Quantitative immunohistochemistry of local tissues was used to quantify the common inflammatory mediators TNF-α, IL-1β, and IL-6.
Results
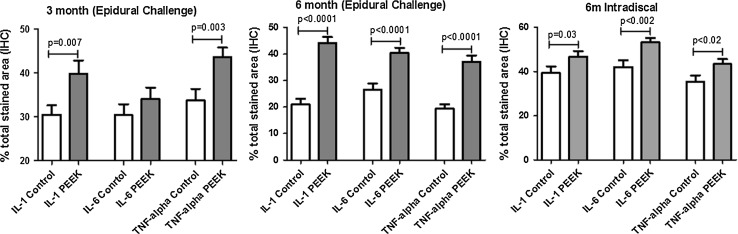
Both treatments did not alter the normal appearance of the dura mater and vascular structures; however, limited epidural fibrosis was observed. Epidural challenge of PEEK particles resulted in a significant (30 %) increase (p < 0.007) in TNF-α and IL-1β at both 3 and 6 months compared to that of controls, and IL-6 at 6 months (p < 0.0001). Intradiscal challenge of PEEK particles resulted in a significant increase in IL-1β, IL-6 and TNF-α at 6-months post-challenge (p ≤ 0.03). However, overall there were only moderate increases in the relative amount of these cytokines when compared with surgical controls (10–20 %). In contrast, epidural challenge resulted in a 50–100 % increase.
Conclusions
The results of this study are similar to past investigations of PEEK, whose results have not been shown to elicit an aggressive immune response. The degree to which these results will translate to the clinical environment remains to be established, but the pattern of subtle elevations in inflammatory cytokines indicated both a mild persistence of responses to PEEK debris, and that intradiscal implant debris will likely result in less inflammation than epidural implant debris.
Keywords: PEEK, Wear particles, Biocompatibility, Disc arthroplasty, Immune response
Introduction
The survival of total joint arthroplasty has been restrained primarily by the generation of wear debris and its subsequent biologic sequela, aseptic loosening caused by wear particle-mediated inflammation and an osteolytic cascade [1–5]. Thus, given the historical development parallels, the increasing role of disc arthroplasty devices as an alternative to fusion remains a clinical concern [6–8]. Although disc arthroplasty has over a 20-year clinical history, much remains unknown particularly when compared with the understanding of large joint arthroplasty. As a consequence, there is lack of scientific evidence regarding the bioreactivity and potential consequence of an adverse host response to these devices within and around the spinal environment.
The articulating materials for disc arthroplasty devices have predominantly been metal–polymer or metal–metal [9]. However, early simulator studies have shown the potential of other candidate materials, such as poly-ether-ether-ketone (PEEK) [10, 11], as a wear resistant alternative to these materials for articulating bearing surfaces. Consequently, self-mating PEEK implants are now in clinical use in the form of lumbar nucleus replacement [12] and cervical total disc arthroplasty devices [13]. However, only recently has the proinflammatory potential of implantable grade PEEK been evaluated, where human monocyte/macrophage responses to PEEK particles generally demonstrated similar inflammatory cytokine responses when compared with UHMWPE [14], with similar results reported for titanium with regard to osteoblast activity [15]. However, given the relative novelty of self-mating PEEK as a bearing material in spine applications, it remains critically important to evaluate the potential inflammatory reactivity of PEEK wear debris. Past study of metal and polymer particulates within the epidural space has demonstrated increased inflammatory reactivity (e.g., persistent increases in cytokines such as IL-6 and TNF-α), increased osteoclastic activity and cellular apoptosis [16, 17]. Clinical case reports of total disc replacements have shown elevated serum metal ions [18], gross metallosis [19] and what appears to be elevated immune responses [20, 21]. However to date, few investigations have reported on the peri-spine effects of PEEK particles [22], and none have reported the inflammatory effects associated with PEEK particle challenge intradiscally or with clinically relevant sizes and dose of particles.
The current study seeks to elucidate the relative histopathological effects and assesses the local and systematic histological response of PEEK particulate implanted within the epidural versus intervertebral disc space. We hypothesized that clinically relevant amounts and sizes of PEEK implant debris from spinal disc arthroplasty devices would be more reactive when placed epidurally versus intradiscally due to the added barriers to immune cells (e.g., macrophages) given the avascular nature of the intervertebral disc. This hypothesis was tested using an in vivo rabbit model to investigate the possible histopathological effects and assess the local and systematic histological response of PEEK particulate implanted within the epidural and intradiscal spaces.
Methods
Preparation of particulate
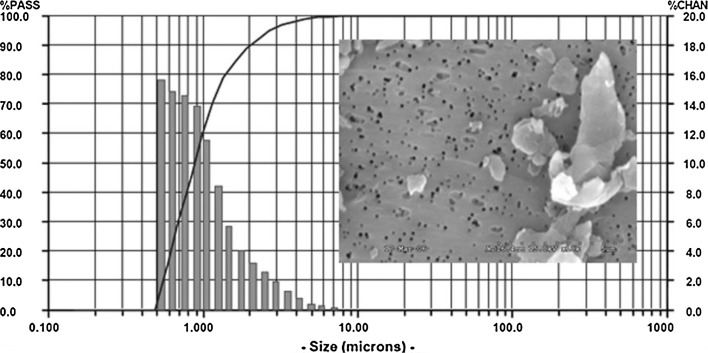
Commercially available particles (BioEngineering Solutions, Oak Park, IL) were produced from implant-grade non-sterilized PEEK bar stock (PEEK-OPTIMA LT1, Invibio Biomaterial Solutions) using proprietary techniques involving custom cryomilling and cryopulverization in liquid nitrogen, suspended in distilled water and analyzed via laser light diffraction and scanning electron microscope analysis (ASTM 1877-05). Several past investigations have shown that for a given amount of mass, smaller particles (e.g., <1 μm) generally produce greater inflammation than larger particles (e.g., 10 μm), because there is a much higher dose (e.g., for 1 μm vs. 10 μm diameter particles = over 1000 × the total amount of particle dose) [23–25]. The mean particle size used in this study was 1.15 ± 0.51 μm with a range from 0.5 to 70.0 μm diameter with 99 % ≤5.0 μm diameter (Fig. 1), where particle size/diameter is defined here as equivalent spherical diameter (ECD) based on the average diameter of an equivalent circular cross-sectional area. Because particles of ≤5.0 μm have been found to be readily phagocytosable by local immune cells, i.e., macrophages, this size range is considered to be more bioreactive and clinically relevant [26] compared to particulate debris <10 μm, which are still phagocytosable by macrophages, but not as clinically prevalent or important for articulating implants [1, 7, 14, 23–25]. All particles used in this study had an aspect ratio of approximately 1.5, similar to particles found in vivo [17, 23–25]. What exactly are the most bioreactive particle sizes remains controversial; thus the size and dose in this study were determined to be clinically relevant based on published data from wear simulator studies of self-mating PEEK devices [10, 11, 27, 28]. These studies showed >95 % of the wear particles were <5.0 μm on a number basis, with average sizes from 0.7 to 2.8 μm ECD, and that the wear rates varied from approximately 0.26 to 1.27 mm3/million cycles [10, 11, 27, 28]. Prior to implantation, the PEEK samples were packaged dry in vials to deliver approximately 3 mg PEEK particles (epidural or intradiscal) and ETO sterilized. Prior to packaging, the particles were endotoxin cleaned (Pyroclean™) and tested to be free of endotoxin using Kinetic Quantitative Colorimetric LAL (QCL) testing for endotoxin analysis (<0.05 EU/ml).
Fig. 1.
The PEEK particulate size number-based distribution using low-angle laser light scattering (LALLS) shows >99 % of the particles <10 µm and >95 % of particles <5 µm (ECD). Flake-like and granular particles can be seen in the corresponding SEM image. Size = equivalent spherical diameter, based on cross-sectional area (microns), %PASS = the cumulative % of particles below each size (line) and %CHAN = the relative % of particles within each size range (bars)
Surgery
After approval by the Institutional Animal Care and Use Committee (IACUC), 30 skeletally mature New Zealand white rabbits (approximately, 5 kg) were assigned to three groups: epidural, epidural control and intradiscal (Table 1). Following normal health status determination, each animal was sedated with subcutaneous injections of ketamine (35 mg/kg), xylazine (5 mg/kg) and acepromazine (0.75 mg/kg) anesthetic medications, followed by endotracheal intubation and general anesthesia using 0.5–1.0 % isoflurane.
Table 1.
Summary of treatment groups
| Treatment groups | 3 months | 6 months |
|---|---|---|
| Group 1—epidural PEEK particulate | 1A (n = 5) | 1B (n = 5) |
| Group 2—epidural control | 2A (n = 5) | 2B (n = 5) |
| Group 3—intradiscal PEEK and control | 3A (n = 5) | 3B (n = 5) |
For Groups 1 and 2, a single midline skin incision of approximately 5 cm in length was centered over the L6L7 operative level. The L7 spinous process and L6L7 supraspinous/interspinous ligament were then exposed using a periosteal elevator and electrocautery, as necessary. The L7 spinous process and ligamentum flavum at L6L7 was excised, permitting interlaminar exposure of the dural sac. The membranous coverings and neural structures of the spinal canal were then accessible (Fig. 2). For Group 1, 3.0 mg of PEEK particulate was implanted in sterile, dry format in ten animals (Fig. 2). The remaining ten served as operative controls (Group 2) and consisted of epidural exposure alone and saline injection.
Fig. 2.
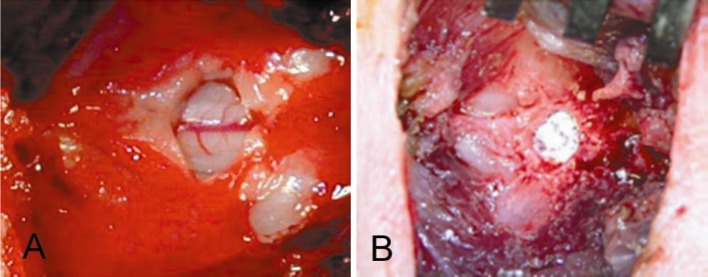
Epidural exposure (a) and application of 3.0 mg of PEEK particulate in dry format (b)
For Group 3, surgical exposure consisted of a 4- to 5-cm incision beginning at the iliac crest and extending along the palpable borders of the lumbar transverse processes. The anterolateral aspects of the L2L3 and L4L5 vertebral bodies and intervertebral discs were exposed leaving the L3L4 disc intact. The annulus fibrosis and nucleus pulposus were then perforated using an 18-gauge needle. This was followed by implantation of 3.0 mg PEEK particulate in dry, sterile format at the L4L5 experimental level (Fig. 3). The L2L3 intervertebral disc level was perforated using the same surgical procedure and served as a control.
Fig. 3.
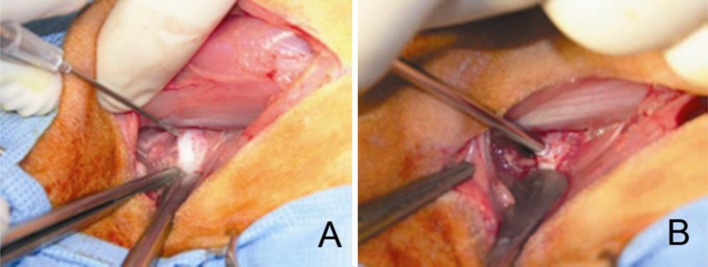
Annular perforation using an 18-gauge needle (a) followed by application of 2.0 mg of PEEK particulate in dry format (b)
For wound closure, the muscles and fascia were approximated using 2-0 Vicryl and the skin closed with staples. All animals were observed and kept on antibiotics for a period of 10 days. Particular attention was given to the surgical site with an emphasis on wound healing and signs of infection. All animals were carefully monitored for signs of neurologic dysfunction, severe pain or other adverse reactions to the surgical procedure throughout the course of the study.
At the appropriate time interval, the animals were killed using an overdose (150 mg/kg) of concentrated pentobarbital solution (390 mg/ml). The spinal cord and operative disc levels were carefully removed and prepared for routine histological and immunocytochemical analyses. A total of ten organs and lymph node structures from the animal’s reticuloendothelial and systemic system were harvested and placed in 10 % neutral buffered formalin solution. These consisted of the axillary, periaortic and mesenteric lymph nodes, liver, lung, kidney, spleen, pancreas, heart and spinal cord.
Histology/immunohistochemistry preparation
The spinal cord and overlying tissue band was processed to quantify the levels of local cytokines using standard immunocytochemistry techniques. The specimens were trimmed and excess bone fragments were removed to produce a 2-cm2 piece of tissue. Tissues were then placed in cassettes and covered with OCT embedding compound, placed in cooled isopentane, sectioned with a cryostat, fixed in anhydrous acetone for 20 s and stored at −70 °C. The endogenous peroxides within the samples were blocked with peroxide (H202) using a two-step method. Using primary and secondary antibodies combined with the avidin–biotin complex (ABC)-horseradish peroxidase technique, macrophage intracellular and membrane-bound TNF-a, IL-1b, and IL-6 cytokines were stained for and included the following antibodies: anti-rabbit TNF-a, IL-1b, and IL-6 (SantaCruz Biochem, Santa Cruz, CA).
Systemic tissue analysis
For all animals, the soft tissue organs and structures were resected, sectioned, and prepared by a veterinarian pathologist. The specimens were fixed in a 10 % formalin solution and subjected to routine paraffin processing and slide preparation. Using thin-sectioning microtomy, the paraffin-embedded sections were cut 3–5 μm thick, and then slide mounted and stained using standard hematoxylin and eosin (H&E). Pathological assessment for all tissues included, but was not limited to, comments on the architecture of the tissues, presence of wear debris, osteolysis as well as any signs of particulate debris, foreign body giant cell/granulomas inflammatory reactions, degenerative changes, or autolysis.
Intervertebral discs
For Group 3, the operative and control intervertebral discs and adjacent vertebral bodies were harvested, prepared using decalcified technique, embedded in paraffin, and sectioned along the mid-sagittal plane. Specimens then underwent routine H&E staining.
Quantitative immunohistochemistry (IHC)
The spinal cord and overlying tissue layer were processed to quantitatively evaluate the levels of local cytokines using immunohistochemical (IHC) and image processing for TNF-α, IL-1b, and IL-6, challenged both epidurally and intradiscally. Non-treated controls were compared with treated animals at 3- and 6-month time points for both epidural and intradiscal PEEK particle challenge. Due to practical experimental limitations, intradiscal challenge with PEEK particles was only conducted in a 6-month group of animals (n = 10 treated segments and n = 10 non-treated distal segments). Controls for the intradiscal challenge were at the adjacent disc in the same animal. Quantitative IHC was performed using two different techniques for corroborative purposes, where Method 1 measured the relative amount of staining by manually selecting random sections along the tissue border and then conducting image analysis, and Method 2 utilized an automated histology image analysis of the entire section, as described below.
IHC method 1
Quantitative IHC analysis was conducted using previously reported quantitative IHC techniques [17]. Briefly for each cytokine/time point/control, five slides per tissue specimen were analyzed from five randomly selected microscope (of the sample periphery) fields per slide resulting in 25 fields per tissue sample (at 200×) that were used to quantify local cytokines (Scion Image, Scion Corporation, Frederick, MD). The area of staining was compared to the total to yield a percentage of stained area.
IHC method 2
Validation of the 6-month quantitative IHC results using the above technique was performed using the BioQuant Image Analysis System (R&M Biometrics, Nashville, TN), where one section per specimen was analyzed using 50 consecutive fields per sample at 200× magnification. At this magnification and field number, the entire soft tissue region overlying the dura and spinal cord itself was evaluated.
Statistical analysis
All data were calculated as mean ± one standard deviation. Standard two-tailed t testing was used to determine significance (p < 0.05) in cytokine expression (after normality was established using both Kolmogorov–Smirnov and D’Agostino and Pearson normality testing) between independent rabbit groups at each time point (3 and 6 months).
Results
General
There was no incidence of intraoperative or perioperative neurologic, infectious, or vascular complications in any of the 30 cases. All animals were characterized as having a normal recovery throughout the 3- and 6-month postoperative periods. By 24 h postoperatively, all animals were fully ambulating and demonstrating normal behavior. Gross histopathology of the spinal cord for Group 1 was compared to Group 2 at 3 and 6 months. Both treatments showed normal appearance of the dura mater and vascular structures, with limited epidural fibrosis. At the time of scheduled necropsy, there were no gross signs of infection in any animals. For Group 3, there was no gross evidence of significant histopathological changes in the local tissues.
Systemic tissue analysis
Histopathologic analysis of the systemic tissues at the 3- and 6-month intervals indicated no significant pathologic changes induced in either the control or PEEK experimental treatments when observing tissue sections of the axillary, periaortic and mesenteric lymph nodes, and the liver, lung, kidney, spleen, pancreas, heart, or spinal cord. Pathological assessment characterized all systemic organs and organ systems as having normal tissue architecture, without the presence of foreign body materials, foreign body giant cell, granulomatous inflammatory reactions, degenerative changes, or autolysis. There was no evidence of PEEK particulates in the systemic tissues analyzed.
Histomorphometric analyses: epidural and control groups
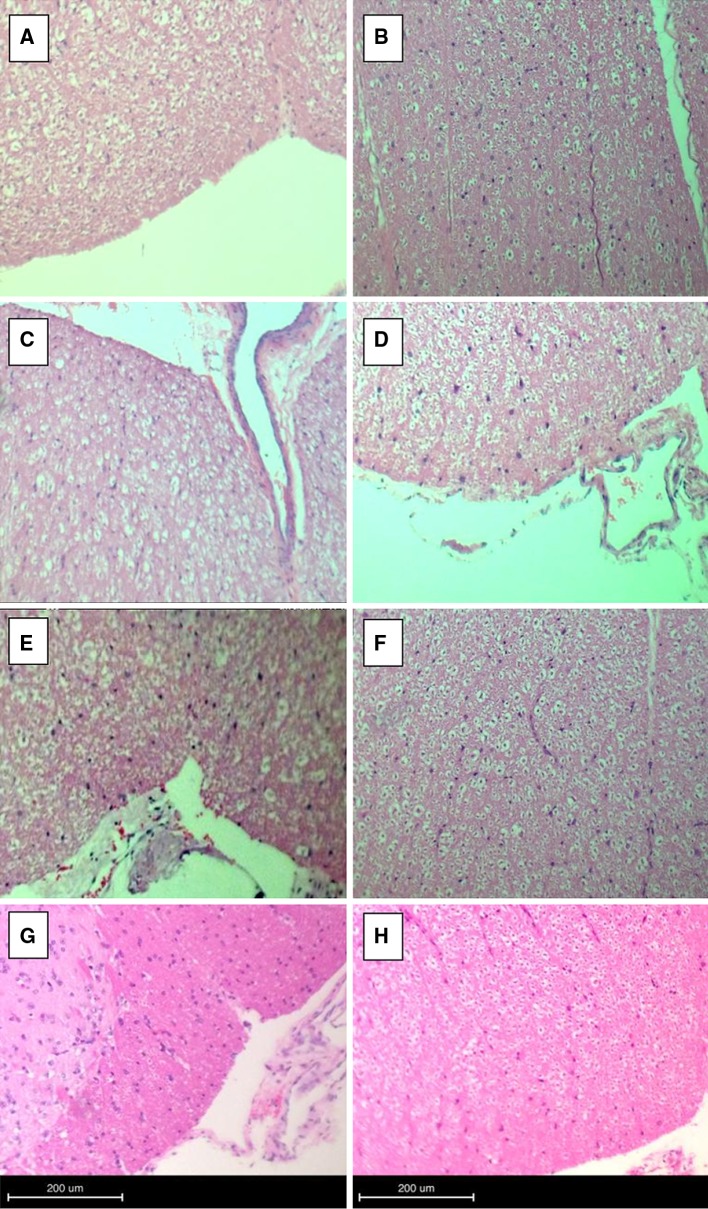
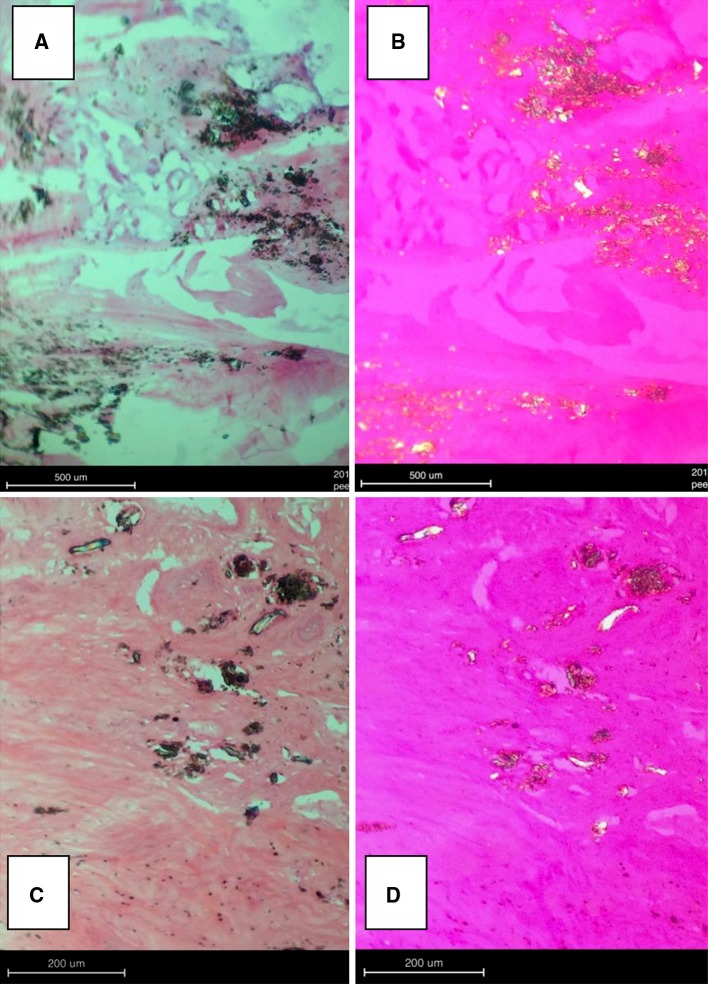
Plain and polarized light microscopy of the histologic cross sections from the control and experimental PEEK groups indicated a very mild reaction at the dura mater and spinal cord. The surgical procedure itself resulted in increased concentrations of cytokines and macrophages; however, the extent of epidural fibrosis was limited when compared with the experimental group (Fig. 4). Based on plain and polarized light microscopy, PEEK particles were visible in 10/10 (100 %) of the epidural treatments for the PEEK groups (Fig. 5a, b). The H&E stains indicated normal distribution of myelin and the intracellular neurofibrilla network, and characterized both treatments as without significant pathological changes at both the 3- and 6-month time intervals. There was no evidence of giant cell reaction or other significant pathological changes for either the control or PEEK groups.
Fig. 4.
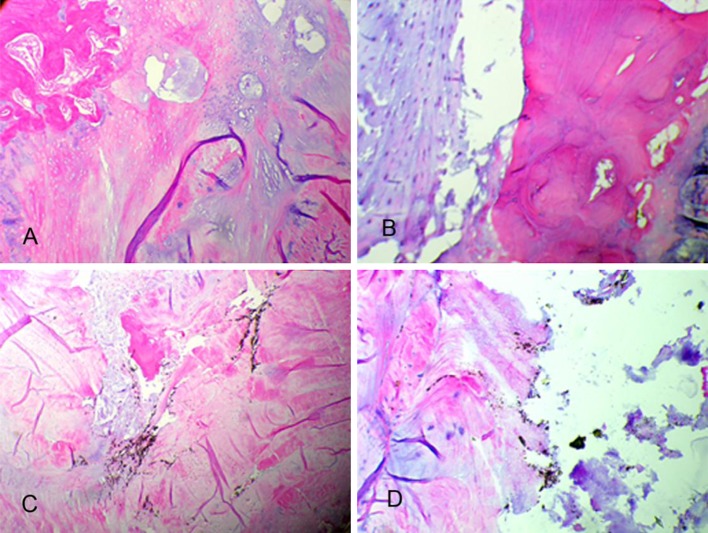
Plain light microscopy demonstrating spinal cord histologic cross sections from representative 3- and 6- month epidural (a, b), 3- and 6-month intradiscal (c, d), and representative 3- and 6-month epidural control (e, f) and intradiscal control (g, h). No significant pathological changes are observed in either case (H&E stain)
Fig. 5.
PEEK particles were visible in 10/10 (100 %) of the epidural (a, b) and intradiscal (c, d) PEEK treatment groups as verified by plain (left) and polarized (right) light microscopy
Histomorphometric analyses: intradiscal group
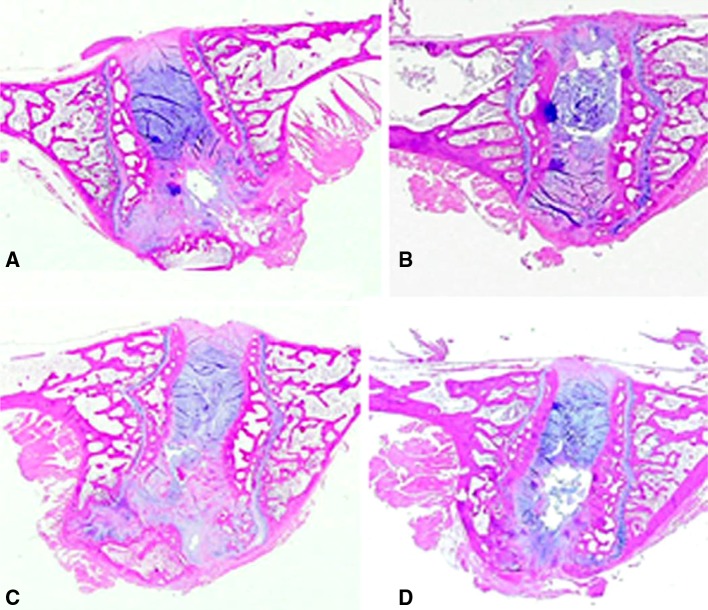
Plain light microscopy of the decalcified histologic sagittal sections from the control and experimental PEEK groups indicated a very mild reaction to the implanted material. Based on plain and polarized light microscopy, PEEK particles were visible in 10/10 (100 %) of the intradiscal PEEK treatment groups at the 3- and 6-month time points (Fig. 5c, d). The elicited histiocytic response to the PEEK treatments was localized within the nucleus pulposus. There was evidence of particulate dissemination into the adjacent interstitial spaces, bone matrix, and bone marrow with no adverse cellular response. The H&E stains indicated normal morphology of the nucleus pulposus and annulus fibrosis, and characterized both the control and PEEK treatments as without significant pathological changes at both the 3- and 6-month time intervals (Figs. 6, 7).
Fig. 6.
Plain light microscopic image of histologic sagittal sections from representative 3- (a) and 6-month (b) operative control and experimental (c, d) intradiscal groups. No significant pathological changes were observed (posterior, top; anterior, bottom) (H&E stain)
Fig. 7.
Light microscopy views of the local intervertebral disc of specimens in Fig. 6 demonstrated unremarkable cartilaginous endplate changes in the 3- (a) and 6-month (b) postoperative control treatments and the 3- and 6-month experimental groups (c, d). PEEK particulate is evident by the dark staining in images c and d (H&E stain)
Quantitative immunocytochemical analyses
Epidural particle challenge
Analysis of epidural challenge with PEEK particles using quantitative IHC analysis (Method 1) of epidural tissue resulted in a significant increase (p < 0.007) of TNF-α and IL-1β at both 3 and 6 months over that of controls (Fig. 8). This was an approximately 30 % increase in particle-challenged animals over controls for IL-1β and TNF-α at 3 months in the epidurally particle-challenged group. At 6 months, there continued to be a significant increase (p < 0.001) in PEEK-challenged epidural fibrosis tissues compared to surgical controls. At the 6-month time point, IL-6 in addition to IL-1β and TNF-α was significantly elevated. Analysis of 6-month epidurally challenged specimens using the additional method of quantitative IHC (Method 2), demonstrated increases in cytokines (IL-1β, IL-6, and TNF-α), but these were non-significant (p > 0.1). This lack of statistical significance at 6 months was due in part to the high background staining associated with the surgical controls and the high variability within each sample set as represented by the high standard deviations.
Fig. 8.
Epidural and intradiscal challenge with PEEK particles evaluated using quantitative IHC analysis (Method 1). Epidural challenge resulted in a significant increase of TNF-α and IL-1 at both 3 and 6 months and IL-6 at 6 months over that of controls. Intradiscal challenge with PEEK particles resulted in a significant increase in IL-1, IL-6, and TNF-α at 6 months post-challenge
Intradiscal challenge
Intradiscal challenge with 2.0 mg of PEEK particles resulted in a significant increase in IL-1β, IL-6, and TNF-α at 6 months post-challenge (p < 0.03, Fig. 8) using quantitative IHC (Method 1). However, there were only moderate increases in the relative amount of these cytokines when compared with surgical controls (10–20 %). When comparing the relative increase in treated to associated untreated control tissues, there was a greater inflammatory effect of PEEK particles when placed in epidural tissues compared to intradiscal challenge (i.e., there was a 50–100 % increase in normalized cytokine expression in epidural tissues compared to approximately 20 % increases intradiscally; Fig. 8).
Discussion
The current study sought to elucidate the histopathological effects and assess the local and systematic histological response of PEEK particulate implanted within the epidural and intervertebral disc space using an in vivo rabbit model. Our results support the hypothesis that clinically relevant amounts and sizes of PEEK implant debris from spinal arthroplasty devices would be more reactive when placed epidurally versus intradiscally. We found that these results also corroborate with the findings by previous investigators that PEEK does not elicit an aggressive immune response [14, 22]. The direct epidural and intradiscal application of PEEK particulate wear debris was shown to elicit a mild histiocytic reaction localized primarily within the epidural fibrous layers, which was most pronounced at the 3-month time interval based on qualitative histological review. By 6 months post-operatively, the histiocytic and cytokine response had markedly decreased compared to the 3-month time period. Overall, the results indicated that there was no evidence of an acute neuropathic or systemic histopathologic response to PEEK particulate. The quantitative IHC performed in this study demonstrated that there were significant increases in the innate immune response associated with cytokines (IL-1β, IL-6 and TNF-α) at both 3 and 6 months post-challenge. These increases are consistent with macrophage recruitment and activation to non-chemically reactive particulate challenge, in general.
This is the first study of inflammatory effects induced by clinically relevant sizes and doses of implantable grade PEEK particulate in the peri-spine region. A dose of 3 mg in a 5-kg rabbit translates roughly to 42 mg in a 70-kg human. However, it is not known to what degree this type of scaling is appropriate for scaling local tissue-relative local tissue responses. Additionally, it is the first study of intradiscal administration of implant debris of any kind, and the first to compare subsequent epidural versus intradiscal inflammation. Our results are consistent with past investigations (Table 2) of the neuropathic peri-spinal effects of PEEK particles [22] and those of metal implant debris such as magnesium particles [29], titanium, and nitinol particles [30]. A similar study involving lumbar epidural challenged with 2.5–10 mg of cobalt alloy particles (average size 0.2 μm ECD, range 1–3.27 μm) demonstrated dose-dependent increases in cytokines (IL-6, TNF-α, p < 0.05) using quantitative immunohistochemistry at 12 and 24 weeks, but there was no gross toxicity or local inflammation evident on macroscopic observation in any CoCr-dosed animals [17]. The current study agrees with these past in vivo investigations of spinal implant particles in that clinically relevant PEEK particles have been shown to also elicit mild chronic peri-spine inflammatory reactions. This is of concern to long-term implant function, because continued mild long-term inflammation can lead to osteolysis and loosening [31].
Table 2.
Comparison of different particulate implant materials with PEEK particles tested in the current investigation
| Particle type | Challenge location | Average particle size mn (μm) (range) |
Dose | Time in situ | Reaction | Ref |
|---|---|---|---|---|---|---|
| PEEK | Epidural | 1.2 μm (0.5–70) | 3 mg | 3 and 6 months | No systemic toxicity or organ pathology. Elevated IL-6 and TNF-α in peri-challenge tissue at 3 and 6 months | Current study |
| PEEK | Intradiscal | 1.2 μm (0.5–70) | 3 mg | 3 and 6 months | No systemic toxicity or organ pathology. Elevated IL-6 and TNF-α in peri-challenge tissue at 6 months | Current study |
| UHMWPE | Epidural | 1.2 μm (0.5–10) | 4 mg | 3 and 6 months | No systemic toxicity or organ pathology. Elevated IL-6 in peri-challenge tissue at 3 months (not at 6 months) | 36 |
| Stainless steel (316L) | Epidural | 0.1 μm (0.1–10) | 4 mg | 3 and 6 months | No systemic toxicity or organ pathology. Elevated IL-6 in peri-challenge tissue at 3 months (not at 6 months) | 36 |
| Titanium | Epidural | 0.2 μm (0.1–10) | 4 mg | 3 and 6 months | No systemic toxicity or organ pathology. Elevated IL-6 in peri-challenge tissue at 3 months (not at 6 months) | 36 |
| Nitinol (Ti35 %Ni) | Epidural | 100–300 μm (100–300) | 12 mg | 1, 4, 12, 26, and 52 weeks | No systemic toxicity or organ pathology. Moderate inflammation at 1–4 weeks progressing to mild inflammation by 52 weeks (histological evaluation of lymphocytes, plasma cells, macrophages and giant cells) | 30 |
| Nickel | Epidural | 0.6 μm (0.3–19) | 20 mg | 3 Days | Severe toxicity: neuropathy | 17 |
| Cobalt alloy | Epidural | 0.2 μm (0.1–3.3) | 2.5–10 mg | 3 and 6 months | No systemic toxicity or organ pathology. Elevated IL-6 and TNF-α in peri-challenge tissue at 3 and 6 months for >5 mg of particle challenge | 17 |
| Cobalt alloy | Epidural | 0.1 μm (0.1–10) | 4 mg | 3 and 6 months | No systemic toxicity or organ pathology. No elevation ins local tissue cytokine levels (IL-6, TNF-α, IL-1b, and IL-8) | 36 |
The relative increase in inflammatory cytokines at 6 months compared to 3 months indicated the non-biodegradable nature of the PEEK polymeric challenge and persistence of response after 3 months in situ. The amount of inflammation was evaluated relative to surgical controls (with no particle challenge). The actual amount of % cytokine staining did not increase from 3 to 6 months, but remained relatively consistent at approximately 40 %. However, as expected the surgery only demonstrated a reduction in inflammation over time in controls from approximately 30 % at 3 months to 20 % at 6 months. Thus, the appearance of relative increases over 3 to 6 months are attributable to decreased inflammation over time of epidural and intradiscal sham particle challenge. This observed decrease supports the use of quantitative IHC to measure in vivo innate immune inflammation in response to particle challenge over time.
It is important to note that the percentages of the areas reported in Figs. 5, 6 and 7 do not represent the actual amounts (i.e., pg/mL) of cytokines, but reflect a relative measure of expression compared to control samples. Thus these cytokine results imply mild inflammation, but do not rule out the possibility of acute inflammation after 6 months. Whether there is acute inflammation or systemic ramifications is beyond the scope of this investigation.
Important limitations of the current study include the following. In the clinical setting, wear debris is generated continuously rather than as a single bolus of material. Some osteolysis models for total joints have used a continuous infusion of particulate to further gain insight into aseptic loosening and periprosthetic osteolysis [32]. However, it is unknown what amount of particulate injected on a consistent basis would represent the clinical situation for disc arthroplasty applications. Additional limitations involve needle puncture that is often used to induce disc degeneration in rabbit models [33]. This was partially accounted for by the use of sham-injected relative controls. In addition, the generalized staining of the tissues where surgical control samples demonstrated a high background level of inflammation using quantitative IHC may have resulted in the non-significant increases observed in the cytokines studied utilizing Method 2 as compared to Method 1.
The clinical relevance of implant-generated wear debris in the spine remains poorly understood. As a consequence of device wear in the lumbar spine, common inflammatory mediators, such as IL-1β, IL-6 and TNF-α, have been observed after total disc replacement [34–36]. We found that clinically relevant amounts and sizes of PEEK implant debris are more reactive when placed epidurally versus intradiscally, resulting in significant increases in these common inflammatory mediators. However, overall the results of this study are similar to past investigations where PEEK has not been shown to elicit an aggressive immune response when applied to the epidural tissues, where macroscopic and semi-quantitative histologic analyses showed normal vascularization, and an absence of necrosis or swelling. The degree to which these results will translate to the clinical environment remains to be established, but the pattern of subtle elevations in inflammatory cytokines indicated both a mild persistence of responses to PEEK debris and that intradiscal implant debris will likely result in less inflammation than epidural implant debris.
Acknowledgments
Funding was provided by Pioneer Surgical.
Conflict of interest
Nadim Hallab is a consultant for Pioneer Surgical Technology and an employee of Bioengineering Solutions Inc. Qi-Bin Bao is a former employee of Pioneer Surgical Technology. Tim Brown is an employee of Pioneer Surgical Technology.
Contributor Information
Nadim J. Hallab, Phone: +1-312-9427079, FAX: +1-312-9422101, Email: nhallab@rush.edu
Qi-Bin Bao, Phone: +1-906-2269909, FAX: +1-906-2264455.
Tim Brown, Phone: +1-906-2269909, FAX: +1-906-2264455.
References
- 1.Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007;28(34):5044–5048. doi: 10.1016/j.biomaterials.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallab NJ, Anderson S, Stafford T, et al. Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. 2005;23:384–389. doi: 10.1016/j.orthres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs JJ, Hallab NJ. Loosening and osteolysis associated with metal-on-metal bearings: a local effect of metal hypersensitivity? J Bone Jt Surg Am. 2006;88:1171–1172. doi: 10.2106/JBJS.F.00453. [DOI] [PubMed] [Google Scholar]
- 4.Ren W, Wu B, Peng X, et al. Implant wear induces inflammation, but not osteoclastic bone resorption, in RANK(−/−) mice. J Orthop Res. 2006;24:1575–1586. doi: 10.1002/jor.20190. [DOI] [PubMed] [Google Scholar]
- 5.Shanbhag AS, Kaufman AM, Hayata K, et al. Assessing osteolysis with use of high-throughput protein chips. J Bone Jt Surg Am. 2007;89:1081–1089. doi: 10.2106/JBJS.F.00330. [DOI] [PubMed] [Google Scholar]
- 6.Agins H, Alcock NW, Bansal M, et al. Metallic wear in failed titanium-alloy total hip replacements. A histological and quantitative analysis. J Bone Jt Surg. 1988;70-A:347–356. [PubMed] [Google Scholar]
- 7.Jacobs JJ, Urban RM, Schajowicz F, Gavrilovic J, Galante JO (1992) Particulate-associated endosteal osteolysis in titanium-base alloy cementless total hip replacement. In: St. John KR (ed) Particulate debris from medical implants: mechanisms of formation and biological consequences ASTM STP 1144, American Society for Testing and Materials, Philadelphia, pp 52–60
- 8.Lombardi AV, Jr, Mallory TH, Vaughn BK, et al. Aseptic loosening in total hip arthroplasty secondary to osteolysis induced by wear debris from titanium-alloy modular femoral heads. J. Bone Jt Surg. 1989;71-A:1337–1342. [PubMed] [Google Scholar]
- 9.Hallab NJ. A review of the biologic effects of spine implant debris: fact from fiction. SAS J. 2009;3:143–160. doi: 10.1016/j.esas.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown T, Bao QB. The use of self-mating PEEK as an alternative bearing material for cervical disc arthroplasty: a comparison of different simulator inputs and tribological environments. Eur Spine J. 2012;21(Suppl 5):S717–S726. doi: 10.1007/s00586-012-2252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown T, Bao QB, Kilpela T, et al. An in vitro biotribological assessment of NUBAC, a polyetheretherketone-on-polyetheretherketone articulating nucleus replacement device: methodology and results from a series of wear tests using different motion profiles, test frequencies, and environmental conditions. Spine. 2010;35:E774–E781. doi: 10.1097/BRS.0b013e3181d59e45. [DOI] [PubMed] [Google Scholar]
- 12.Balsano M, Zachos A, Ruggiu A, et al. Nucleus disc arthroplasty with the NUBAC device: 2-year clinical experience. Eur Spine J. 2011;20(Suppl 1):S36–S40. doi: 10.1007/s00586-011-1752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeavons RP, Lakkol SG, Reddy G, et al. A comparison of MRI clarity in titanium and polyetheretherketone (PEEK) cervical disc devices. Spine J. 2012;12:S73. doi: 10.1016/j.spinee.2012.08.209. [DOI] [Google Scholar]
- 14.Hallab NJ, McAllister K, Brady M, et al. Macrophage reactivity to different polymers demonstrates particle size- and material-specific reactivity: PEEK-OPTIMA particles versus UHMWPE particles in the submicron, micron, and 10 micron size ranges. J Biomed Mater Res B Appl Biomater. 2011;100B(2):480–492. doi: 10.1002/jbm.b.31974. [DOI] [PubMed] [Google Scholar]
- 15.Sagomonyants KB, Jarman-Smith ML, Devine JN, et al. The in vitro response of human osteoblast to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials. 2008;29(11):1563–1572. doi: 10.1016/j.biomaterials.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham BW, Orbegoso CM, Dmitriev AE, et al. The effect of titanium particulate on development and maintenance of a posterolateral spinal arthrodesis: an in vivo rabbit model. Spine. 2002;27:1971–1981. doi: 10.1097/00007632-200209150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hallab NJ, Chan FW, Harper ML. Quantifying subtle but persistent peri-spine inflammation in vivo to submicron cobalt–chromium alloy particles. Eur Spine J. 2012;21(12):2649–2658. doi: 10.1007/s00586-012-2251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeh A, Planert M, Siegert G, et al. Time dependent release of cobalt and chromium ions into the serum following implantation of the metal-on-metal Maverick-type artificial lumbar disc (Medtronic Sofamor Danek) Arch Orthop Trauma Surg. 2009;129(6):741–746. doi: 10.1007/s00402-008-0677-8. [DOI] [PubMed] [Google Scholar]
- 19.Francois J, Coessens R, Lauweryns P. Early removal of a Maverick disc prosthesis: surgical findings and morphological changes. Acta Orthop Belg. 2007;73(1):122–127. [PubMed] [Google Scholar]
- 20.Cavanaugh DA, Nunley PD, Kerr EJ, et al. Delayed hyper-reactivity to metal ions after cervical disc arthroplasty: a case report and literature review. Spine. 2009;34(7):E262–E265. doi: 10.1097/BRS.0b013e318195dd60. [DOI] [PubMed] [Google Scholar]
- 21.Guyer RD, Shellock J, MacLennan B, et al. Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction: diagnosis and treatment experience in four cases. Spine. 2011;36(7):E492–E497. doi: 10.1097/BRS.0b013e31820ea9a2. [DOI] [PubMed] [Google Scholar]
- 22.Rivard CH, Rhalmi S, Coillard C. In vivo biocompatibility testing of peek polymer for a spinal implant system: a study in rabbits. J Biomed Mater Res. 2002;62(4):488–498. doi: 10.1002/jbm.10159. [DOI] [PubMed] [Google Scholar]
- 23.Green TR, Fisher J, Matthews JB, et al. Effect of size and dose on bone resorption activity of macrophages by in vitro clinically relevant ultra high molecular weight polyethylene particles. J Biomed Mater Res. 2000;53(5):490–497. doi: 10.1002/1097-4636(200009)53:5<490::AID-JBM7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Matthews JB, Besong AA, Green TR, et al. Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J Biomed Mater Res. 2000;52(2):296–307. doi: 10.1002/1097-4636(200011)52:2<296::AID-JBM8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Matthews JB, Green TR, Stone MH, et al. Comparison of the response of primary murine peritoneal macrophages and the U937 human histiocytic cell line to challenge with in vitro generated clinically relevant UHMWPE particles. Biomed Mater Eng. 2000;10(3–4):229–240. [PubMed] [Google Scholar]
- 26.Jacobs JJ, Roebuch KA, Archibeck M, et al. Osteolysis: basic science. Clin Orthop Relat Res. 2001;393:71–77. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Brown T, Bao QB, Agrawal CM, et al. An in vitro assessment of wear particulate generated from NUBAC, a PEEK on PEEK articulating nucleus replacement device: methodology and results from a series of wear tests using different motion profiles, test frequencies and environmental conditions. Spine. 2011;36(26):E1675–E1685. doi: 10.1097/BRS.0b013e31821ac8a0. [DOI] [PubMed] [Google Scholar]
- 28.Brown T, Bao Q-B, Yuan HA. The use of PEEK in spine arthroplasty. In: Foker S, editor. Recent advances in arthroplasty. 1. Croatia: InTech; 2012. pp. 211–234. [Google Scholar]
- 29.Kaya RA, Cavusoglu H, Tanik C, et al. The effects of magnesium particles in posterolateral spinal fusion: an experimental in vivo study in a sheep model. J Neurosurg Spine. 2007;6(2):141–149. doi: 10.3171/spi.2007.6.2.141. [DOI] [PubMed] [Google Scholar]
- 30.Rhalmi S, Charette S, Assad M, et al. The spinal cord dura mater reaction to nitinol and titanium alloy particles: a 1-year study in rabbits. Eur Spine J. 2007;16(7):1063–1072. doi: 10.1007/s00586-007-0329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gristina AG. Implant failure and the immuno-incompetent fibro-inflammatory zone. Clin Orthop Relat Res. 1994;298:106–118. [PubMed] [Google Scholar]
- 32.Ren P, Irani A, Huang Z, et al. Continuous infusion of UHMWPE particles induces increased bone macrophages and osteolysis. Clin Ortho Relat Res. 2011;469(1):113–122. doi: 10.1007/s11999-010-1645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoki Y, Akeda K, An H, et al. Nerve fiber ingrowth into scar tissue formed following nucleus pulposus extrusion in the rabbit anular-puncture disc degeneration model: effects of depth of puncture. Spine. 2006;31(21):E774–E780. doi: 10.1097/01.brs.0000238681.71537.41. [DOI] [PubMed] [Google Scholar]
- 34.Devin CJ, Myers TG, Kang JD. Chronic failure of a lumbar total disc replacement with osteolysis. Report of a case with 19-year follow-up. J Bone Jt Surg Am. 2008;90(10):2230–2234. doi: 10.2106/JBJS.G.01712. [DOI] [PubMed] [Google Scholar]
- 35.Punt IM, Austen S, Cleutjens JP, et al. Are periprosthetic tissue reactions observed after revision of total disc replacement comparable to the reactions observed after total hip or knee revision surgery? Spine. 2009;37(2):150–159. doi: 10.1097/BRS.0b013e3182154c22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham BW. Basic scientific considerations in total disc arthroplasty. Spine J. 2004;4(6 Suppl):219S–230S. doi: 10.1016/j.spinee.2004.07.015. [DOI] [PubMed] [Google Scholar]