Abstract
Introduction
Anterior lumbar interbody fusion (ALIF) is an established treatment for structural instability associated with symptomatic disk degeneration (SDD). Stand-alone ALIF offers many advantages, however, it may increase the risk of non-union. Recombinant human bone morphogenetic protein-2 (BMP-2) may enhance fusion rate but is associated with postoperative complication. The optimal dose of BMP-2 remains unclear. This study assessed the fusion and subsidence rates of stand-alone ALIF using the SynFix-LR interbody cage with 6 ml/level of BMP-2.
Methods
Thirty-two ALIF procedures were performed by a single surgeon in 25 patients. Twenty-five procedures were performed for SDD without spondylolisthesis (SDD group) and seven procedures were performed for SDD with grade-I olisthesis (SDD-olisthesis group). Patients were followed-up for a mean of 17 ± 6 months.
Results
Solid fusion was achieved in 29 cases (90.6 %) within 6 months postoperatively. Five cases of implant subsidence were observed (16 %). Four of these occurred in the SDD-olisthesis group and one occurred in the SDD group (57 % vs. 4 % respectively; p = 0.004). Three cases of subsidence failed to fuse and required revision. The body mass index of patients with olisthesis who developed subsidence was higher than those who did not develop subsidence (29 ± 2.6 vs. 22 ± 6.5 respectively; p = 0.04). No BMP-2 related complications occurred.
Conclusion
The overall fusion rate of stand-alone ALIF using the SynFix-LR system with BMP-2 was 90.6 %, comparable with other published series. No BMP-2 related complication occurred at a dose of 6 mg/level. Degenerative spondylolisthesis and obesity seemed to increase the rate of implant subsidence, and thus we believe that adding posterior fusion for these cases should be considered.
Keywords: ALIF, Spine fusion, BMP-2, PEEK cage, Subsidence
Introduction
Anterior lumbar interbody fusion (ALIF) is frequently used to treat structural instability in the setting of symptomatic disc degeneration (SDD) [1, 2]. The anterior approach to the spine offers many advantages over the posterior approach, including sparing of the lumbar para-spinal musculature, improved postoperative mobility, decreased chronic muscle pain and the ability to expand the interbody device within the predominant load-bearing column of the spine enabling to re-establish its normal anatomical alignment [2–4].
Despite numerous advantages, ALIF with autogenous bone graft (e.g. iliac crest) as a stand-alone procedure has been associated with high rates of non-union (~44 %) [5], subsidence, and graft extrusion, due to its inability to achieve adequate stability necessary for vertebral interbody fusion [3, 5, 6]. Adding posterior instrumented fusion led to improved stability increased fusion rate and has gained acceptance by many surgeons as the standard of care [3]. Naturally, combined anterior-posterior approach has been associated with prolonged operation time, increased blood loss and complications rate [7].
To overcome the need for posterior stabilization new interbody cages made of metal or composite materials have been devised. However, despite the improved mechanical stability of these cages, when used with bone graft in stand-alone ALIF the reported fusion rate was only 16–70 % [7, 8]. In an attempt to enhance fusion, an osteoinductive growth factor (i.e. Bone Morphogenetic Protein-2 [BMP-2]) was used and, indeed, increased fusion rate of stand-alone ALIF using a metal cage to 91–94 % [3, 9–12].
Despite the encouraging fusion outcomes metal cages subsidence into the vertebral bodies described [13–15]. Interbody cages made of the non-absorbable, biocompatible material polyetheretherketone (PEEK) are radiolucent and have modulus of elasticity similar to bone [13, 16]. Using PEEK cages may offer an advantage over metal cages in load-bearing that may reduce subsidence rate. Also, due to its radiolucency interbody cages made of PEEK may enable easier assessment of fusion in radiographs [17, 18].
The objectives of this study were to evaluate fusion and subsidence rate following stand-alone ALIF using a PEEK interbody cage (Synthes, SynFix-LR) with BMP-2 and to identify factors affecting union. No previous study has evaluated fusion rate in this setting to the best of our knowledge.
Patients and methods
Study design
This was a retrospective radiographic outcome evaluation of stand-alone ALIF procedures with BMP-2 augmentation. All procedures were performed for lumbar SDD by a single team in our spine surgery unit.
Selection of participants
We reviewed the medical records and lumbar spine imaging (X-rays, CT and MRI scans) of all patients who underwent ALIF procedures in our spine surgery unit between December 2008 and December 2011. Patients were included in the study based on the following criteria: (1) lumbar SDD with mechanical disabling low back pain over a period of at least 6 months without improvement under conservative treatment, (2) no other spinal pathology apart from grade-I degenerative spondylolisthesis, (3) involvement of one or more of the following discs: L3-4, L4-5, L5-S1, (4) minimum of 12-month follow-up from surgery, (5) MRI of lumbar spine with Modic changes in vertebral end plates and disc height <7 mm [19, 20].
Patients with a medical condition affecting bone healing (e.g. diabetes mellitus), previous instrumented lumbar spine operations, patients who smoked or were taking medication that may affect bone healing (e.g. corticosteroids, non-steroidal anti-inflammatory medications) were excluded from the study.
Study protocol
All patients underwent a full clinical evaluation including a thorough physical examination and MRI scan of their lumbar spine prior to the surgery. Whenever a multi-level pathology was found, a diagnostic discography was made to help determine the symptomatic level [21]. ALIF was done through an open approach under fluoroscopy control with pulse oxygen meter placed on left foot [22, 23]. Patients were placed in the supine position and a standard retroperitoneal approach was carried out with ligation of the segmental vessels. The great vessels were mobilized, exposing the anterior surface and lateral borders of the disc space. The midpoint of the disc space was identified with radiographic markers and fluoroscopy. Then, an incision was made in the anterior portion of the annulus, removing the anterior longitudinal ligament and the anterolateral borders of the annulus fibrosus. Under direct visualization the entire contents of the disc space were removed, including the nucleus pulposus and the cartilaginous endplates. Two perforations were made with a curette in the central endplate area coinciding with the area in the cage which accommodates the BMP2 sponges. The disc space was sequentially distracted to the height of normal adjacent disc space height. Final amount of intra-discal distraction and cage size was determined by intraoperative assessment of annular tension and direct visualization of the disc space dimensions.
A Synthes SynFix-LR cage was used (Synthes Inc, West Chester, PA, USA) in all operations. Each cage was filled with sponge soaked with 6 mg of BMP-2 (Medtronic Infuse, Minneapolis, USA). The cages were inserted into the prepared intervertebral disc space. Cage placement was assessed under fluoroscopy in both the antero-posterior and lateral views [24]. Cage fixation was performed with four screws inserted through pre-made cage bores, two screws to the vertebral body above the cage, and two screws to the vertebra below.
Postoperatively, the patients were allowed to mobilize as pain allowed. No bracing was given. Isometric strengthening and an exercise program were started at 6 weeks following surgery.
Outcome measures
Patients were evaluated for fusion at 3, 6, 12 and 18 months after surgery. Independent orthopedic and radiologist consultant interpreted all radiographs MRI’s and CT scans. A third independent orthopedic consultant adjudicated controversial fusion findings.
Fusion was assessed by high-quality spine radiographs based on the following criteria: (1) visible bridging bone either through the cage or surrounding it as seen on anterior-posterior or lateral radiographs [25]; (2) vertebral body translation of <3 mm on lateral radiographs [9]; (3) lack of substantial sclerotic changes in the recipient bone bed [7]. Brantigan et al. [25] found that the sensitivity, positive predictive value and overall accuracy of spine radiographs to detect bone union following spinal fusion with PEEK cage was 97, 94 and 93 % respectively. (Figs. 1, 2).
Fig. 1.
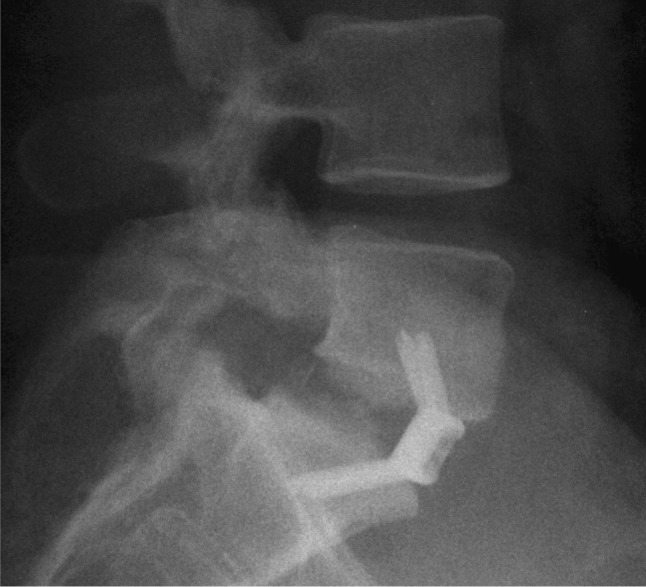
Lateral radiograph of L5-S1 level showing non-union following spinal fusion with a PEEK cage
Fig. 2.
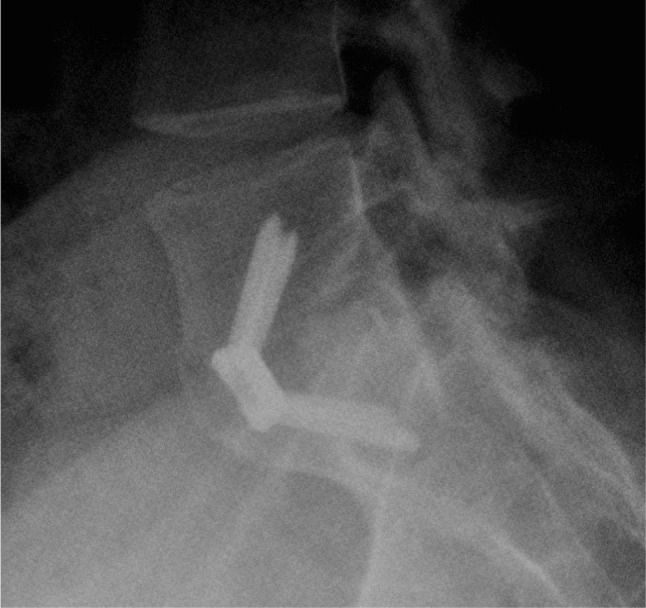
Lateral spine radiographs of L5-S1 level showing solid bone union following spinal fusion with PEEK cage
Subsidence was assessed based on the following criteria: (1) disc space height loss of >1 mm (indicating cage protrusion into the cancellous vertebral bone) [7]; (2) visible fracture of the vertebral body endplate [7]. Spondylolisthesis was measured using the Meyerding method [26].
A thin layer slicing CT scan (<1 mm) was performed in all cases where bone fusion on plain radiographs was in doubt (CT scan was performed in 8 patients). Assessment of fusion was done according to the protocol of Williams et al. [27]: (1) lack of any lucency at the cage margins; (2) lack of any visible fracture of the cage or vertebrae; (3) lack of any cystic changes within the endplates adjacent to the cage; (4) lack of any linear defects (fracture) through the intervertebral new bone formation within or surrounding the cage; (5) lack of subsidence or dislocation of cage; (6) bridging bone surrounding or within the cage. (Fig. 3)
Fig. 3.
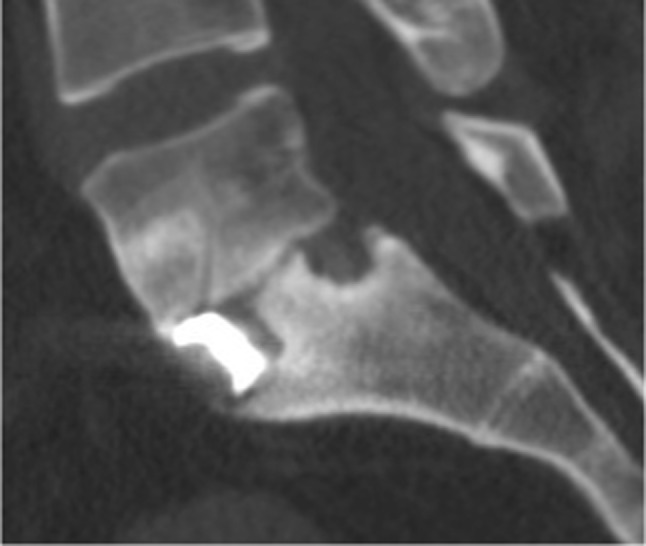
A sagittal CT image of L5-S1 level showing non-union following spinal fusion with a PEEK cage
Data analysis
Continuous parameters were described as the mean and the standard deviation (SD) with 95 % confidence intervals (CI). Categorical parameters were described with proportions and 95 % confidence intervals. Comparisons between procedures performed for SDD without olisthesis (SDD group) and procedures performed for SDD with olisthesis (SDD-olisthesis group) were performed using two-tailed unpaired t test for continuous parameters and the Fisher exact test for categorical parameters. Statistical analysis was performed using SPSS for Windows (version 16.0; IBM, Chicago, Illinois). Significance level was set at p < 0.05.
Results
Twenty-five patients who underwent ALIF in our spine surgery unit met all study criteria and were available for data analysis. Eighteen of them were females (72 %). The average age of the study group was 52 ± 14 years (range 23–71 years). A total of 32 ALIF operations was performed: three fusions of L3-4 level (9.4 %), 15 fusions of L4-5 level (46.9 %) and 14 fusions of L5-S1 level (43.7 %). The average post-operative follow-up was 17 ± 6 months (range 12–31 months). The patients’ characteristics and radiographic outcomes are summarized in Table 1.
Table 1.
Study cohort and radiographic outcomes
| Number of patients | 25 |
| Age at surgery, (years ± SD) | 52 ± 14 |
| Gender, n (%) | |
| Males | 7 (28 %) |
| Females | 18 (72 %) |
| Number of levels fused, n (%) | |
| Patients who had 1-level fusion | 18 (72 %) |
| Patients who had 2-level fusion | 7 (28 %) |
| Number of procedures performed | 32 |
| Follow-up (months ± SD) | 16.7 ± 5.8 |
| Subsidence rate, n (% of procedures) | 5 (15.6 %) |
| Fusion Rate, n (% of procedures) | 29 (90.6 %) |
Solid asymptomatic fusion was achieved in 29 of the 32 procedures (90.6 %). Four cases fused within 3-month (12.5 %) and 25 cases (78.1 %) fused within 6-month follow-up. Implant subsidence was observed in five operated levels (in five patients, four females and one male). Two subsidence cases united uneventfully in 6-month follow-up. The remaining three cases of subsidence (in three patients, two females and one male) resulted in symptomatic non-union (9.4 % of procedures), and underwent revision surgery with postero-lateral instrumented fusion.
Twenty-five ALIF procedures (in 18 patients) were performed for SDD without olisthesis (SDD-group) and seven procedures (in seven patients) for SDD with grade I olisthesis (SDD-olisthesis group). Patients with olisthesis were older than patients without olisthesis (66 ± 4 vs. 51 ± 13 years respectively, difference 15 years, 95 % CI 4 to 25; p = 0.007). Gender distribution was similar in both groups (72 % females in the SDD group vs. 57 % in the SDD-olisthesis group, difference 15, 95 % CI −18 to 49; p = 0.64). The follow-up duration was similar for both groups as well (16 ± 6 vs. 19 ± 5 months respectively, difference 3 months, 95 % CI −2 to 8; p = 0.25).
Subsidence was observed in 1 procedure in the SDD group and in 4 procedures in the SDD-olisthesis group (4 vs. 57 %, difference 53, 95 CI 17–80 %; p = 0.004). Two subsidence cases in the SDD-olisthesis group united uneventfully within 6 months. The remaining two subsidence cases in the SDD-olisthesis group and the single subsidence case in the SDD group resulted in symptomatic non-union and required revision surgery as mentioned above. Union was achieved in 24 of 25 cases in the SDD group (96 %) and in 5 of 7 cases (72 %) in the SDD-olisthesis group (difference 24, 95 % CI −1 to 60 %; p = 0.11). Nevertheless, due to the small size of these subgroups the study was underpowered to detect a difference in fusion rates. Therefore, the statistical difference between the groups could not be claimed confidently.
The body mass index (BMI) of both SDD-group and SDD-olisthesis group was similar (24.8 ± 4.5 vs. 25.6 ± 5.6, difference 0.8 kg, 95 % CI −3.3 to 4.9; p = 0.69). However, in the SDD-olisthesis group the four patients who had subsidence had significantly higher BMI than the rest of the group (29.2 ± 2.6 vs. 22.1 ± 6.5, difference 7.1 BMI, 95 % CI 0.2–14; p = 0.04). (Table 2)
Table 2.
Characteristics and outcomes of procedures performed for non-olisthetic compared to olisthetic levels
| SDD (25 procedures, 18 patients) | SDD-olisthesis (7 procedures, 7 patients) | Difference (95 % CI) | p value | |
|---|---|---|---|---|
| Age at surgery, years ± SD | 51 ± 13 | 66 ± 4 | 15 (4, 25) | 0.005 |
| Gender, n (%) | ||||
| Males | 7 (28 %) | 3 (43 %) | 15 % (−18 %, 49 %) | 0.64 |
| Females | 18 (72 %) | 4 (57 %) | ||
| BMI ± SD | 24.8 ± 4.5 | 25.6 ± 5.6 | 0.8 (−3, 5) | 0.69 |
| Index operation level, n (%) | ||||
| L3-4 | 3 (12 %) | 0 (0 %) | 12 % (−24 %, 30 %) | 0.99 |
| L4-5 | 8 (32 %) | 7 (100 %) | 68 % (27 %, 83 %) | 0.002 |
| L5-S1 | 14 (56 %) | 0 (0 %) | 56 % (16 %, 73 %) | 0.01 |
| Number of levels fused, n (%) | ||||
| Patients who had 1-level fusion | 11 (61 %) | 7 (100 %) | ||
| Patients who had 2-level fusion | 7 (39 %) | 0 (0 %) | ||
| Follow-up, months ± SD | 16 ± 6 | 19 ± 5 | 3 (−2, 8) | 0.23 |
| Subsidence rate, n (%) | 1 (4 %)a | 4 (57 %)b | 53 % (17 %, 80 %) | 0.004 |
| Fusion rate, n (%) | ||||
| Fused in 3 months | 2 (8 %) | 2 (29 %) | 21 % (−5 %, 56 %) | 0.20 |
| Fused in 6 months | 22 (88 %) | 3 (43 %) | 45 % (8 %, 73 %) | 0.02 |
| Overall fusion rate | 24 (96 %) | 5 (72 %) | 24 % (−1 %, 60 %) | 0.11 |
| Non-union (18-month follow-up)c | 1 (4 %) | 2 (28 %) | 24 % (−1 %, 60 %) | 0.11 |
SDD procedures performed for symptomatic disc degeneration without olisthesis, SDD-olisthesis procedures performed for symptomatic disc degeneration with grade-I olisthesis
aCage subsidence into L4 vertebra following L3-4 procedure
bCage subsidence into L5 vertebra following L4-5 procedure in all cases
cAll three cases of non-union underwent revision surgery with postero-lateral instrumented fusion
Discussion
Achieving early, solid and long-lasting bone union is the goal of any spinal fusion regardless of the surgical approach and technique [2]. Posterior and postero-lateral approaches to the lumbar spine have been traditionally used for spinal fusion. However, the extensive muscle striping involved in these approaches may compromise the functional outcome and complicate the rehabilitation process [28, 29]. The advantages of ALIF without posterior fusion have been extensively documented [6, 30].
The use of the osteoinductive protein BMP-2 in spine surgery has been previously studied and was found to enhance fusion rate [4, 9, 31, 32]. However, stand-alone ALIF using BMP-2 with allograft without cage implantation showed 62 % subsidence rate due to massive resorption of the allograft [33]. A second generation interbody cages made of non-absorbable material have been developed to increase the structural stability in spinal fusion and to improve fusion rate.
The aim of this study was to evaluate the fusion rate following stand-alone ALIF using the SynFix-LR system with BMP-2. The SynFix-LR cage is a polyetheretherketone (PEEK) implant with an integrated anterior plate, which additionally stabilizes the motion segment using four angle-locked screws. Biomechanical studies showed that the stability achieved with this cage is comparable with the stability of the traditional combined anterior-posterior approach [34, 35] and is adequate biomechanically for stand-alone anterior fusion [7, 34, 35]. The PEEK cage with its large area carries most of the forces in flexion, whereas the integrated plate with the four diverging cortically anchored screws neutralizes the forces in extension and rotation. A recent study by Strube et al. [7] reported 70.6 % fusion rate using this cage in stand-alone operation without BMP-2 and 68.7 % fusion when posterior stabilization was added, therefore arguing against the need of additional posterior stabilization.
In our study stand-alone ALIF with the use of BMP-2 resulted in fusion rate of 90.6 % (29 of 32 procedures) in 18-month follow-up. Similar findings were reported by Burkus et al. [4] who found 94.4 % fusion rate using the Medtronic LT-Cage device (metal cage) at 24-month follow-up and 91 % fusion rate at 6-year follow-up [9] following stand-alone ALIF with BMP-2. Five cases of implant subsidence into the adjacent vertebra occurred in our cohort (15.6 %) and seems comparable with (or ever lower than) subsidence rates of 25 % reported in other series [15, 36]. Three of our subsidence cases resulted in symptomatic non-union which require revision surgery. Significantly more subsidences (4 of the 5 subsidence cases) occurred in patients with SDD and olisthesis. The higher subsidence rate in the SDD-olisthesis group resulted in lower fusion rate (72 %) compared to the SDD group (96 %). However due to the small cohort we were not able to determine the significance of this difference. To our knowledge, the influence of olisthetic on fusion rate and subsidence following stand-alone ALIF was not reported previously.
Complications related to BMP-2 use in ALIF procedures were reported in previous studies. Postoperative leg pain without MRI evidence of root compression was reported in 17 % of patients [37], urinary retention and retrograde ejaculation were reported in 9 % of males [38]. Interestingly, no BMP-2 related complications were documented in our cohort. This lack of complications may be explained by the lower dose of BMP-2 used in our cohort, compared to previous reports (6 mg/level vs. 12 mg/level respectively [36]), which may have reduced the dose dependent hyper-inflammatory nature of BMP-2. Furthermore, we refrained from placing BMP-2 anterior or posterior to the cage and placed it only inside and lateral to it, but still contained within the annulus. We also irrigated the surgical site thoroughly to wash out traces of BMP-2 not incorporated into the sponge. Our findings suggest that the use of 6 mg/level of BMP-2 in spinal fusion is safe and effective. However, recognizing our limited cohort we believe that larger studies are required to determine the optimal dose of BMP in spinal fusion.
Several factors seem to affect the significantly higher subsidence rate in our SDD-olisthesis group: (1) significantly older SDD-olisthesis patients (66 years) as compared to SDD patients (51 years). Since degenerative spondylolisthesis is a late sign on the symptomatic degenerated disc continuum [39] it is not surprising that decreased bone mass density associated with aging may explain the higher cage subsidence into the brittle vertebral body [40, 41]. (2) Patients in the SDD-olisthesis group with subsidence were found to have significantly higher BMI (29.2 BMI) than patients who did not have subsidence (22.1 BMI). It has been shown that increased BMI is associated with factors such as accelerated disc degeneration [42], increased sacral slope and increased L1-S1 lordosis, this postural changes may lead to lumbar olisthesis, increased instability and cage subsidence [43].
Our study had several potential limitations. First, this was a retrospective study with a limited cohort, which reduced the power of the study to detect differences between the study subgroups and to determine the significance of these differences. Second, patients’ ages differ significantly between the SDD and SDD-olisthesis groups. The study did not assess bone mineral density and age-related differences in bone density could potentially affect our results. Finally, we excluded patients with risk factors that could affect bone union (e.g. diabetes, smoking, corticosteroids use, etc.). Excluding these patients may lead to underestimation of non-union rate.
In conclusion, we found that the overall fusion rate of stand-alone ALIF using the SynFix-LR system with BMP-2 was 90.6 %, comparable with other published series. No BMP-2 related complication occurred with the use of 6 mg/level BMP-2. Subgroup analysis revealed higher subsidence rate in procedures performed for degenerative spondylolisthesis in overweight patients. We believe that for these cases adding posterior stabilization should be considered.
Acknowledgments
I hereby declare that no funding or grants were given to conduct this study.
Conflict of interest
None.
References
- 1.Czerwein JK, Jr, et al. Complications of anterior lumbar surgery. J Am Acad Orthop Surg. 2011;19(5):251–258. doi: 10.5435/00124635-201105000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Quraishi NA, et al. Access related complications in anterior lumbar surgery performed by spinal surgeons. Eur Spine J. 2013;22(Suppl 1):16–20. doi: 10.1007/s00586-012-2616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkus JK, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15(5):337–349. doi: 10.1097/00024720-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Burkus JK, et al. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16(2):113–122. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Flynn JC, Hoque MA. Anterior fusion of the lumbar spine. End-result study with long-term follow-up. J Bone Joint Surg Am. 1979;61(8):1143–1150. [PubMed] [Google Scholar]
- 6.Stauffer RN, Coventry MB. Anterior interbody lumbar spine fusion. Analysis of Mayo Clinic series. J Bone Joint Surg Am. 1972;54(4):756–768. [PubMed] [Google Scholar]
- 7.Strube P, et al. Stand-alone anterior versus anteroposterior lumbar interbody single-level fusion after a mean follow-up of 41 months. J Spinal Disord Tech. 2012;25(7):362–369. doi: 10.1097/BSD.0b013e3182263d91. [DOI] [PubMed] [Google Scholar]
- 8.Pellise F, et al. Low fusion rate after L5-S1 laparoscopic anterior lumbar interbody fusion using twin stand-alone carbon fiber cages. Spine (Phila Pa 1976) 2002;27(15):1665–1669. doi: 10.1097/00007632-200208010-00015. [DOI] [PubMed] [Google Scholar]
- 9.Burkus JK, et al. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181–1189. doi: 10.2106/JBJS.G.01485. [DOI] [PubMed] [Google Scholar]
- 10.Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine (Phila Pa 1976) 2003;28(4):372–377. doi: 10.1097/01.BRS.0000048469.45035.B9. [DOI] [PubMed] [Google Scholar]
- 11.Boden SD, et al. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine (Phila Pa 1976) 2000;25(3):376–381. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 12.Burkus JK, et al. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 2002;27(21):2396–2408. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 13.Schimmel JJ et al (2012) PEEK Cages in Lumbar Fusion: Mid-term Clinical Outcome and Radiological Fusion. J Spinal Disord Tech. doi:10.1097/BSD.0b013e31826eaf74
- 14.Spruit M, et al. The in vitro stabilising effect of polyetheretherketone cages versus a titanium cage of similar design for anterior lumbar interbody fusion. Eur Spine J. 2005;14(8):752–758. doi: 10.1007/s00586-005-0961-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Galbusera F, Schmidt H, Wilke H-J. Lumbar interbody fusion: a parametric investigation of a novel cage design with and without posterior instrumentation. Eur Spine J. 2012;21(3):455–462. doi: 10.1007/s00586-011-2014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions. Spine (Phila Pa 1976) 1993;18(9):1186–1189. doi: 10.1097/00007632-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 18.McAfee PC, et al. Symposium: a critical discrepancy-a criteria of successful arthrodesis following interbody spinal fusions. Spine (Phila Pa 1976) 2001;26(3):320–334. doi: 10.1097/00007632-200102010-00020. [DOI] [PubMed] [Google Scholar]
- 19.Modic MT, et al. Imaging of degenerative disk disease. Radiology. 1988;168(1):177–186. doi: 10.1148/radiology.168.1.3289089. [DOI] [PubMed] [Google Scholar]
- 20.Modic MT, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 21.Manchikanti L, et al. Systematic review of lumbar discography as a diagnostic test for chronic low back pain. Pain Phys. 2009;12(3):541–559. [PubMed] [Google Scholar]
- 22.Konig MA, et al. The routine intra-operative use of pulse oximetry for monitoring can prevent severe thromboembolic complications in anterior surgery. Eur Spine J. 2011;20(12):2097–2102. doi: 10.1007/s00586-011-1900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brau SA. Expert’s comment concerning Grand Rounds case entitled “The routine intra-operative use of pulse oximetry for monitoring can prevent severe thromboembolic complications in anterior surgery” (by M.A. Konig, Y. Leung, S. Jurgens, S. MacSweeney and B.M. Boszczyk) Eur Spine J. 2011;20(12):2103–2104. doi: 10.1007/s00586-011-1947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakshmanan P, et al. Sagittal endplate morphology of the lower lumbar spine. Eur Spine J. 2012;21(Suppl 2):S160–S164. doi: 10.1007/s00586-012-2168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brantigan JW, et al. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine (Phila Pa 1976) 2000;25(11):1437–1446. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Hresko MT. Radiographic analysis of spondylolisthesis and sagittal spinopelvic deformity. J Am Acad Orthop Surg. 2012;20(4):194–205. doi: 10.5435/JAAOS-20-04-194. [DOI] [PubMed] [Google Scholar]
- 27.Williams AL, Gornet MF, Burkus JK. CT evaluation of lumbar interbody fusion: current concepts. AJNR Am J Neuroradiol. 2005;26(8):2057–2066. [PMC free article] [PubMed] [Google Scholar]
- 28.Kahanovitz N, Viola K, Gallagher M. Long-term strength assessment of postoperative diskectomy patients. Spine (Phila Pa 1976) 1989;14(4):402–403. doi: 10.1097/00007632-198904000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Beimborn DS, Morrissey MC. A review of the literature related to trunk muscle performance. Spine (Phila Pa 1976) 1988;13(6):655–660. [PubMed] [Google Scholar]
- 30.Penta M, Sandhu A, Fraser RD. Magnetic resonance imaging assessment of disc degeneration 10 years after anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1995;20(6):743–747. doi: 10.1097/00007632-199503150-00018. [DOI] [PubMed] [Google Scholar]
- 31.Glassman SD, et al. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine (Phila Pa 1976) 2011;36(22):1849–1854. doi: 10.1097/BRS.0b013e3181d133d0. [DOI] [PubMed] [Google Scholar]
- 32.Valdes MA, et al. Recombinant bone morphogenic protein-2 in orthopaedic surgery: a review. Arch Orthop Trauma Surg. 2009;129(12):1651–1657. doi: 10.1007/s00402-009-0850-8. [DOI] [PubMed] [Google Scholar]
- 33.Vaidya R, et al. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342–345. doi: 10.1302/0301-620X.89B3.18270. [DOI] [PubMed] [Google Scholar]
- 34.Cain CM, et al. A new stand-alone anterior lumbar interbody fusion device: biomechanical comparison with established fixation techniques. Spine (Phila Pa 1976) 2005;30(23):2631–2636. doi: 10.1097/01.brs.0000187897.25889.54. [DOI] [PubMed] [Google Scholar]
- 35.Schleicher P, et al. Biomechanical comparison of two different concepts for stand alone anterior lumbar interbody fusion. Eur Spine J. 2008;17(12):1757–1765. doi: 10.1007/s00586-008-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mroz TE, et al. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9S):S86–S104. doi: 10.1097/BRS.0b013e3181d81ef2. [DOI] [PubMed] [Google Scholar]
- 37.Rowan FE, O’Malley N, Poynton A. RhBMP-2 use in lumbar fusion surgery is associated with transient immediate post-operative leg pain. Eur Spine J. 2012;21(7):1331–1337. doi: 10.1007/s00586-011-2113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tepper G, et al. Quantitative assessment of retrograde ejaculation using semen analysis, comparison to a standardized qualitative questionnaire and investigating the impact of rhBMP-2. Spine J. 2012;12(9):8. doi: 10.1016/j.spinee.2012.08.251. [DOI] [PubMed] [Google Scholar]
- 39.Majid K, Fischgrund JS. Degenerative lumbar spondylolisthesis: trends in management. J Am Acad Orthop Surg. 2008;16(4):208–215. doi: 10.5435/00124635-200804000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa K, et al. An experimental study on the interface strength between titanium mesh cage and vertebra in reference to vertebral bone mineral density. Spine (Phila Pa 1976) 2001;26(8):957–963. doi: 10.1097/00007632-200104150-00022. [DOI] [PubMed] [Google Scholar]
- 41.Okuyama K, et al. Influence of bone mineral density on pedicle screw fixation: a study of pedicle screw fixation augmenting posterior lumbar interbody fusion in elderly patients. Spine J. 2001;1(6):402–407. doi: 10.1016/S1529-9430(01)00078-X. [DOI] [PubMed] [Google Scholar]
- 42.Liuke M, et al. Disc degeneration of the lumbar spine in relation to overweight. Int J Obes (Lond) 2005;29(8):903–908. doi: 10.1038/sj.ijo.0802974. [DOI] [PubMed] [Google Scholar]
- 43.Schuller S, Charles YP, Steib JP. Sagittal spinopelvic alignment and body mass index in patients with degenerative spondylolisthesis. Eur Spine J. 2011;20(5):713–719. doi: 10.1007/s00586-010-1640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


