Abstract
Objective
Recently, interspinous process devices have attracted much attention since they can be implanted between the lumbar spinous processes (LSP) of patients with degenerative disc disease (DDD) and degenerative spondylolisthesis (DLS) using a minimally invasive manner. However, the motion characters of the LSP in the DLS and DDD patients have not been reported. This study is aimed at investigating the kinematics of the lumbar spinous processes in patients with DLS and DDD.
Methods
Ten patients with DDD at L4–S1 and ten patients with DLS at L4–L5 were studied. The positions of the vertebrae (L2–L5) at supine, standing, 45° trunk flexion, and maximal extension positions were determined using MRI-based models and dual fluoroscopic images. The shortest ISP distances were measured and compared with those of healthy subjects that have been previously reported.
Results
The shortest distance of the interspinous processes (ISP) gradually decreased from healthy subjects to DDD and to DLS patients when measured in the supine, standing, and extension positions. During supine-standing and flexion–extension activities, the changes in the shortest ISP distances in DDD patients were 2 ± 1.2 and 4.8 ± 2.1 mm at L4–L5; in DLS patients they were 0.5 ± 0.4 and 2.8 ± 1.7 mm at L4–L5, respectively. The range of motion is increased in DDD patients but decreased in DLS patients when compared with those of the healthy subjects. No significantly different changes were detected at L2–L3 and L3–L4 levels.
Conclusion
At the involved level, the hypermobility of the LSP was seen in DDD and hypomobility of the LSP in DLS patients. The data may be instrumental for improving ISP surgeries that are aimed at reducing post-operative complications such as bony fracture and device dislocations.
Keywords: Lumbar spine, Kinematics, Spinous process, Degenerative lumbar spondylolisthesis, Disc degeneration
Introduction
The Lumbar spinous process (LSP) plays an important role in stabilizing the segmental spine unit and protecting the neural structure in the spinal canal. Recently, the LSP has attracted much attention as the sites for implantation of the interspinous process devices (ISPD) [1–5]. The minimally invasive surgery has been reported for the treatment of the patients with degenerative disc disease (DDD) [5] or symptomatic lumbar spinal stenosis caused by degenerative lumbar spondylolisthesis (DLS) [2]. Therefore, a quantitative knowledge on the biomechanical characteristics of the spinous processes of the patients with DDD and DLS is important for improvement of the surgical treatment efficacy.
Previous biomechanical studies have primarily focused on the motion of the vertebral bodies, intervertebral discs, and facet joints [6–9]. Limited data have been reported on the motion characteristics of the LSP using cadaveric specimens [10], anterior–posterior (AP) radiographs [11], and Computed tomography (CT) [12]. However, the spinous process is a complex 3-D structure and most previous studies are based on the two-dimensional (2D) imaging techniques that make it difficult to accurately determine the three-dimensional (3D) motion characters of the LSP. Xia et al. [13] reported 3D motion characteristics of LSP under in vivo weight-bearing conditions of healthy subjects. A literature review revealed that no data have been reported to describe the motion characteristics of LSP in patients with DDD and DLS.
In this study, we measured the motion of the LSP during non-weight bearing, weight-bearing standing, and flexion–extension of the trunk in patients with DDD or DLS. The data were compared with those of healthy subjects published previously [13]. We hypothesized that the spinous processes at the DDD and DLS levels and at immediately adjacent levels would demonstrate distinct motion patterns during active in vivo function of the body when compared with those of healthy subjects.
Materials and methods
Patient recruitment
Ten patients with DDD at L4–S1 (7 men and 3 women; mean age = 51.8 years; mean height = 169 cm; mean weight = 65.7 kg) and ten patients diagnosed with lumbar spinal stenosis caused by DLS at L4–L5 (3 men and 7 women; mean age = 72.6 years; mean height = 162 cm; mean weight = 65.2 kg) were recruited from a single academic center. Eight healthy participants (3 male and 5 female participants; mean age = 54.4 years; mean height = 163.5 cm; mean weight = 63.5 kg) tested in our previous studies were used as normal controls [13]. Based on the clinical radiographic assessments, degeneration of the disc was graded using Pfirrmann classification for all subjects [14] (Table 1). The vertebral slippage of all DLS patients was categorized as Grade I using the Meyerding classification method [15]. In addition, anterior disc height (ADH) and posterior disc height (PDH) were, respectively, retrieved by measuring the perpendicular distance between the two opposite points of the endplate rims (Table 2).
Table 1.
Disc degeneration graded with Pfirrmann system for normal participants, DDD, and DLS patients
| L2–L3 | L3–L4 | L4–L5 | |
|---|---|---|---|
| Normal | 1.1 ± 0.4 | 1.6 ± 0.5 | 1.9 ± 0.6 |
| Range of grade | 1–2 | 1–3 | 1–3 |
| DDD | 1.4 ± 0.5 | 1.6 ± 0.8 | 4.2 ± 0.8 |
| Range of grade | 1–2 | 1–3 | 3–5 |
| DLS | 2.4 ± 0.7 | 2.6 ± 1.1 | 4.7 ± 0.8 |
| Range of grade | 1–3 | 2–4 | 4–5 |
The value were presented as mean (SD)
Normal normal subject (N = 8), DDD degenerative disc disease (N = 10), DLS degenerative lumbar spondylolisthesis (N = 10)
Table 2.
Disc height under different postures
| Posture | Disc height | Normal | DDD | DLS |
|---|---|---|---|---|
| Supine | ADH | 10.82 ± 1.79 | 10.59 ± 1.61 | 8.24 ± 3.13 |
| PDH | 6.58 ± 1.78 | 6.13 ± 2.02 | 3.66 ± 1.66#* | |
| Stand upright | ADH | 9.94 ± 1.87 | 9.83 ± 1.85 | 6.60 ± 2.81#* |
| PDH | 5.74 ± 1.53 | 6.07 ± 1.97 | 3.09 ± 1.40#* | |
| Extension | ADH | 11.02 ± 1.05 | 11.13 ± 1.55 | 8.05 ± 3.22#* |
| PDH | 5.50 ± 0.87 | 6.05 ± 2.28 | 2.81 ± 1.70#* | |
| Flexion | ADH | 9.42 ± 2.54 | 9.20 ± 2.34 | 6.39 ± 3.21 |
| PDH | 6.62 ± 2.11 | 6.46 ± 2.00 | 3.32 ± 1.14#* |
ADH anterior disk height, PDH posterior disk height. The value were presented as mean (SD)
Normal normal subject (N = 8), DDD degenerative disc disease (N = 10), DLS degenerative lumbar spondylolisthesis (N = 10)
#,*Both the ADH and PDH were shorter in the DLS group than in the normal and DDD groups
Approval by our institutional review board was obtained prior to the initiation of this study. We obtained informed consent from each patient before any testing was performed.
Imaging technique
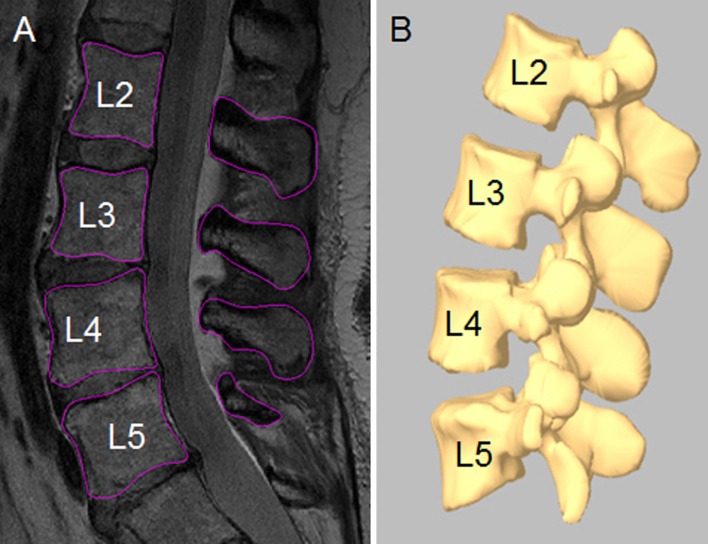
Each patient was scanned in a non-weight bearing supine position using a 3-T MRI scanner (Siemens Medical Solutions MAGNETOM Trio) with a spine surface coil and a T2-weighted fat-suppressed 3-D spoiled gradient recalled sequence. The MRI images of the spinal segments were then imported into a solid modeling software (Rhinoceros®, Robert McNeel & Associates, Seattle, WA, USA) to construct 3-D anatomical vertebral models of L2–L5 using a protocol established in our laboratory [6, 16]. Mesh models of the vertebrae were created from bony contours (Fig. 1a, b).
Fig. 1.
a Digitized contours of lumbar vertebrae in the sagittal plane. b Three-dimensional anatomical vertebral model of L2–L5 from the magnetic resonance imaging
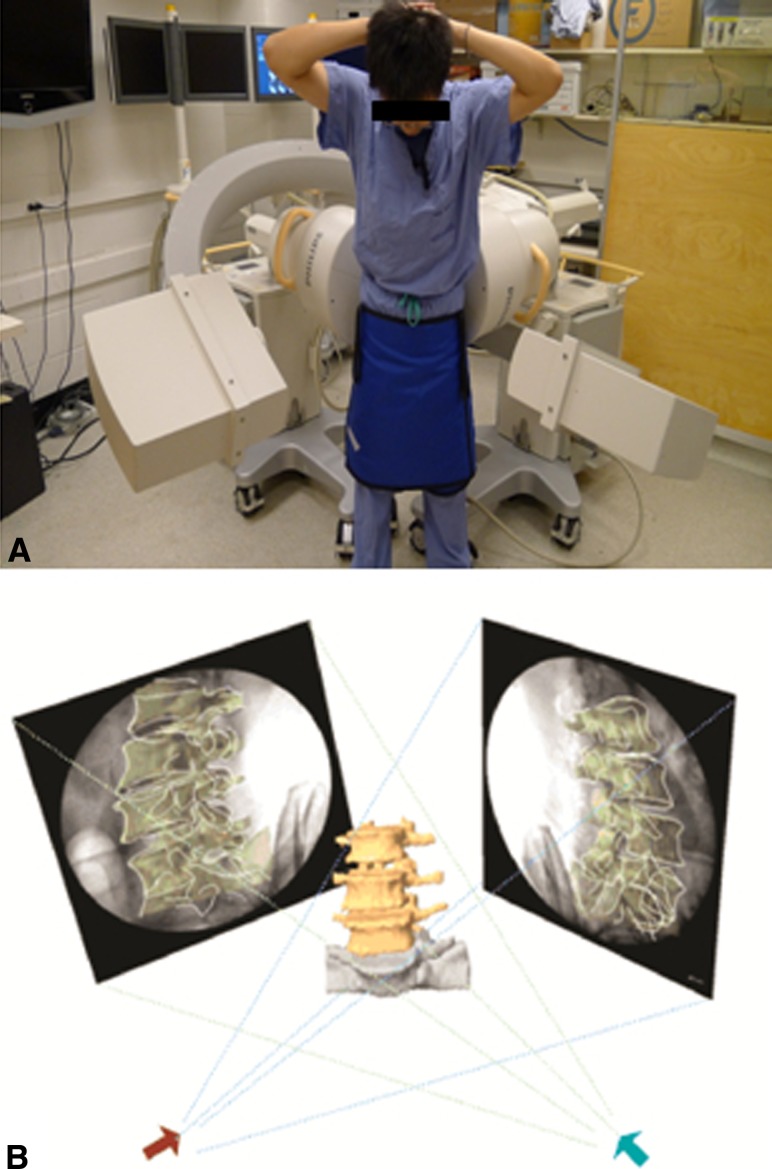
The lumbar spine of each patient was then imaged with different weight-bearing poses of the body: standing position, 45° trunk flexion, and maximal extension (Fig. 2a, b) using a dual fluoroscopic imaging system (DFIS) [6, 16]. The subject was asked to hold each pose for about 1 s while two fluoroscopes took simultaneous images from two orthogonal directions. The poses were monitored by an orthopedic surgeon to reduce inter-subject variation. Every subject was asked to minimize his/her hip motions to maximize the lumbar motion.
Fig. 2.
a The experimental setup of the dual fluoroscopic imaging system for capturing the lumbar spine positions of living subjects. b Virtual reproduction of the DFIS and the vertebral positions
The in vivo positions of the vertebrae at various weight-bearing body positions were reproduced in the Rhinoceros software using the MRI-based 3D models and the corresponding pairs of orthogonal fluoroscopic images of the vertebrae [6, 16]. A model of the vertebrae can be independently translated and rotated in 6DOF until its projections match the osseous outlines captured on the fluoroscopic images in the virtual fluoroscopy system in the computer. Therefore, the vertebral positions during in vivo weight-bearing activities at each selected pose were determined. In our previous validation paper [6], the accuracy of this technique is 0.3 mm for determination of translation and 0.6° for rotation.
Measurement of the shortest interspinous process (ISP) distance
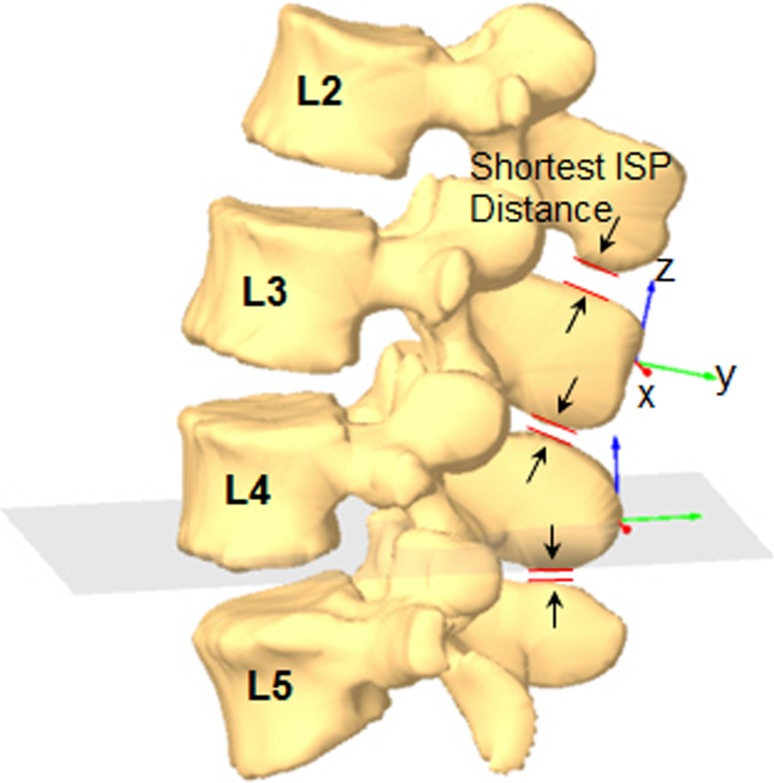
After obtaining the positions of the vertebrae, the shortest ISP distances were automatically calculated using a software code (Matlab v. 7.0, Mathworks, Natick, MA, USA) based on iterative closest point method [6, 17]. In this study, the shortest ISP distances were measured at the adjacent levels (L2–L3 and L3–L4) and the diseased level (L4–L5) when the subject was in the non-weight bearing supine position, weight-bearing standing position, 45° trunk flexion, and maximal extension positions (Fig. 3).
Fig. 3.
Measurement of the shortest interspinous process using iterative closest point method
Statistical analysis
Multi-way analysis of variance (ANOVA) was used to compare the ISP distances at L2–L3, L3–L4, and L4–L5 vertebral levels during various body poses of each subject. Another multi-way ANOVA was also used to compare the ISP distances among the healthy subjects, DDD and DLS patients at the same level and same body posture. The subject group was the categorical factor and the level and the body position were the dependent variables. The level of significance was set at p < 0.05. When a statistically significant difference was detected, a Newman–Keuls post hoc test was performed. The statistical analysis was performed using the Statistica software (StatSoft version 8.0, Tulsa, Ok, USA).
Results
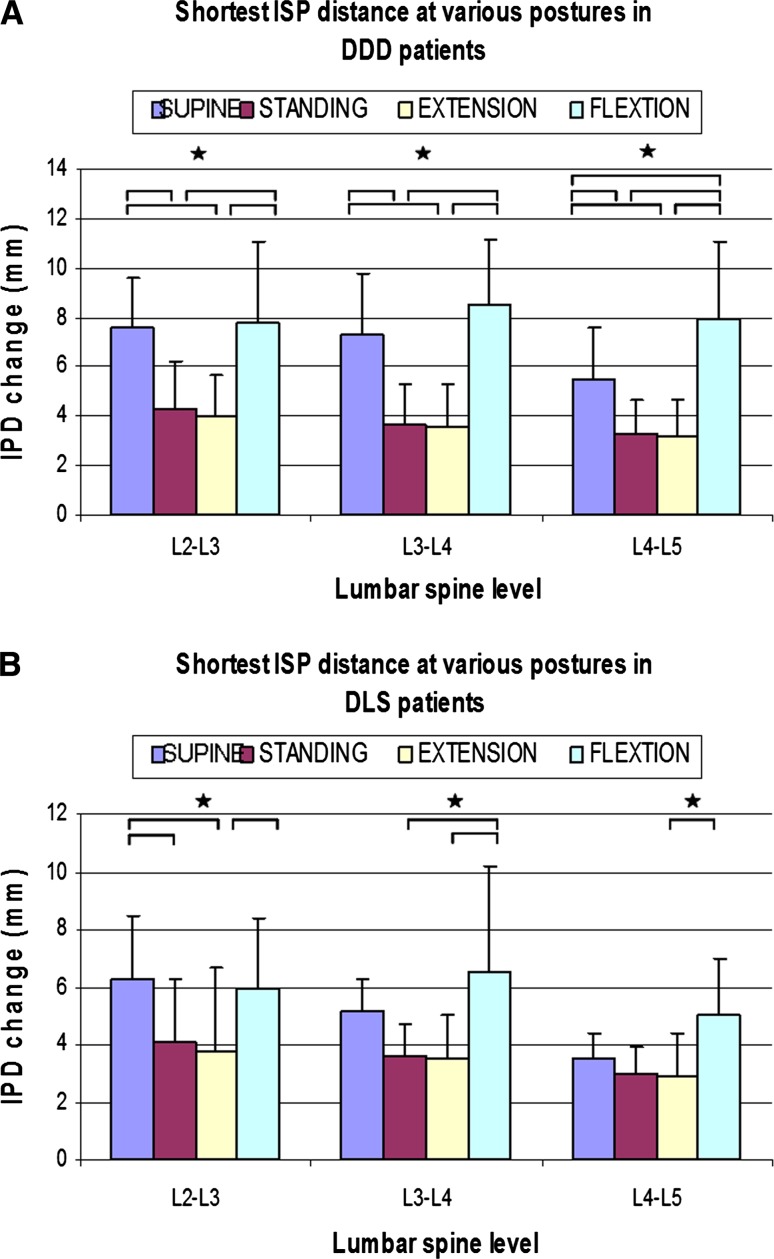
DDD patients (Fig. 4a)
Fig. 4.
Shortest interspinous process distance at various postures and different levels in a DDD, and b DLS patients. (*p < 0.05)
At L2–L3 level, the shortest ISP distance was 7.8 ± 3.3 mm at maximal flexion position, that is significantly higher than that at standing (4.3 ± 1.9 mm) (p = 0.009) and extension (4.0 ± 1.7 mm) (p = 0.005) positions, but similar to that of supine (7.6 ± 2.0 mm) (p = 0.86). The shortest ISP distance at supine position was also significantly higher than that at standing position (p = 0.001) and extension (p = 0.001) positions.
At L3–L4 level, the shortest ISP distance was 8.5 ± 2.7 mm at maximal flexion position, significantly higher than that at standing (3.7 ± 1.6 mm) (p = 0.001) and extension (3.6 ± 1.7 mm) (p = 0.001) positions, but similar to that of supine (7.3 ± 2.5 mm) (p = 0.201) position. The shortest ISP distance at supine position was also significantly higher than that at standing position (p = 0.001) and extension (p = 0.001) position.
At L4–L5 vertebral levels, the shortest ISP distance was 8.0 ± 3.1 mm at maximal flexion position, significantly higher than that at standing (3.3 ± 1.4 mm) (p = 0.0003), extension (3.2 ± 1.5 mm) (p = 0.0002), and supine (5.5 ± 2.1) (p = 0.012) positions. The shortest ISP distance at supine position was also significantly higher than that at standing (p = 0.039) and extension (p = 0.023) positions.
Interspinous process distances were also compared between different vertebral levels. The only significant difference was found in the supine position, where the distance L4–L5 was significantly smaller than that of the L2–L3 level (p = 0.032).
DLS patients (Fig. 4b)
At L2–L3 level, the shortest ISP distance was 6.0 ± 2.4 mm at maximal flexion position, which was significantly higher than that of extension (3.8 ± 2.9 mm) (p = 0.031), but similar to that of supine (6.3 ± 2.0 mm) (p = 0.205) and standing (4.1 ± 2.2 mm) (p = 0.361) positions. The shortest ISP distance at supine position was significantly higher than those at standing (p = 0.031) and extension (p = 0.015) positions.
At L3–L4 level, the shortest ISP distance was 6.5 ± 3.7 mm at maximal flexion position, which was significantly higher than that at standing (3.6 ± 1.1 mm) (p = 0.006) and extension (3.5 ± 1.5 mm) (p = 0.004) positions, but was similar to that of supine (5.2 ± 1.5 mm) (p = 0.214) position.
At L4–L5 vertebral levels, the shortest ISP distance was 5.0 ± 2.0 mm at maximal flexion position, which was significantly higher than that at extension (2.9 ± 1.5 mm) (p = 0.048) position, but was similar to those of supine (3.5 ± 1.0 mm) (p = 0.144) and standing (3.0 ± 0.9 mm) (p = 0.054) positions.
At different vertebral levels, the only significant difference was found in the supine position, where the L4–L5 distance was found to be significantly smaller than that of L2–L3 (p = 0.007).
Comparison with healthy participants
The shortest ISP distances were compared among the healthy subjects, DDD and DLS patients at various postures (Table 2). Significantly shorter distances for DLS patients were found in the supine position at the L4–L5 level compared with the healthy participants (DLS: 3.5 ± 1.0 vs. normal: 5.6 ± 3.0 mm p = 0.04) and DDD patients (DDD: 5.5 ± 2.1 mm p = 0.02). Significant shorter distances for DLS patients were also found in the flexion position at the L4–L5 level compared with DDD patients (DLS: 5.0 ± 2.0 vs. DDD: 8.0 ± 3.1 mm p = 0.03).
During supine-standing activities (Fig. 5a)
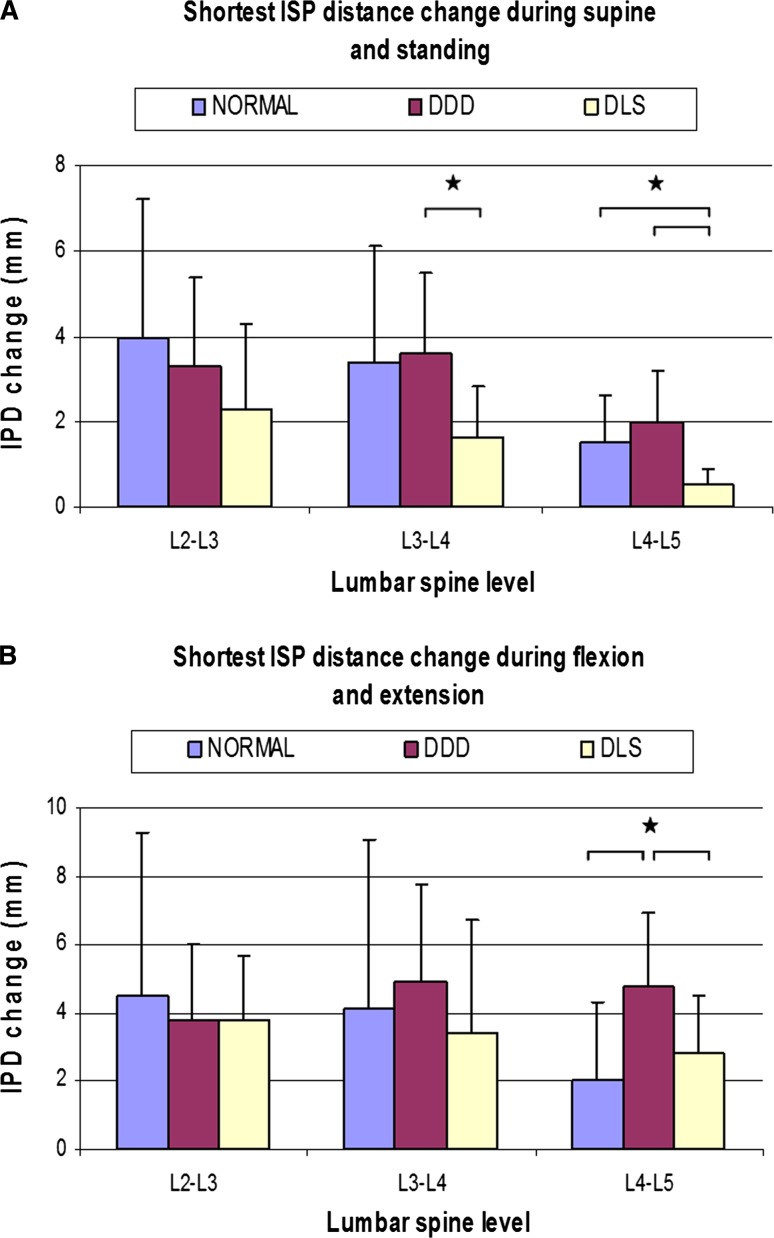
Fig. 5.
Distance change between processes (a) during supine and standing and (b) during flexion and extension at different level. (*p < 0.05)
At L2–L3 level, no significant difference was found among the three groups. At L3–L4 level, the shortest ISP distance change in DLS patients was significantly smaller than those of DDD patients (DDD: 3.6 ± 1.9 vs. DLS: 1.6 ± 1.2 mm p = 0.035). At L4–L5 level, the shortest ISP distance change in DLS patients was significantly smaller than those of healthy participants (DLS: 0.5 ± 0.4 vs. normal: 1.5 ± 1.1 mm p = 0.02) and DDD patients (DDD: 2.0 ± 1.2 mm p = 0.035).
During flexion–extension activities (Fig. 5b)
At adjacent L2–L3 and L3–L4 level, no significant difference was found among the three groups. At L4–L5 level, the shortest ISP distance change in DDD patients was significantly higher than those of healthy participants (DDD: 4.8 ± 2.1 vs. normal: 2.0 ± 2.3 mm p = 0.036) and DLS patients (DLS: 2.8 ± 1.7 mm p = 0.032).
Discussion
In this study, we investigated the changes of the shortest ISP distances of DDD and DLS patients during different body positions and compared the data with those of the healthy subjects reported previously [13]. We found that in general the shortest ISP distance showed a decreasing trend from healthy subjects to DDD and to DLS patients. During supine-standing activities, the change of the shortest ISP distance is significantly smallest in DLS patients at the diseased level (L4–L5). During the flexion–extension activity, the change of the shortest ISP distance in DDD patients is the largest at the diseased level (L4–L5). The data on adjacent levels were similar to that observed from healthy subjects. Overall, these findings indicated that disc degeneration and spondylolisthesis correlated to distinct alterations in movements of the ISP at the involved level [18].
Few studies have reported on the kinematics of the LSP [10–12]. Ihm et al. [19] measured the lumbar ISP distance and found that there is a decreasing trend in the ISP distance with advancing ages. Sobottke et al. [12] examined the anatomic proportions of the interspinous space and the spinous processes and found that the best anatomic position for a stand alone interspinous spacer is anteriorly positioned. However, the 2D classification methods are limited by the inaccurate identification of the same anatomic landmarks on multiple planer radiographs [20, 21], resulting in a great variability among the results. Besides, the image registration method proved to be more accurate image matching [22]. Xia et al. [13] reported 3D motion characteristics of healthy subjects during upright standing, flexion and extension, and found that changes in ISP distances were dependent on body postures and vertebral levels. Wan et al. [17, 23] indicated the shortest ISP distances significantly increased in patients with lumbar spine stenosis after X-stop implantation from 3D lumbar models. No data were reported on the kinematics of the LSP in patients with DDD and DLS during in vivo physiologic weight-bearing activities (Table 3).
Table 3.
Shortest distance between processes in normal subjects, DDD, and DLS patients
| L2–L3 | L3–L4 | L4–L5 | |
|---|---|---|---|
| Supine | |||
| Normal | 8.5 ± 3.1 | 7.5 ± 3.0 | 5.6 ± 3.0 |
| DDD | 7.6 ± 2.0 | 7.3 ± 2.5 | 5.5 ± 2.1 |
| DLS | 6.3 ± 2.0 | 5.2 ± 1.5 | 3.5 ± 1.0*,# |
| Standing | |||
| Normal | 4.9 ± 4.0 | 4.3 ± 3.9 | 4.8 ± 3.2 |
| DDD | 4.3 ± 1.9 | 3.7 ± 1.6 | 3.3 ± 1.4 |
| DLS | 4.1 ± 2.2 | 3.6 ± 1.1 | 3.0 ± 0.9 |
| Extension | |||
| Normal | 3.5 ± 1.8 | 4.2 ± 1.9 | 4.1 ± 4.7 |
| DDD | 4.0 ± 1.7 | 3.6 ± 1.7 | 3.2 ± 1.5 |
| DLS | 3.8 ± 2.9 | 3.5 ± 1.5 | 2.9 ± 1.5 |
| Flexion | |||
| Normal | 8.1 ± 5.4 | 8.3 ± 6.1 | 6.4 ± 4.5 |
| DDD | 7.8 ± 3.3 | 8.5 ± 2.7 | 8.0 ± 3.1 |
| DLS | 6.0 ± 2.4 | 6.5 ± 3.7 | 5.0 ± 2.0* |
The values were presented as mean (SD) in millimeter
* <0.05 when compared with DDD patients, # <0.05 compared with normal subject at the same level and position
ISP distance change in essence correlated with the segmental motion. Passias et al. [24] indicated that the range of motion at L4–L5 was significantly larger in the DDD group than in the normal group. Miao [9] indicated no statistically significant difference in the ROMs between the normal and DLS groups. These motion data agreed with our ISP change findings between groups. ISP distance change also correlated with the change in size of the spinous process as degeneration progress. Aylott et al. [20] reported that the LSP increases in size with advancing age in both vertical and axial dimensions, and vertical dimension increases of the LSP have the potential to reduce the distance of the ISP. Additionally, abutment of the LSP or “kissing spines” is well recognized in the elderly [21]. Furthermore, Paholpak et al. indicated a significant increase in the height of the L4 and L5 spinous process. In this study, the average age of DLS patients was higher than the ages of the normal subjects and the DDD patients. We found that the shortest distance of the ISP gradually decreased from healthy subjects to DDD and DLS patients in supine, standing, extension positions. In our data, we also observed that the ADH was smaller in the DLS group than in the normal and DDD groups in all the four postures. The changes of disc heights in DLS group indicated that the affected disc not only had increased vertical laxity and decreased elasticity but also the morphological changes of an increase in the height of the spinous process may be a kind of biological defense reaction to stabilize it. This result corresponds with the results of earlier studies showing that degenerated intervertebral discs lead to decreased height of the posterior structures [25], but also implying that age might be another factor that leads to the reduced ISP distances.
Kirkaldy-Willis and Farfan [26] divided the process of degenerative lumbar diseases into three phases: (1) temporary dysfunction, (2) unstable phase, and (3) stabilization. However, it is difficult to examine this pathway by following a patient group through the entire process of lumbar vertebral degeneration. In this study, we selected a normal subject group from our previous studies as a comparison group. The data indicated that the shortest ISP distance change during flexion and extension and supine-standing activities increased in DDD patients with moderate disc degeneration. Furthermore, the data also indicated that the shortest ISP distance change decreased in DLS patients with increasing severity of the disc degeneration. Therefore, the segmental motion of LSP may also imply a course from healthy subjects to DDD and to DLS patients that follows from stable to dysfunctional, unstable, and to restabilization. These results seem to be consistent with previous assumptions on the structural and biomechanical changes in the lumbar disc that occur with degeneration of the vertebral segment [9, 18, 26].
Because the role of the LSP in pathogenesis of low back pain remains unclear, the findings of our study may have valuable clinical implications. Recently, ISPD have been used as an alternative solution to the conventional decompressive surgery for DDD, lumbar spinal stenosis caused by degenerative lumbar spondylolisthesis (DLS) [27, 28]. Promising clinical outcomes have been reported. However, there are also studies reporting that the interspinous implants may result in complications, including implant migration and spinous process fracture [4, 27, 28]. Kim et al. [27, 29] indicated that degenerative lumbar spondylolisthesis appears strongly associated with the occurrence of spinous process fracture after interspinous process spacer (IPS) surgery. Current kinematic studies indicate that for elderly patients with Grade 1 DLS, not only the shortest ISP distance but also the range of the shortest ISP distance change decreased during active in vivo function of the body. Therefore, the interspinous implants that were inserted in the best fitting sizes of DDD patients may become larger than the interspinous distance in DLS patients during functional activities. This situation may cause overloading of a relevant segment that could result in a spinous process fracture or implant dislocation breakage. These complications may be exaggerated with elderly patients due to senile osteoporosis. Verhoof et al. [28] do not recommend the use of an ISP device for the treatment of DLS patients. In DDD patients, the shortest ISP distance decreased but the range of the shortest ISP distance change during flexion and extension increased. The use of interspinous implant in DDD group should restrict the hypermobility to avoid a loosely fitted device falling out from the ISP. These complications may be prevented by establishing clear indications and developing sophisticated implants with regard to motion of process. Currently as a rule of thumb, compressible devices are more intended for DDD whereas rigid ones for spinal stenosis. The data on the motion of LSP could provide more information for choosing compressible or rigid devices from a biomechanical point of view.
There are several limitations to this study that need to be noted. The patients involved in the study were specifically selected with DLS at the L4–L5 level and DDD at the L4–L5 level and L5–S1 level, which only represented a portion of all the DDD and DLS patients. It is possible that in groups with different degenerative patterns, the result might be different. The limited number of subjects is due to a large effort to collect the patients in each group to meet the enrollment requirements, as well as the time-consuming analysis to obtain accurate 3D kinematic data. However, we were still able to observe statistical significance with the number of tested subjects. Future studies should include patients with DDD and DLS at different segments. Another limitation of our finding is that we have yet to test those who have undergone interspinous process device implantations. This makes the comparison of our findings with the currently available data difficult. Despite these limitations, the present study provides new data on aberrant spinous process motion characteristics in DDD and DLS patients under various physiologic loading conditions.
In conclusion, the present study used an in vivo technique to quantify abnormal motion of the ISP in DDD and DLS patients with 3D model during non-weight bearing, weight-bearing standing, and flexion–extension of the trunk. The data could provide baseline information to help identify and characterize the pathologic motion of the ISP under in vivo physiological conditions. This information may help develop modifications of surgical technique and evaluate the effect of new surgical modalities for treatment of DDD and DLS patients.
Acknowledgments
This study was partially supported by the NIH (R21AR057989), research grant from Depuy Synthes (USA), the National Natural Science Foundation of China (81000796) and Beijing Nova program (2011085).
Conflict of interest
None.
Footnotes
Q. Yao and S. Wang contributed equally to the study.
References
- 1.Moojen WA, Arts MP, Bartels RH, Jacobs WC, Peul WC. Effectiveness of interspinous implant surgery in patients with intermittent neurogenic claudication: a systematic review and meta-analysis. Eur Spine J. 2011;20:1596–1606. doi: 10.1007/s00586-011-1873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson PA, Tribus CB, Kitchel SH. Treatment of neurogenic claudication by interspinous decompression: application of the X STOP device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2006;4:463–471. doi: 10.3171/spi.2006.4.6.463. [DOI] [PubMed] [Google Scholar]
- 3.Christie SD, Song JK, Fessler RG. Dynamic interspinous process technology. Spine. 2005;30:S73–S78. doi: 10.1097/01.brs.0000174532.58468.6c. [DOI] [PubMed] [Google Scholar]
- 4.Zucherman JF, Telles CJ. Commentary: interspinous devices, spondylolisthesis, and spinous process-related complications. Spine J. 2012;12:473–475. doi: 10.1016/j.spinee.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Kabir SM, Gupta SR, Casey AT. Lumbar interspinous spacers: a systematic review of clinical and biomechanical evidence. Spine. 2010;35:E1499–E1506. doi: 10.1097/BRS.0b013e3181e9af93. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Passias P, Li G, Wood K. Measurement of vertebral kinematics using noninvasive image matching method-validation and application. Spine. 2008;33:E355–E361. doi: 10.1097/BRS.0b013e3181715295. [DOI] [PubMed] [Google Scholar]
- 7.Kozanek M, Wang S, Passias PG, Xia Q, Li G, Bono CM, Wood KB. Range of motion and orientation of the lumbar facet joints in vivo. Spine. 2009;34:E689–E696. doi: 10.1097/BRS.0b013e3181ab4456. [DOI] [PubMed] [Google Scholar]
- 8.McGregor AH, McCarthy ID, Dore CJ, Hughes SP. Quantitative assessment of the motion of the lumbar spine in the low back pain population and the effect of different spinal pathologies of this motion. Eur Spine J. 1997;6:308–315. doi: 10.1007/BF01142676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao J, Wang S, Wan Z, Park WM, Xia Q, Wood K, Li G. Motion characteristics of the vertebral segments with lumbar degenerative spondylolisthesis in elderly patients. Eur Spine J. 2013;22:425–431. doi: 10.1007/s00586-012-2428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsey DP, Swanson KE, Fuchs P, Hsu KY, Zucherman JF, Yerby SA. The effects of an interspinous implant on the kinematics of the instrumented and adjacent levels in the lumbar spine. Spine. 2003;28:2192–2197. doi: 10.1097/01.BRS.0000084877.88192.8E. [DOI] [PubMed] [Google Scholar]
- 11.Neumann P, Wang Y, Karrholm J, Malchau H, Nordwall A. Determination of inter-spinous process distance in the lumbar spine. Evaluation of reference population to facilitate detection of severe trauma. Eur Spine J. 1999;8:272–278. doi: 10.1007/s005860050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobottke R, Koy T, Rollinghoff M, Siewe J, Kreitz T, Muller D, Bangard C, Eysel P. Computed tomography measurements of the lumbar spinous processes and interspinous space. Surg Radiol Anat. 2010;32:731–738. doi: 10.1007/s00276-010-0686-5. [DOI] [PubMed] [Google Scholar]
- 13.Xia Q, Wang S, Passias PG, Kozanek M, Li G, Grottkau BE, Wood KB. In vivo range of motion of the lumbar spinous processes. Eur Spine J. 2009;18:1355–1362. doi: 10.1007/s00586-009-1068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 15.Ganju A. Isthmic spondylolisthesis. Neurosurg Focus. 2002;13:E1. doi: 10.3171/foc.2002.13.1.2. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Wang S, Passias P, Xia Q, Wood K. Segmental in vivo vertebral motion during functional human lumbar spine activities. Eur Spine J. 2009;18:1013–1021. doi: 10.1007/s00586-009-0936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan Z, Wang S, Kozanek M, Passias PG, Mansfield FL, Wood KB, Li G. Biomechanical evaluation of the X-stop device for surgical treatment of lumbar spinal stenosis. J Spinal Disord Tech. 2011 doi: 10.1097/BSD.0b013e318227eb84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue N, Espinoza Orias AA (2011) Biomechanics of intervertebral disk degeneration. Orthop Clin North Am 42:487–499, vii. doi: 10.1016/j.ocl.2011.07.001 [DOI] [PMC free article] [PubMed]
- 19.Ihm EH, Han IB, Shin DA, Kim TG, Huh R, Chung SS. Spinous process morphometry for interspinous device implantation in Korean patients. World neurosurgery. 2011 doi: 10.1016/j.wneu.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Aylott CE, Puna R, Robertson PA, Walker C. Spinous process morphology: the effect of ageing through adulthood on spinous process size and relationship to sagittal alignment. Eur Spine J. 2012;21:1007–1012. doi: 10.1007/s00586-011-2029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bywaters EG, Evans S. The lumbar interspinous bursae and Baastrup’s syndrome. An autopsy study. Rheumatol Int. 1982;2:87–96. doi: 10.1007/BF00541251. [DOI] [PubMed] [Google Scholar]
- 22.Penning L, Irwan R, Oudkerk M. Measurement of angular and linear segmental lumbar spine flexion-extension motion by means of image registration. Eur Spine J. 2005;14:163–170. doi: 10.1007/s00586-004-0761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan Z, Wang S, Kozanek M, Xia Q, Mansfield FL, Lu G, Wood KB, Li G. The effect of the X-Stop implantation on intervertebral foramen, segmental spinal canal length and disc space in elderly patients with lumbar spinal stenosis. Eur Spine J. 2012;21:400–410. doi: 10.1007/s00586-011-2021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passias PG, Wang S, Kozanek M, Xia Q, Li W, Grottkau B, Wood KB, Li G. Segmental lumbar rotation in patients with discogenic low back pain during functional weight-bearing activities. J Bone Joint Surg Am. 2011;93:29–37. doi: 10.2106/JBJS.I.01348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cinotti G, De Santis P, Nofroni I, Postacchini F. Stenosis of lumbar intervertebral foramen: anatomic study on predisposing factors. Spine. 2002;27:223–229. doi: 10.1097/00007632-200202010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Kirkaldy-Willis WH, Farfan HF (1982) Instability of the lumbar spine. Clin Orthop Relat Res 165:110–123 [PubMed]
- 27.Kim DH, Shanti N, Tantorski ME, Shaw JD, Li L, Martha JF, Thomas AJ, Parazin SJ, Rencus TC, Kwon B. Association between degenerative spondylolisthesis and spinous process fracture after interspinous process spacer surgery. Spine J. 2012 doi: 10.1016/j.spinee.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 28.Verhoof OJ, Bron JL, Wapstra FH, van Royen BJ. High failure rate of the interspinous distraction device (X-Stop) for the treatment of lumbar spinal stenosis caused by degenerative spondylolisthesis. Eur Spine J. 2008;17:188–192. doi: 10.1007/s00586-007-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DH, Tantorski M, Shaw J, Martha J, Li L, Shanti N, Rencu T, Parazin S, Kwon B. Occult spinous process fractures associated with interspinous process spacers. Spine. 2011;36:E1080–E1085. doi: 10.1097/BRS.0b013e318204066a. [DOI] [PubMed] [Google Scholar]