Abstract
Purpose
Vertebral body defects represent one of the most common orthopedic challenges. In order to advance the transfer of stem cell therapies into orthopedic clinical practice, we performed this study to evaluate the safety and efficacy of a composite bioartificial graft based on a hydroxyapatite bone scaffold (CEM-OSTETIC®) combined with human mesenchymal stem cells (MSCs) in a rat model of vertebral body defects.
Methods
Under general isoflurane anesthesia, a defect in the body of the L2 vertebra was prepared and left to heal spontaneously (group 1), implanted with scaffold material alone (group 2), or implanted with a scaffold together with 0.5 million MSCs (group 3) or 5 million MSCs (group 4). The rats were killed 8 weeks after surgery. Histological and histomorphometrical evaluation of the implant as well as micro-CT imaging of the vertebrae were performed.
Results
We observed a significant effect on the formation of new bone tissue in the defect in group 4 when compared to the other groups and a reduced inflammatory reaction in both groups receiving a scaffold and MSCs. We did not detect any substantial pathological changes or tumor formation after graft implantation.
Conclusions
MSCs in combination with a hydroxyapatite scaffold improved the repair of a model bone defect and might represent a safe and effective alternative in the treatment of vertebral bone defects.
Keywords: Vertebral body defect, Mesenchymal stem cells, Hydroxyapatite scaffold, Rat model
Introduction
Vertebral body fractures represent one of the most common orthopedic disorders. Depending on the type and severity of the injury, both conservative and/or surgical treatment is applied. Severe vertebral bone defects requiring vertebral body replacement by an autologous graft or vertebroplasty occur mainly due to (1) traumatic comminute fractures, (2) compressive fractures due to osteoporosis, (3) vertebral osteolytic malignant tumor metastases or (4) vertebral hemangioma [1]. Traumatic comminute fractures affect mostly young people and often have a severe impact on the integrity of the spinal column, in a significant number of cases complicated by spinal cord injury [2, 3]. Moreover, the integrity of the spine can be disrupted by other pathological processes, for example metastatic foci of malignant tumors or metabolic disorders (osteoporosis, hyperparathyroidism, Paget disease) [1].
As a standard surgical treatment, autologous bone grafts are used to promote healing of the vertebral body. However, various problems are associated with graft harvesting, including donor site morbidity and often a limited amount of autologous grafting material. The possible use of allografts is accompanied by significant drawbacks including immunogenicity and possible microbial contamination. An unpredictable portion of the free grafting material is also lost by resorption. Finding an alternative treatment strategy and the development of bioartificial bone graft substitutes are therefore one of the major challenges in orthopedics. For the treatment of bone defects, synthetic calcium-based bone materials are used due to their similarity to the mineral phase of bone, their osteoconductivity and good biocompatibility [4, 5]. However, for the successful repair of large bone defects, the grafted material should also have osteoinductive or even osteogenic properties. In order to enhance bone formation within the implanted synthetic grafts, various growth factors such as bone morphogenic protein 2 (BMP-2), tumor necrosis factor beta (TGF-β) or insulin-like growth factor-1 (IGF-1) have been used in combination with the synthetic bone material [6, 7]. Additionally, methods of tissue engineering using mesenchymal stem cells (MSCs) have been studied as a potential therapeutic tool for bone tissue regeneration [8, 9]. The osteogenic and immunological properties of MSCs, together with the possibility to relatively easily obtain, cultivate and produce these cells in large amounts, represent advantages over other types of cells. MSCs are particularly attractive for their low immunogenicity [10]. They were shown to suppress the proliferation of immune cells (T and B lymphocytes, dendritic cells, natural killer cells) and to modulate the fate and function of macrophages [11, 12]. Combined with hydroxyapatite scaffolds, they enhanced the osteoinductivity of calcium-based scaffolds and promoted bone healing in various experimental bone defects including long bone fractures [13], spinal fusion [14] and craniofacial defects [15]. However, the specific biomechanical properties of vertebrae do not allow generalizing the results from other types of bones to those of vertebral body defects treated with a tissue engineered graft. In response to this, animal models of vertebral body defects in rats or sheep were recently used to study the effect of bone replacement materials [16, 17]. Nevertheless, reports discussing the effect of stem cell-based bone substitutes in the treatment of vertebral body defects are rare in the literature, although this approach represents a promising tool for vertebral bone regeneration. In particular, the possibility of using autologous human MSCs to improve bone healing without the necessity for long precultivation of the cells on a scaffold prior to transplantation might significantly simplify the treatment of such bone defects and provide a viable alternative to traditional surgery.
In order to implement stem cell therapies into orthopedic clinical practice, we have conducted a study to evaluate the safety and efficacy of a hydroxyapatite bone scaffold combined with defined human bone marrow-derived mesenchymal stem cells in a rat model of vertebral body defect.
Materials and methods
Animals
We used 30 Wistar rats (Velaz, Prague, Czech Republic) with body weights of 300–350 g. We included only males in our study to minimize the effects of hormone levels on the variability of the healing as well as bone regeneration [2, 18]. This study was performed in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) regarding the use of animals in research and was approved by the Ethical Committee of the Institute of Experimental Medicine ASCR, Prague, Czech Republic. All efforts were made to minimize the number of animals used in the study.
Experimental groups
Rats were randomly divided into one of the following groups: (1) rats with a vertebral defect only (group 1) (n = 8); (2) rats with a vertebral defect and with an implanted hydroxyapatite scaffold (group 2) (n = 7); (3) rats with a vertebral defect and with a hydroxyapatite scaffold as well as 0.5 × 106 human MSCs implanted (group 3) (n = 7); (4) rats with a vertebral defect and with a hydroxyapatite scaffold as well as 5 x 106 human MSCs implanted (group 4) (n = 8).
Cell isolation and cultivation
Human MSCs were isolated from the bone marrow of four different human donors aged between 28 and 66 years. All procedures for MSC preparation were performed under good manufacturing practice conditions (GMP) in Bioinova, Ltd. (Prague, Czech Republic) and approved by the State Institute for Drug Control of the Czech Republic (SUKL, Czech Republic). The mononuclear fraction containing MSCs was separated from the blood by gradient centrifugation using 25 % Gelofusin® (B. Braun, Melsungen, Germany) and seeded on plastic dishes at a concentration of 5–10 × 106 cells/75 cm2. The cells were cultivated in media containing Alpha MEM Eagle without deoxyribonucleotides, ribonucleotides and UltraGlutamin (Lonza, Basel, Switzerland) supplemented with 5 % thrombocyte lysate (Bioinova, Prague, Czech Republic) and 10 μg/ml gentamicine (Lek Pharmaceuticals, Ljublanja, Slovenia); non-adherent cells were washed out by changing the medium. When the cells reached 80 % confluence, they were detached from the surface of the dishes with 1 ml/75 cm2 of TrypLE CTS Select™ solution (Gibco, Ca, USA) and expanded. Cells of the second passage were analyzed and used in further experiments. The expression of specific surface markers was assessed using fluorescent-activated cell sorting (FACS) analysis (FACSAria flow cytometer, BD Biosciences, San Diego, USA). The cells expressed CD105, CD73 and CD90 and were negative for CD45, CD34, CD14 or CD11b, CD79alpha and HLA-DR surface molecules. In order to verify the differentiation potential of the MSCs, the cells were differentiated into osteogenic, chondrogenic and adipogenic lineages using standard differentiation media as described previously [19]. Cell viability (more than 95 %) was evaluated by using trypan blue staining, and the cultures were tested for the presence of bacterial, fungal and mycoplasmatic contamination. Though the ages of the donors ranged over almost 40 years, cell cultures grown under standard operating procedures in GMP facilities did not show any significant differences in cell proliferation or differentiation between the donors. The cells were frozen in aliquots in saline containing 7.5 % dimethylsulfoxide (DMSO) and 5 % albumin and stored in liquid nitrogen at −160 °C until use.
Preparation of bone implants
MSCs were thawed, centrifuged and washed three times with pre-warmed PBS to remove the residual freezing solution. Cell suspensions at a concentration of 0.5 or 5 × 106 cells/ml were prepared and transferred to vials. A pre-wetted hydroxyapatite bone scaffold CEM-OSTETIC® (Berkeley Advanced Biomaterials, Inc., Berkeley, USA) (0.02 g) was soaked in the cell suspension, and the suspension containing the material was centrifuged at 1,000 rpm. After centrifugation, the excess PBS was removed, and the content of the vial was mixed with a small sterile spatula to form a homogenous cell–material mixture and applied to the lesion of the animal immediately.
Surgery
After the induction of anesthesia with 5 % isoflurane in room air (flow 300 mL/min), the animals were maintained in 2 % isoflurane anesthesia (flow 300 mL/min) via a face mask throughout the operation. Under aseptic conditions, a 2-cm lateral skin incision at the L1–L3 level was made. The dorsal muscles were shifted laterally, and the body of the L2 vertebra was exposed from the ventrolateral side. A bone defect (2 × 5 × 1.5 mm) was created using a microdrill from a ventral approach. The defect was either left empty, filled with CEM-OSTETIC® material only, or filled with the material loaded with 0.5 or 5 × 106 MSCs and covered with a Hyprosorb® (Hypro, Otrokovice, Czech Republic) resorbable collagen membrane. The soft tissues and skin were sutured with nonresorbable thread. Transplanted animals (with a scaffold only or a scaffold loaded with cells) were immunosuppressed daily with 10 mg/kg intramuscular cyclosporine (10 mg/kg, Sandimmun®, Novartis, Basel Switzerland), and bacterial infection was prevented by Gentamicine (0.5 ml, Gentamicine Lek®, Lek Pharmaceuticals, Ljublanja, Slovenia). The rats were killed 8 weeks after surgery.
Histological analysis
At the end of the experiment, the animals were intracardially perfused under deep anesthesia (pentobarbital 150 mg/kg) with 4 % formaldehyde in 0.1 M PBS. The vertebrae were dissected, postfixed in 10 % formaldehyde and further decalcified with formic acid. From each sample three transversal blocks were embedded in paraffin, cut in 4-μm-thin sections and stained with hematoxylin–eosin (H&E) or naftol AS-D chloroacetate esterase for myeloid cells including polymorphonuclear granulocytes. Sections were examined under a light microscope and histomorphometrical analysis was performed using NIS-Elements software (Nikon Instruments, Inc., USA). Immunofluorescent staining for human mitochondria (anti-cytochrome c oxidase subunit II antibody, MT-CO2, ABcam, Cambridge, UK) was used to identify possible surviving transplanted cells. This antibody has frequently been used successfully for human cell detection in animal models [20–23]. Antigen–antibody complexes were visualized using goat anti-mouse IgG secondary antibody conjugated with Alexa-Fluor 488 (Molecular Probes). The samples were examined using a spectral confocal microscope (Carl-Zeiss, Germany).
Micro-CT analysis
From each group one specimen was selected for microtomographic analysis of its microstructure. The specimens were scanned using a previously developed setup [24]. The specimens were irradiated using a micro-focus X-ray tube L8601-01 (Hamamatsu Photonics K.K., Japan) with 5 μm emission spot, tungsten anode and divergent cone beam. For the imaging a flat panel X-ray detector C7942CA-22 (Hamamatsu Photonics K.K., Japan) with resolution 2,368 × 2,240 pixels and physical dimensions 120 × 120 mm was used. For the acquisition we used 360 projections with 1° increment. Maximum possible magnification was used, corresponding to source–object distance 170 mm and source–detector distance 500 mm. Because the L8601-01 source produces X-ray beam with continuous energy spectrum, beam hardening correction was applied to the acquired radiographs to account for the non-uniform attenuation of the samples. The images were reconstructed using a cone-beam backprojection algorithm which has been previously proven suitable for precise imaging of trabecular microarchitecture of whole-bone samples [25, 26]. Resolution of the reconstructed three-dimensional images is approximately 30 μm3.
Statistical analysis
The values are reported as mean values ± SEM. One-way ANOVA with a post hoc honestly significant difference (HSD) test was used for the comparison among individual groups.
Results
Histological examination
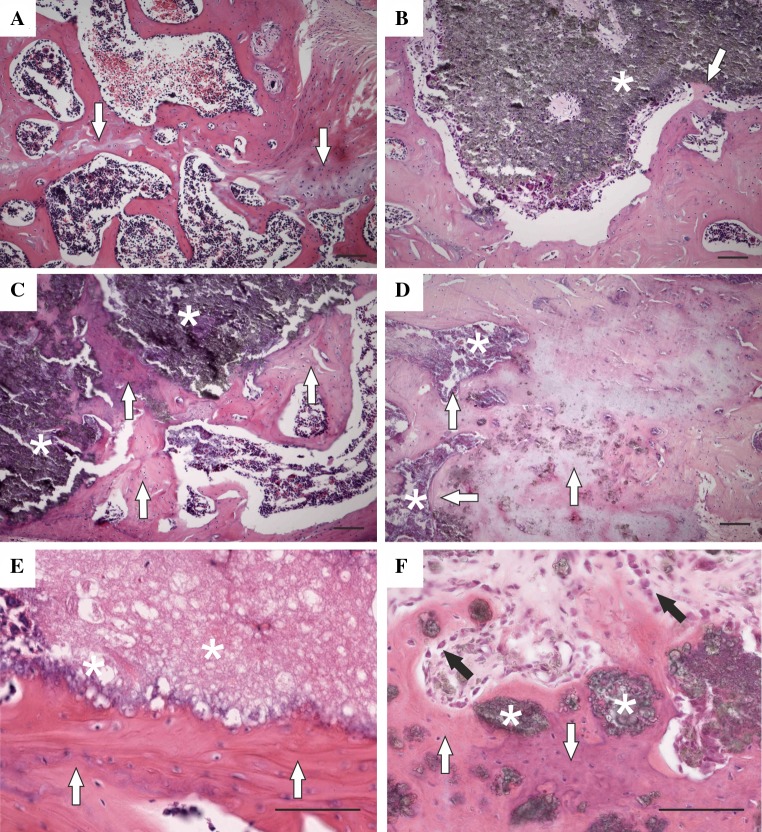
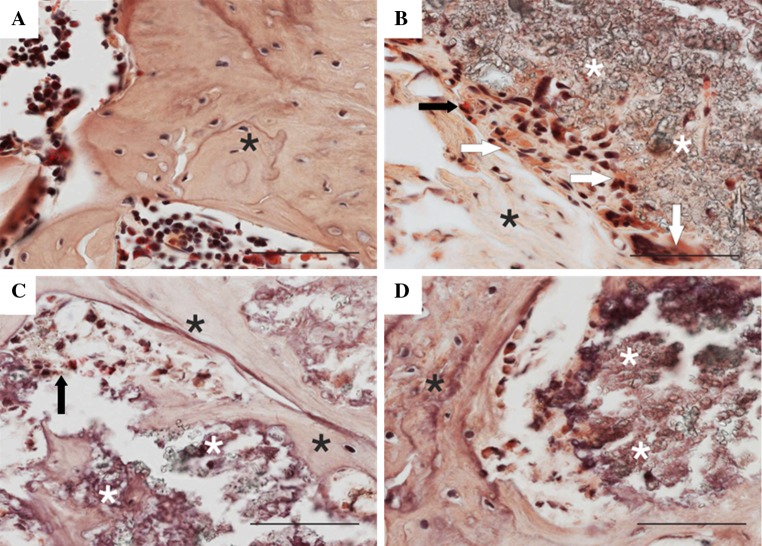
A description of representative sections is given for each group. In the group with a bone defect only, histological analysis revealed signs of bone healing with reactive osteoplasia and focal cartilaginous metaplasia (Fig. 1a). In the group receiving a hydroxyapatite bone scaffold alone, deposits of granular refringent material were clearly observable with an occasional granulomatous reaction against the exogenous material. Isolated regions of new bone and osteoid formation were found on the surface of the material (Fig. 1b). Qualitative differences were found in the groups receiving a hydroxyapatite bone scaffold loaded with MSCs. Larger trabeculae were formed around the implant, and the material was partially incorporated in the newly formed bone in the group receiving 0.5 million MSCs (Fig. 1c). More interestingly, significant formation of bone tissue in the defect was found in the group receiving a hydroxyapatite bone scaffold loaded with 5 million MSCs (Fig. 1d–f). The scaffold was apparently incorporated in the bone tissue and partially resorbed. In the vicinity of the implantation site, reactive osteoplasia (unrelated to the implanted material) and scar formation was present in some cases, probably as a consequence of surgical trauma. No significant granulomatous reaction, inflammatory changes or tumor formation were observed in any group transplanted with MSCs. Figure 2 shows representative sections stained with naftol AS-D chloroacetate esterase. In the bone defect only, the intratrabecular space contained hematopoietic bone marrow with multiple myeloid cells stained positively for naftol AS-D chloroacetate esterase (brightly red) (Fig. 2a). In the group treated with augmentation material alone, the material was typically surrounded by a rim of macrophages, giant cells and polymorphonuclear granulocytes (Fig. 2b). In the group treated with a combination of material loaded with 0.5 million cells, isolated polymorphonuclear granulocytes positive for naftol AS-D chloroacetate esterase were present (Fig. 2c). In the group treated with the hydroxyapatite bone scaffold loaded with 5 million cells, no polymorphonuclear granulocytes were present at the bone–material interface (Fig. 2d).
Fig. 1.
Representative micrographs of vertebral defects 8 weeks after surgery. a The image shows reactive osteoplasia with foci of cartilaginous metaplasia (white arrows) in the group with a bone defect only. b In the group treated with material alone, occasional new bone formation (white arrows) is apparent on the surface of the material (asterisk). c Trabeculae of mixed woven and lamellar (white arrows) on the surface of the augmentation material (asterisks) were formed in the group treated with 0.5 million cells. d In the group treated with 5 million MSCs, the scaffold (asterisks) was incorporated into a large amount of lamellar bone tissue (white arrows). e In the group treated with 5 million MSCs, broader trabeculae (white arrows) on the surface of the augmentation material were formed. f Areas of bone tissue (white arrows) with incorporated granules of augmentation material (asterisks) were seen in the group treated with 5 million MSCs. Rims of active osteoblasts (black arrows) are observable on the trabeculae surface (H&E, scale bar 100 μm)
Fig. 2.
a Defect only. Broad trabecula of predominantly woven bone with multiple cement lines as a result of the healing process (black asterisks). Intertrabecular spaces contain hematopoietic bone marrow with multiple myeloid cells stained positively for naftol AS-D chloroacetate esterase (brightly red). b Augmentation material (white asterisks) surrounded by a rim of macrophages and giant cells (white arrows) and fibrous tissue (black asterisks) in the group treated with material alone. Only isolated polymorphonuclear granulocytes positive for naftol AS-D chloroacetate esterase (black arrow) are present. c Isolated polymorphonuclear granulocytes positive for naftol AS-D chloroacetate esterase (arrow) in association with augmentation material (white asterisks) and newly formed bone trabeculae (black asterisks) in the group treated with 0.5 million cells. d Bone trabecula with a rim of active osteoblasts (black asterisks) and augmentation material (white asterisks). No polymorphonuclear granulocytes are present in the group treated with 5 million MSCs. Staining for naftol AS-D chloroacetate esterase, scale bars 100 μm
A few transplanted cells positive for the human mitochondrial marker (MT-CO2) were found at the site of the lesion 8 weeks after surgery (Fig. 3), suggesting only a limited survival of the transplanted cells. The staining for MT-CO2 was negative in animals transplanted with material alone.
Fig. 3.
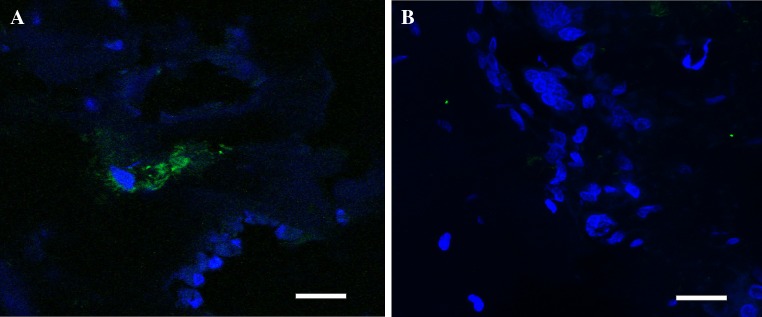
a Transplanted cells positive for the human mitochondria marker MTCO2 (green; DAPI, blue) were found at the site of the lesion 8 weeks after surgery in the group receiving 5 million MSCs. b The staining for MTCO2 was negative in animals transplanted with a scaffold alone. Scale bars 20 μm
Histomorphometry
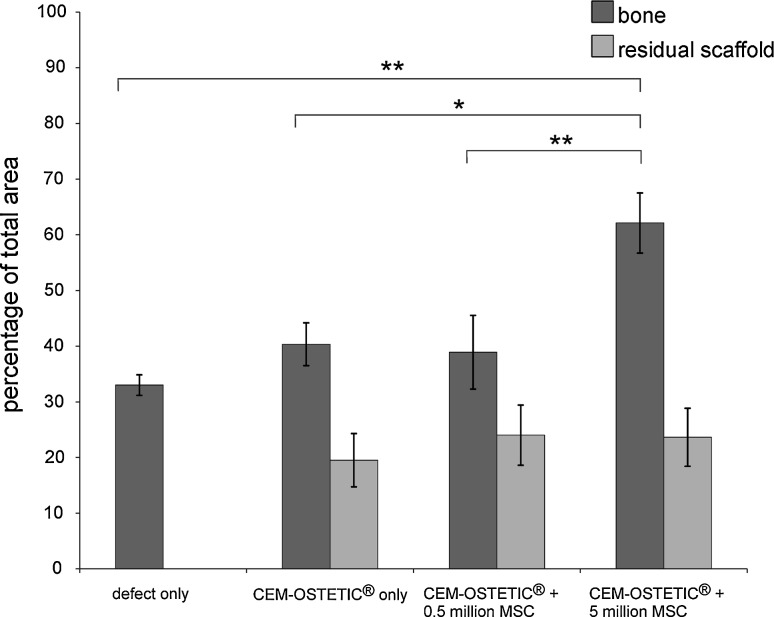
Quantitative analysis of the samples did not reveal significant differences in the percentage of new bone formation between the groups with a defect alone (33.84 ± 1.84 %) and with a defect treated by CEM-OSTETIC® alone (40.34 ± 3.85 %), suggesting the poor osteoinductive properties of the hydroxyapatite bone scaffold alone. However, significantly (p < 0.01) greater bone formation was found in the group treated with CEM-OSTETIC® combined with 5 million MSCs (65.60 ± 4.89 %) in comparison to those groups treated with a scaffold loaded with 0.5 million MSCs (38.91 ± 5.76 %) or a scaffold (p < 0.05) or a defect alone (p < 0.01). The amount of the residual scaffold material in the group treated with CEM-OSTETIC® plus 5 million MSCs was not significantly different from that seen in the other groups (Fig. 4).
Fig. 4.
Histomorphometrical analysis of new bone formation in vertebral bone defects. Significant bone formation was observed in the group transplanted with CEM-OSTETIC® and 5 million MSCs (n = 8) in comparison to CEM-OSTETIC® and 0.5 million MSCs (**p < 0.01) (n = 7), CEM-OSTETIC® alone (*p < 0.05) (n = 7) or a defect only (**p < 0.01) (n = 8). The amount of residual scaffold in the group treated with CEM-OSTETIC® and 5 million MSCs was not significantly different from the other groups
Micro-CT imaging
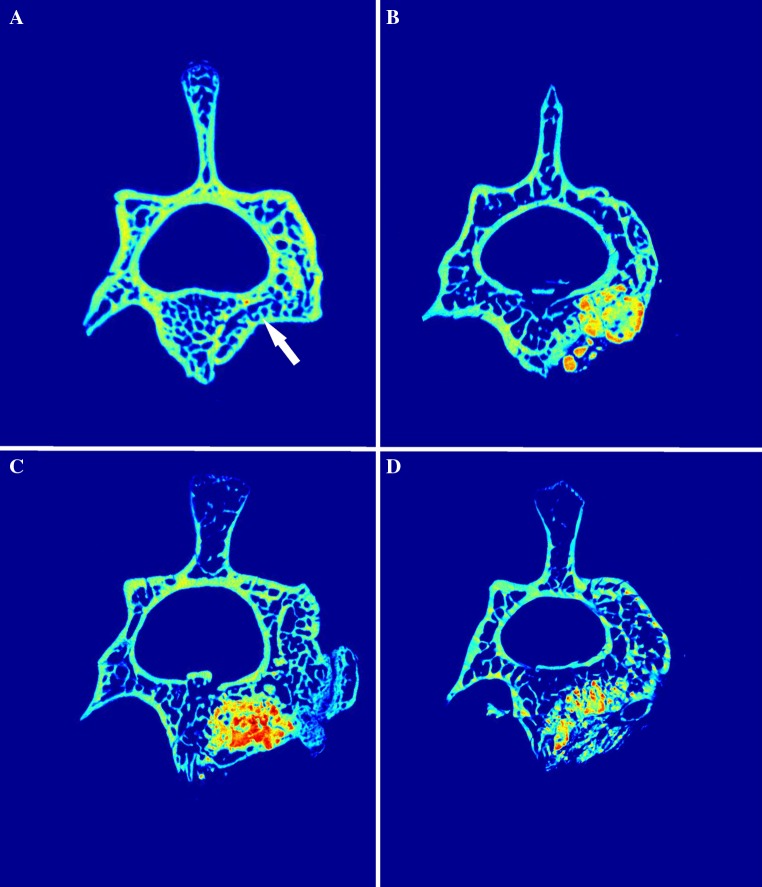
Eight weeks after the onset of the injury, in the group with a bone defect only, representative micro-CT scans showed the incomplete regeneration of the vertebral body in the defect area. The location of the vertebral defect was clearly observable (Fig. 5a). In the group with a defect filled with CEM-OSTETIC® alone, rather diffuse/noncompact deposits of the material were observable (high-density signal) (Fig. 5b). In contrast, in the group with CEM-OSTETIC® combined with 0.5 million MSCs, we found compact filling of the defect with hypertrophy above the niveau of the surrounding bone (Fig. 5c). This corresponds to our histological findings, in which we observed greater bone formation and incorporation of the material and a compact interface between the bone substitute material and the newly formed bone in this group. In the group with material combined with 5 million MSCs, we found a higher resorption rate of the scaffold with apparent new bone formed above the level of the original defect (Fig. 5d). These findings correspond to the histological (Figs. 1, 2) and histomorphometrical results (Fig. 4), where we found significantly greater bone formation and slightly increased material resorption.
Fig. 5.
Representative micro-CT scans of vertebrae extracted 8 weeks after the induction of a defect and the implantation of the material. a The image shows an incompletely regenerated empty defect (white arrow), b a defect filled with CEM-OSETIC® only, c with CEM-OSTETIC® combined with 0.5 million MSCs and d with CEM-OSTETIC® and 5 million MSCs
Discussion
In our study, we evaluated the effect of MSCs in combination with a hydroxyapatite-based scaffold (CEM-OSTETIC®) on bone regeneration in the vertebral body. We have observed the significant formation of bone tissue in the defect in animals receiving a hydroxyapatite bone scaffold loaded with 5 million MSCs. The scaffold was apparently incorporated in the bone tissue and partially resorbed. It was previously shown that the combination of MSCs and hydroxyapatite scaffolds improves bone formation when implanted in vivo. However, the healing process and bone formation are different in various types of bones. Therefore, several efforts have been made recently to establish a model of vertebral body defect for testing bioartificial substitutes and novel materials [27, 28]. In the recent study of Liang et al. [16], the authors established a rat model of a vertebral body defect and used a combination of a poly(lactic-co-glycolic acid) (PLGA) scaffold and BMP to treat the bone defect; they concluded that the model reflects the physiology of bone defects caused by a bone fracture or after the removal of a neoplasm from the vertebral body.
Due to their properties, MSCs represent a potential tool to improve the healing of bone defects in orthopedics. Before clinical application, the safety and functionality of the transplanted cells must be tested in appropriate animal models, and the cell and transplant preparation should be optimized. The process used in our study to cultivate and characterize the MSCs was carried out in accordance with good manufacturing practice (GMP) and approved for preclinical and clinical studies by the State Institute for Drug Control. The characteristics of the cell phenotype complied with the standards defined by the International Society for Cellular Therapy (ISCT) [29]. In order to simplify the preparation procedure of the graft as much as possible and to demonstrate its potential clinical applicability, we avoided lengthy cultivation, differentiation and expansion of the cells prior to implantation. Instead, we used the cells directly after thawing and washing out the freezing media. In the literature, various protocols for the preparation of MSC-based bone grafts have been reported [13, 30]. However, for clinical use, the graft preparation should be easy and feasible in hospital settings. Our results showed significantly greater bone formation in the group transplanted with the material loaded with 5 million MSCs in comparison to the groups treated with material loaded with 0.5 million cells or a scaffold alone, suggesting that high-density cell loading is necessary to promote the osteoinductive activity of the graft. Choi et al. [13] showed significant bone formation in a femoral defect model after the transplantation of a fibronectin-coated HA/TCP scaffold combined with 7.5 million adipose tissue-derived MSCs, but not with 0.75 million, in comparison to material alone and pointed out the cell-loading density-dependent manner of bone formation. Interestingly, when they loaded the material with 75 million MSCs, they found only a minor increase in bone formation in comparison to 7.5 million MSCs.
In our experiments, the amount of residual scaffold in the group transplanted with 5 million MSCs was not significantly different from the amounts found in the other groups. Interestingly, Zerbo et al. [31] reported that the degradation of hydroxyapatite after transplantation is likely to be due to chemical dissolution rather than due to the activity of osteoclasts, which is in agreement with our findings. We did not find any histological signs of osteoclastic resorption of the hydroxyapatite scaffold in our experiments.
The mechanisms by which the transplanted cells contribute to bone formation are still not completely understood. Huang et al. [14] showed that mesenchymal stem cells combined with a hydroxyapatite/PLGA/collagen I scaffold differentiate into osteoblasts and produce extracellular matrix within the graft for posterolateral spinal fusion. Other authors have shown that transplanted MSCs associated with a biomaterial survive only for a limited time after transplantation and recruit cells from the host tissue, thus contributing increased bone formation and bone healing rather indirectly [32].
We observed reactive osteoplasia with foci of cartilaginous metaplasia in the group with an empty defect and those animals treated with hydroxyapatite only, possibly as a result of the trauma-related activation and differentiation of mesenchymal stem cells normally present in the bone marrow. The presence of cartilaginous areas in the center of bony trabecules may be interpreted as a result of early endochondral ossification. Additionally, the micro-CT scans of the defect showed incomplete healing. This could lead to the decreased firmness of the bone, which in clinical practice could result in higher post-operative morbidity. However, when the scaffold was combined with MSCs, such cartilaginous tissue was not observed, and the deposition of bone matrix occurred on the surface of the bone substitute material. The endochondral type of ossification via the remodeling of cartilage occurs in a low oxygen environment with a poor blood supply [33] and is considered as a primary mechanism of bone fracture healing. Interestingly, Tortelli et al. [34] showed in a murine model of ectopic bone formation that the implantation of a hydroxyapatite scaffold material loaded with MSCs led to endochondral ossification and that the obtained bone was of host origin. On the other hand, the intramembranous ossification route does not involve cartilage formation and remodeling and has been observed after the transplantation of differentiated osteoblasts in a murine model [34]. In our experiments, the bone tissue was apparently of host origin, and we found only very few human cells in the bone lesion at the end of the experiment. We can speculate that after the MSCs were transplanted, they provided various growth factors and cytokines [35] and supported the attraction of host osteoprogenitor cells via paracrine signalling, thus creating a favorable environment for the healing of the bone defect via endochondral ossification.
A cellular immunological reaction might have negative effects on bone healing, lead to the formation of fibrous tissue and in turn influence the mechanical properties of the new bone, which may contraindicate the use of hydroxyapatite-based scaffolds for vertebral bone repair. In our study, we observed a granulomatous foreign body reaction at the material–bone interface in animals transplanted with the material alone. In contrast, such an inflammatory reaction around the transplant was apparently reduced in the groups receiving MSCs, likely due to the immunomodulatory and immunosuppressive properties of MSCs [36], which might significantly improve the bone healing process [37]. In our experiments, we used cyclosporine A as an immunosuppressant. Several groups observed potentiation between cyclosporin A and the immunosuppressive effect of human MSCs in vitro [38, 39]. However, since the animals with an HA scaffold only also received immunosuppression, the effect observed in animals transplanted with a scaffold loaded with cells is most likely due to the presence of the cells rather than just immunosuppression. Different experimental approaches to xenogenic MSC transplantation in terms of using immunosuppressive drugs are discussed in the literature. Tcacencu et al. [40] implanted human MSCs seeded on a peptide hydrogel in the mandible of immunosuppressed rats (with cyclosporine A) and reported significantly better alveolar bone density and decreased osteoclast numbers at the site of the injury, which they ascribed to the immunomodulatory effect of the MSCs and their interaction with host monocytes and macrophages. Kim et al. [10] showed that transplanted xenogenic bone marrow MSCs loaded onto an HA/β-TCP scaffold generated new bone formation in the posterolateral lumbar spine of non-immunosuppressed rabbits and pointed out the low immunogenicity and good survival of the transplanted MSCs.
Consistent with other studies that used MSCs for orthopedic treatment, we did not observe any signs of neoplasm formation [41]. Also, no significant bone deformation or spinal cord compression was observed in the transplanted animals, suggesting the safety of the transplantation procedure.
Conclusion
Our study showed that the implantation of a hydroxyapatite scaffold combined with human MSCs, cultivated and expanded according to a large-scale manufacturing protocol, was safe and significantly increased bone formation in a rat model of vertebral body defect. MSCs combined with a hydroxyapatite scaffold might represent a safe and effective alternative in the treatment of vertebral bone defects. We tested a simple procedure of graft preparation, which might be easily reproduced in hospital settings. Our results will contribute to the translation of cell therapies into clinical practice.
Acknowledgments
This work was supported by the Grant Agency of the Czech Republic (GACR 304/10/0320, GACR304/12/0259), and Grant Agency of the Ministry of Health of the Czech Republic (NT13477). The authors thank James Dutt for his critical reading of the manuscript.
Conflict of interest
The authors report no conflicts of interest.
References
- 1.Barsa P. Vertebroplasty—treatment options for structurally insufficient vertebras. Cesk Slov Neurol N. 2012;75:8–17. [Google Scholar]
- 2.Sedy J, Urdzikova L, Jendelova P, Sykova E. Methods for behavioral testing of spinal cord injured rats. Neurosci Biobehav Rev. 2008;32:550–580. doi: 10.1016/j.neubiorev.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Sykova E, Homola A, Mazanec R, Lachmann H, Konradova SL, Kobylka P, Padr R, Neuwirth J, Komrska V, Vavra V, Stulik J, Bojar M. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transpl. 2006;15:675–687. doi: 10.3727/000000006783464381. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Lu B, Chen L, Chang J. Evaluation of an osteostimulative putty in the sheep spine. J Mater Sci Mater Med. 2011;22:185–191. doi: 10.1007/s10856-010-4175-5. [DOI] [PubMed] [Google Scholar]
- 5.Bauer TW, Muschler GF (2000) Bone graft materials. An overview of the basic science. Clin Orthop Relat Res 371:10–27 [PubMed]
- 6.Niu X, Feng Q, Wang M, Guo X, Zheng Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J Control Release. 2009;134:111–117. doi: 10.1016/j.jconrel.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Srouji S, Rachmiel A, Blumenfeld I, Livne E. Mandibular defect repair by TGF-beta and IGF-1 released from a biodegradable osteoconductive hydrogel. J Craniomaxillofac Surg. 2005;33:79–84. doi: 10.1016/j.jcms.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Wang K, Shi Z, Yang H, Dang X, Wang W. Osteogenesis of the construct combined BMSCs with beta-TCP in rat. J Plast Reconstr Aesthet Surg. 2010;63:227–232. doi: 10.1016/j.bjps.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Griffin M, Iqbal SA, Bayat A. Exploring the application of mesenchymal stem cells in bone repair and regeneration. J Bone Joint Surg Br. 2011;93:427–434. doi: 10.1302/0301-620X.93B4.25249. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Park JB, Lee JK, Park EY, Park EA, Riew KD, Rhee SK. Transplanted xenogenic bone marrow stem cells survive and generate new bone formation in the posterolateral lumbar spine of non-immunosuppressed rabbits. Eur Spine J. 2008;17:1515–1521. doi: 10.1007/s00586-008-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noel D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Therapy. 2010;1:2. doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi HJ, Kim JM, Kwon E, Che JH, Lee JI, Cho SR, Kang SK, Ra JC, Kang BC. Establishment of efficacy and safety assessment of human adipose tissue-derived mesenchymal stem cells (hATMSCs) in a nude rat femoral segmental defect model. J Korean Med Sci. 2011;26:482–491. doi: 10.3346/jkms.2011.26.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang JW, Lin SS, Chen LH, Liu SJ, Niu CC, Yuan LJ, Wu CC, Chen WJ. The use of fluorescence-labeled mesenchymal stem cells in poly(lactide-co-glycolide)/hydroxyapatite/collagen hybrid graft as a bone substitute for posterolateral spinal fusion. J Trauma. 2011;70:1495–1502. doi: 10.1097/TA.0b013e318216b9ee. [DOI] [PubMed] [Google Scholar]
- 15.Miura M, Miura Y, Sonoyama W, Yamaza T, Gronthos S, Shi S. Bone marrow-derived mesenchymal stem cells for regenerative medicine in craniofacial region. Oral Dis. 2006;12:514–522. doi: 10.1111/j.1601-0825.2006.01300.x. [DOI] [PubMed] [Google Scholar]
- 16.Liang H, Wang K, Shimer AL, Li X, Balian G, Shen FH. Use of a bioactive scaffold for the repair of bone defects in a novel reproducible vertebral body defect model. Bone. 2010;47:197–204. doi: 10.1016/j.bone.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Zhu XS, Zhang ZM, Mao HQ, Geng DC, Zou J, Wang GL, Zhang ZG, Wang JH, Chen L, Yang HL. A novel sheep vertebral bone defect model for injectable bioactive vertebral augmentation materials. J Mater Sci Mater Med. 2011;22:159–164. doi: 10.1007/s10856-010-4191-5. [DOI] [PubMed] [Google Scholar]
- 18.Luize DS, Bosco AF, Bonfante S, de Almeida JM. Influence of ovariectomy on healing of autogenous bone block grafts in the mandible: a histomorphometric study in an aged rat model. Int J Oral Maxillofac Implants. 2008;23:207–214. [PubMed] [Google Scholar]
- 19.Turnovcova K, Ruzickova K, Vanecek V, Sykova E, Jendelova P. Properties and growth of human bone marrow mesenchymal stromal cells cultivated in different media. Cytotherapy. 2009;11:874–885. doi: 10.3109/14653240903188947. [DOI] [PubMed] [Google Scholar]
- 20.Abdouh M, Facchino S, Chatoo W, Balasingam V, Ferreira J, Bernier G. BMI1 sustains human glioblastoma multiforme stem cell renewal. J Neurosci. 2009;29:8884–8896. doi: 10.1523/JNEUROSCI.0968-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amemori T, Romanyuk N, Jendelova P, Herynek V, Turnovcova K, Prochazka P, Kapcalova M, Cocks G, Price J, Sykova E. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Res Therapy. 2013;4:68. doi: 10.1186/scrt219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cocks G, Romanyuk N, Amemori T, Jendelova P, Forostyak O, Jeffries AR, Perfect L, Thuret S, Dayanithi G, Sykova E, Price J. Conditionally immortalized stem cell lines from human spinal cord retain regional identity and generate functional V2a interneurons and motorneurons. Stem Cell Res Therapy. 2013;4:69. doi: 10.1186/scrt220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruzicka J, Romanyuk N, Hejcl A, Vetrik M, Hruby M, Cocks G, Cihlar J, Pradny M, Price J, Sykova E, Jendelova P. Treating spinal cord injury in rats with a combination of human fetal neural stem cells and hydrogels modified with serotonin. Acta Neurobiol Exp. 2013;73:102–115. doi: 10.55782/ane-2013-1925. [DOI] [PubMed] [Google Scholar]
- 24.Jakubek J, Holy T, Jakubek M, Vavrik D, Vykydal Z. Experimental system for high resolution X-ray transmission radiography. Nucl Instr Methods Phys Res Sect A Accel Spectrom Detect Assoc Equip. 2006;563:278–281. doi: 10.1016/j.nima.2006.01.033. [DOI] [Google Scholar]
- 25.Kytyr D, Jirousek O, Dammer J (2011) High resolution X-ray imaging of bone-implant interface by large area flat-panel detector. J Instrum 6:1–5
- 26.Vavrik D, Dammer J, Jakubek J, Jeon I, Jirousek O, Kroupa M, Zlamal P. Advanced X-ray radiography and tomography in several engineering applications. Nucl Instr Methods Phys Res Sect A Accel Spectrom Detect Assoc Equip. 2011;633:S152–S155. doi: 10.1016/j.nima.2010.06.152. [DOI] [Google Scholar]
- 27.Fujishiro T, Bauer TW, Kobayashi N, Kobayashi H, Sunwoo MH, Seim HB, 3rd, Turner AS. Histological evaluation of an impacted bone graft substitute composed of a combination of mineralized and demineralized allograft in a sheep vertebral bone defect. J Biomed Mater Res A. 2007;82:538–544. doi: 10.1002/jbm.a.31056. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi H, Fujishiro T, Belkoff SM, Kobayashi N, Turner AS, Seim HB, 3rd, Zitelli J, Hawkins M, Bauer TW. Long-term evaluation of a calcium phosphate bone cement with carboxymethyl cellulose in a vertebral defect model. J Biomed Mater Res A. 2009;88:880–888. doi: 10.1002/jbm.a.31933. [DOI] [PubMed] [Google Scholar]
- 29.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima T, Iizuka H, Tsutsumi S, Kayakabe M, Takagishi K (2007) Evaluation of posterolateral spinal fusion using mesenchymal stem cells: differences with or without osteogenic differentiation. Spine (Phila Pa 1976) 32:2432–2436. doi:10.1097/BRS.0b013e3181573924 [DOI] [PubMed]
- 31.Zerbo IR, Bronckers AL, de Lange G, Burger EH. Localisation of osteogenic and osteoclastic cells in porous beta-tricalcium phosphate particles used for human maxillary sinus floor elevation. Biomaterials. 2005;26:1445–1451. doi: 10.1016/j.biomaterials.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Boukhechba F, Balaguer T, Bouvet-Gerbettaz S, Michiels JF, Bouler JM, Carle GF, Scimeca JC, Rochet N. Fate of bone marrow stromal cells in a syngenic model of bone formation. Tissue Eng Part A. 2011;17:2267–2278. doi: 10.1089/ten.tea.2010.0461. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008;15:53–76. doi: 10.22203/ecm.v015a05. [DOI] [PubMed] [Google Scholar]
- 34.Tortelli F, Tasso R, Loiacono F, Cancedda R. The development of tissue-engineered bone of different origin through endochondral and intramembranous ossification following the implantation of mesenchymal stem cells and osteoblasts in a murine model. Biomaterials. 2010;31:242–249. doi: 10.1016/j.biomaterials.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 35.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 37.Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, Baan C, Dahlke MH. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol. 2010;10:1496–1500. doi: 10.1016/j.intimp.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringden O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 39.Maccario R, Moretta A, Cometa A, Montagna D, Comoli P, Locatelli F, Podesta M, Frassoni F. Human mesenchymal stem cells and cyclosporin a exert a synergistic suppressive effect on in vitro activation of alloantigen-specific cytotoxic lymphocytes. Biol Blood Marrow Transpl. 2005;11:1031–1032. doi: 10.1016/j.bbmt.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 40.Tcacencu I, Karlstrom E, Cedervall J, Wendel M. Transplanted human bone marrow mesenchymal stem cells seeded onto peptide hydrogel decrease alveolar bone loss. Biores Open Access. 2012;1:215–221. doi: 10.1089/biores.2012.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centeno CJ, Schultz JR, Cheever M, Freeman M, Faulkner S, Robinson B, Hanson R. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2011;6:368–378. doi: 10.2174/157488811797904371. [DOI] [PubMed] [Google Scholar]